Mothers with autoimmune diseases (AID) may have exacerbations of their disease during pregnancy and postpartum period, with fetal implications and neonatal complications.
The aim of this study was to describe miscarriages during pregnancy and postpartum problems among mothers with AID and associated neonatal pathology. Retrospective data was recorded from 2004 to 2010. 29 mothers with AID were analyzed, 65% of whom had lupus erythematosus (SLE). There were 52 pregnancies, which resulted in 39 newborns. There were 10 instances of maternal complications (25.6%) during the pregnancies, including 1 with digital vasculitis, 1 with pancreatitis, 1 outbreak of glomerulonephritis, 1 case of gestational diabetes, 2 patients at risk for preterm birth, 3 with preeclampsia and 1 with eclampsia. During the postpartum period, there was one case of SLE exacerbation.
Among the newborns 20.5% had low birth weight and 4 exhibited the transplacental passage of maternal antibodies with one case of neonatal lupus. Among complications beyond the neonatal period, 8 (20.5%) children developed asthma, one presented negative ANA oligoarthritis and another presented immune thrombocytopenic purpura.
In our hospital, the rates of miscarriage, prematurity and LBW among the newborns of mothers with AID are similar to those reported in the literature. The observation of a case of NL with the transplacental passage of anti-Sm is remarkable.
Las madres con enfermedad autoinmunitaria (EAI) pueden presentar exacerbaciones de su enfermedad durante la gestación y el puerperio, con implicaciones fetales y neonatales.
El objetivo del presente estudio fue describir las incidencias de estas madres y la afección neonatal asociada. Se realizó un análisis retrospectivo entre los años 2004 a 2010, controlándose 29 madres con EAI. Se registraron 52 embarazos, 39 RN vivos y 13 abortos.
Durante la gestación se produjeron 10 complicaciones: una vasculitis digital, una pancreatitis, una glomerulonefritis, una diabetes gestacional, 2 amenazas de parto prematuro, 3 preeclampsias y 1 eclampsia. En el posparto, una exacerbación lúpica. Entre los RN 20,5% presentaron bajo peso y 4 transferencia de anticuerpos maternos con un lupus neonatal (LNN). Posteriormente, 8 niños (20,5%) desarrollaron asma, uno oligoartritis ANA negativa y otro púrpura trombocitopénica autoinmunitaria.
En nuestro hospital la tasa de abortos y prematuridad es similar a la descrita en la literatura. Destaca la presencia de un caso de LNN con paso transplacentario de anti-Sm.
Autoimmune diseases (AID) affect mainly young women of childbearing age, so pregnancy is common in these patients. Pregnancy may exacerbate underlying maternal disease, causing significant morbidity and mortality during pregnancy and potentially serious effects on the fetus.1,2
In patients with systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS), pregnancy is recommended only if the patient has inactive disease and has not received cytotoxic treatment in the 6 months prior to pregnancy. The recognition of a disease outbreak during pregnancy can be difficult, because symptoms and signs may be similar to those of a normal pregnancy. For these reasons, pregnancy should be planned and controlled by a multidisciplinary team of obstetricians, rheumatologists, internists and neonatologists.1–3
In SLE patients with good disease control, the risk of kidney disease is 5%–10%. This percentage increases to 50%–60% in patients in whom pregnancy coincides with an outbreak of the disease.3 In patients with systemic sclerosis or mixed connective tissue disease and pulmonary hypertension, pregnancy is not recommended. Patients with systemic sclerosis also may have renal crisis during pregnancy and postpartum.
The children of mothers carrying anti-La/SSB or anti-Ro/SSA antibodies have an increased risk of presenting NNL and/or congenital atrioventricular block. In some series, NNL risk rises to 1.5%–2% of live newborns (RN), especially children of mother with SLE or primary Sjögren's syndrome.4–6
The presence of antiphospholipid antibodies (APS) during pregnancy has been associated with multiple complications such as increased risk of abortions and stillbirth, prematurity, intrauterine growth retardation and low birth weight, preeclampsia, eclampsia and HELLP syndrome7,8 (hemolysis, elevated liver enzymes and thrombocytopenia), among others.
This study describes the incidence of both maternal and neonatal complications in patients with controlled AID in our center.
Materials and MethodsWe retrospectively reviewed the data from the medical records of pregnant patients with controlled AID seen by the departments of Rheumatology, Internal Medicine and Obstetrics of the Hospital General de Granollers (Barcelona) between 2004 and 2010 and also reviewed medical records of newborns and follow-up of these patients until the end of the study.
We included patients with SLE, subacute cutaneous lupus (SACL), primary Sjögren's syndrome, primary APS, mixed connective tissue disease and systemic sclerosis.
Maternal data was collected on: type of underlying disease, number of living children, number of abortions, antibodies present at baseline or during follow-up of pregnancy (anti-Ro, anti-La, anti-RNP, anti-Sm), presence or antiphospholipid antibodies (APLA) and complications during pregnancy, childbirth and postpartum (preterm labor, preeclampsia, eclampsia, HELLP syndrome, proteinuria, glomerulonephritis, fever or other complications).
ANA were determined by IIF, titrated and considered positive if over 1/160. Anti-Ro, anti-La, anti-RNP and anti-Sm were determined by ELISA, considering as positive a value greater than 1.
Among infants, data collected were: gender, gestational age at birth, type of delivery (dystocic, eutocic), birth weight, presence of placental transfer of antibodies, performance of an ECG at birth, result of the ECG, neonatal complications and long-term complications.
At the time of data collection we conducted a telephone survey of patients to see if their children had developed any of the following diseases: bronchial asthma, skin atopy, celiac disease, diabetes, thyroid disease or other autoimmune disease.
ResultsDuring the 7 years analyzed there were 29 mothers with AID under control in our center.
There were 52 pregnancies with 39 live births (75%) and 13 abortions (25%). All patients who had had abortions had APLA and one met criteria for primary APS.
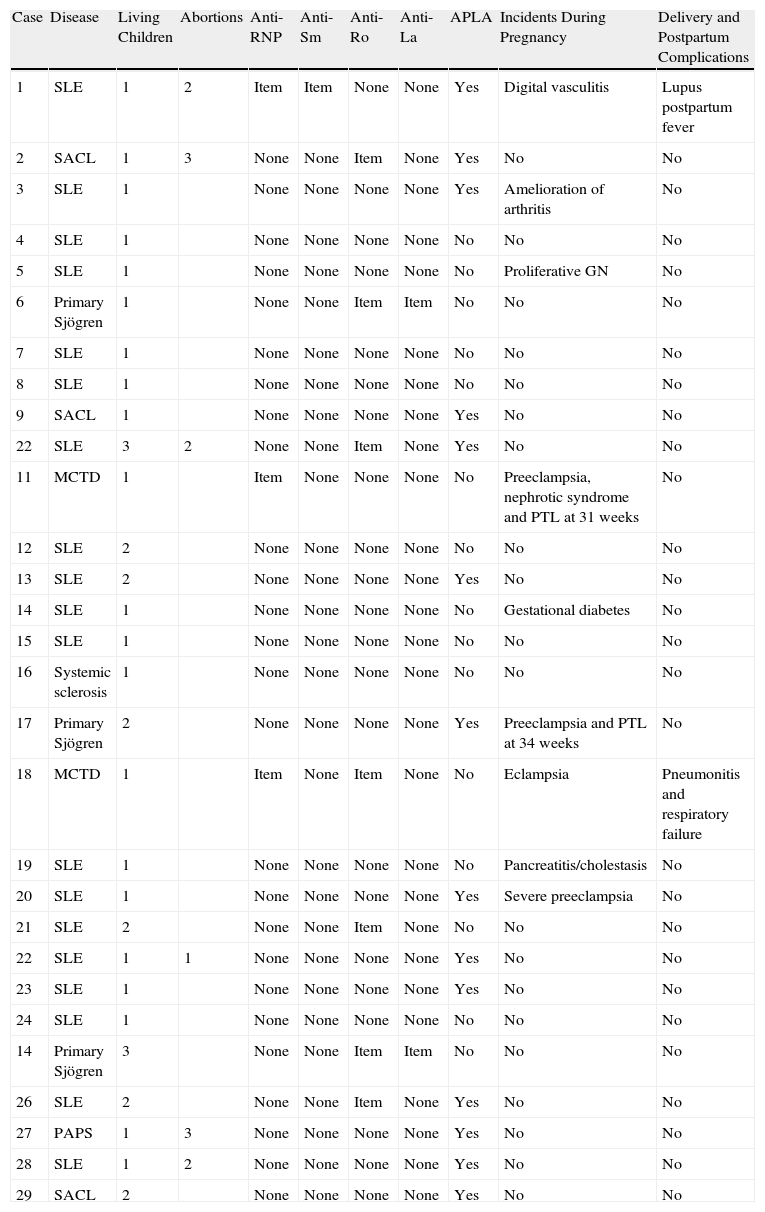
Maternal Data (Table 1)The mean age at the time of pregnancy of the 29 patients was 30.8 years (24–41 years). Maternal AID registered were: 19 SLE (65.5%), 3 LCSA, 3 primary Sjögren's syndrome, 2 mixed connective tissue disease, one primary APS and one limited systemic sclerosis. Cutaneous lupus cases corresponded with LCSA, with the presence of anti-Ro and without systemic manifestations.
Maternal Data.
| Case | Disease | Living Children | Abortions | Anti-RNP | Anti-Sm | Anti-Ro | Anti-La | APLA | Incidents During Pregnancy | Delivery and Postpartum Complications |
| 1 | SLE | 1 | 2 | Item | Item | None | None | Yes | Digital vasculitis | Lupus postpartum fever |
| 2 | SACL | 1 | 3 | None | None | Item | None | Yes | No | No |
| 3 | SLE | 1 | None | None | None | None | Yes | Amelioration of arthritis | No | |
| 4 | SLE | 1 | None | None | None | None | No | No | No | |
| 5 | SLE | 1 | None | None | None | None | No | Proliferative GN | No | |
| 6 | Primary Sjögren | 1 | None | None | Item | Item | No | No | No | |
| 7 | SLE | 1 | None | None | None | None | No | No | No | |
| 8 | SLE | 1 | None | None | None | None | No | No | No | |
| 9 | SACL | 1 | None | None | None | None | Yes | No | No | |
| 22 | SLE | 3 | 2 | None | None | Item | None | Yes | No | No |
| 11 | MCTD | 1 | Item | None | None | None | No | Preeclampsia, nephrotic syndrome and PTL at 31 weeks | No | |
| 12 | SLE | 2 | None | None | None | None | No | No | No | |
| 13 | SLE | 2 | None | None | None | None | Yes | No | No | |
| 14 | SLE | 1 | None | None | None | None | No | Gestational diabetes | No | |
| 15 | SLE | 1 | None | None | None | None | No | No | No | |
| 16 | Systemic sclerosis | 1 | None | None | None | None | No | No | No | |
| 17 | Primary Sjögren | 2 | None | None | None | None | Yes | Preeclampsia and PTL at 34 weeks | No | |
| 18 | MCTD | 1 | Item | None | Item | None | No | Eclampsia | Pneumonitis and respiratory failure | |
| 19 | SLE | 1 | None | None | None | None | No | Pancreatitis/cholestasis | No | |
| 20 | SLE | 1 | None | None | None | None | Yes | Severe preeclampsia | No | |
| 21 | SLE | 2 | None | None | Item | None | No | No | No | |
| 22 | SLE | 1 | 1 | None | None | None | None | Yes | No | No |
| 23 | SLE | 1 | None | None | None | None | Yes | No | No | |
| 24 | SLE | 1 | None | None | None | None | No | No | No | |
| 14 | Primary Sjögren | 3 | None | None | Item | Item | No | No | No | |
| 26 | SLE | 2 | None | None | Item | None | Yes | No | No | |
| 27 | PAPS | 1 | 3 | None | None | None | None | Yes | No | No |
| 28 | SLE | 1 | 2 | None | None | None | None | Yes | No | No |
| 29 | SACL | 2 | None | None | None | None | Yes | No | No |
APLA: antiphospholipid antibodies; PTL: preterm labor; LCSA: Subacute cutaneous lupus; SLE: systemic lupus erythematosus; MCTD: mixed connective tissue disease; Neg: negative; Pos: positive, WG: weeks of gestation.
Of the 39 pregnancies that came to term, there were complications in 10 (25.6%): one case of digital vasculitis, one of the pancreatitis with cholestasis, one with glomerulonephritis, one with gestational diabetes, 2 with preterm labor, 3 with severe preeclampsia and one with eclampsia. All complications were resolved without sequelae. One patient showed improvement of arthritis. Comparatively, patients with SLE had a higher rate of complications than patients with other types of AID.
One case presented postpartum fever and one case had eclampsia with pneumonitis and respiratory failure requiring intubation and admission to intensive care and responded favorably to steroid bolus treatment.
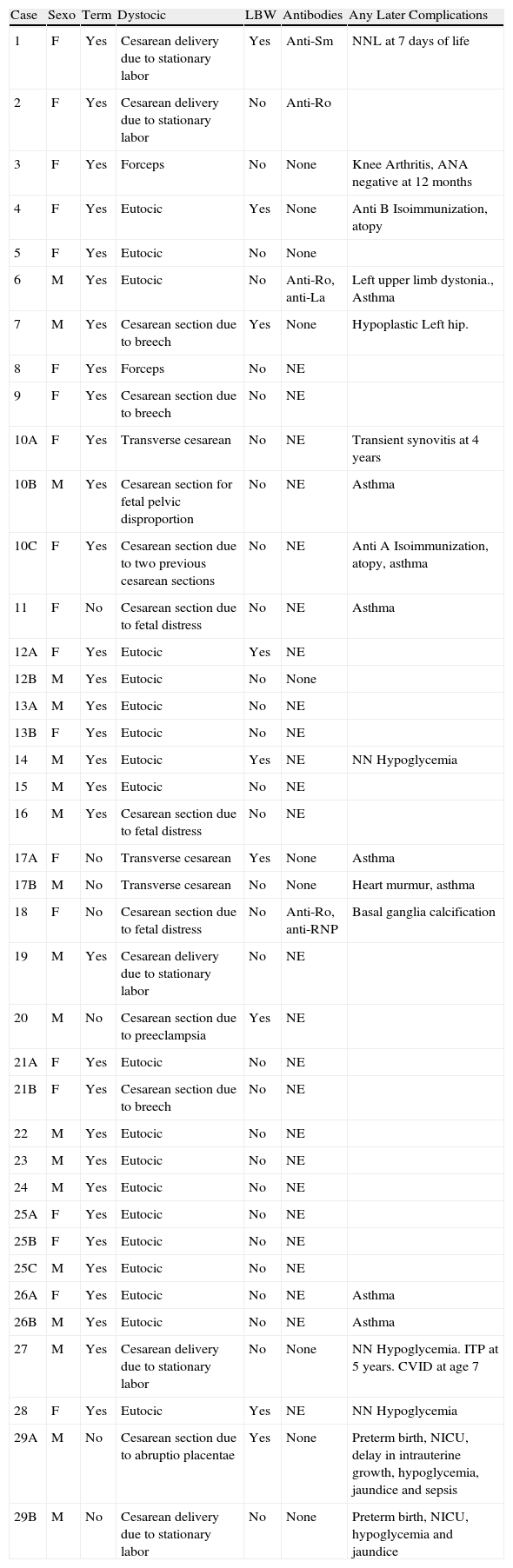
Neonatal Data (Table 2)Among the newborns, 7 were preterm (<37 weeks) (17.9%), 8 had low weight for gestational age (LWGA) (weight below the 3rd percentile in the reference tables for gestational age) (20.5%) and 4 had placental transfer of maternal antibodies: a case of placental transfer of anti-Sm, a case of anti-Ro, a case of anti-Ro and anti-La and a case of anti-Ro and anti-RNP. We performed antibody assays in 14 of the 39 infants born (35.8%).
Neonatal Data.
| Case | Sexo | Term | Dystocic | LBW | Antibodies | Any Later Complications |
| 1 | F | Yes | Cesarean delivery due to stationary labor | Yes | Anti-Sm | NNL at 7 days of life |
| 2 | F | Yes | Cesarean delivery due to stationary labor | No | Anti-Ro | |
| 3 | F | Yes | Forceps | No | None | Knee Arthritis, ANA negative at 12months |
| 4 | F | Yes | Eutocic | Yes | None | Anti B Isoimmunization, atopy |
| 5 | F | Yes | Eutocic | No | None | |
| 6 | M | Yes | Eutocic | No | Anti-Ro, anti-La | Left upper limb dystonia., Asthma |
| 7 | M | Yes | Cesarean section due to breech | Yes | None | Hypoplastic Left hip. |
| 8 | F | Yes | Forceps | No | NE | |
| 9 | F | Yes | Cesarean section due to breech | No | NE | |
| 10A | F | Yes | Transverse cesarean | No | NE | Transient synovitis at 4 years |
| 10B | M | Yes | Cesarean section for fetal pelvic disproportion | No | NE | Asthma |
| 10C | F | Yes | Cesarean section due to two previous cesarean sections | No | NE | Anti A Isoimmunization, atopy, asthma |
| 11 | F | No | Cesarean section due to fetal distress | No | NE | Asthma |
| 12A | F | Yes | Eutocic | Yes | NE | |
| 12B | M | Yes | Eutocic | No | None | |
| 13A | M | Yes | Eutocic | No | NE | |
| 13B | F | Yes | Eutocic | No | NE | |
| 14 | M | Yes | Eutocic | Yes | NE | NN Hypoglycemia |
| 15 | M | Yes | Eutocic | No | NE | |
| 16 | M | Yes | Cesarean section due to fetal distress | No | NE | |
| 17A | F | No | Transverse cesarean | Yes | None | Asthma |
| 17B | M | No | Transverse cesarean | No | None | Heart murmur, asthma |
| 18 | F | No | Cesarean section due to fetal distress | No | Anti-Ro, anti-RNP | Basal ganglia calcification |
| 19 | M | Yes | Cesarean delivery due to stationary labor | No | NE | |
| 20 | M | No | Cesarean section due to preeclampsia | Yes | NE | |
| 21A | F | Yes | Eutocic | No | NE | |
| 21B | F | Yes | Cesarean section due to breech | No | NE | |
| 22 | M | Yes | Eutocic | No | NE | |
| 23 | M | Yes | Eutocic | No | NE | |
| 24 | M | Yes | Eutocic | No | NE | |
| 25A | F | Yes | Eutocic | No | NE | |
| 25B | F | Yes | Eutocic | No | NE | |
| 25C | M | Yes | Eutocic | No | NE | |
| 26A | F | Yes | Eutocic | No | NE | Asthma |
| 26B | M | Yes | Eutocic | No | NE | Asthma |
| 27 | M | Yes | Cesarean delivery due to stationary labor | No | None | NN Hypoglycemia. ITP at 5 years. CVID at age 7 |
| 28 | F | Yes | Eutocic | Yes | NE | NN Hypoglycemia |
| 29A | M | No | Cesarean section due to abruptio placentae | Yes | None | Preterm birth, NICU, delay in intrauterine growth, hypoglycemia, jaundice and sepsis |
| 29B | M | No | Cesarean delivery due to stationary labor | No | None | Preterm birth, NICU, hypoglycemia and jaundice |
LBW: low weight for gestational age; ECG: electrocardiogram; F: female; CVID: common variable immunodeficiency; NNL: neonatal lupus; M: male; Neg: negative; NN: neonatal; NE: Not evaluated; ITP: immune thrombocytopenic purpura; NICU: neonatal intensive care unit.
The infant with transplacental transfer of anti-Sm antibodies developed skin lesions on the face and neck after 7 days, and was diagnosed with transient NNL. We performed an ECG and echocardiography which were normal. The evolution was favorable.
ECG was performed in 33% of infants studied, all normal.
Observations Outside the Neonatal Period in the Children of Mothers With Autoimmune DiseaseWe conducted a telephone survey of 27 of the 29 mothers (97% response rate), with a mean follow-up of children of 4 years (1–7 years).
The son of a patient with SLE developed an RF negative, self-limited oligoarthritis and ANA after one year and the child of a mother with primary APS developed immune thrombocytopenic purpura at age 5, and was diagnosed at age 7 with common variable immunodeficiency.
Eight children of mothers with AID developed bronchial asthma (20%) and 2 developed skin atopy.
No cases of thyroid disease, celiac disease, diabetes or other immune-based diseases were seen.
DiscussionAID often affects women of childbearing age and pregnancy remains common among these patients. Although the diagnosis, treatment and prognosis of these diseases have improved greatly in recent years, pregnancy is still a critical time for patients with AID, as the number and severity of complications, not only both maternal and fetal but also neonatal, are higher than in the general population.1,9–12
In our study, the rate of maternal complications, abortions, prematurity and low birth weight is similar to that described in the literature.1,9,12–14 Ucar et al.,1 conducted a prospective study that followed 35 women with SLE during pregnancy and the postpartum period, registering 87% of liveborn infants and 13% of abortions, with a rate of 8.7% and only one case of LBW and 2 cases of preeclampsia. In another similar study, Le Thi Huong et al.,9 monitored 35 patients with SLE, with or without APS, in which there were 62 pregnancies with 51 live newborns (82%) and 11 abortions (18%). The study showed a prematurity rate of 41%, a single case of a child of LBW from a mother with APS and 3 cases of preeclampsia.
There are fewer studies of pregnancy in other autoimmune diseases. Julkunen et al.,14 conducted a retrospective collection of data from pregnant patients with primary Sjögren's syndrome and observed a higher rate of abortions than in the general population, with a relative risk of fetal loss of 2.7. This study showed no higher incidence of preterm birth or LBW. Lidar et al.,15 have published another study on pregnancy in patients with systemic sclerosis, showing a higher incidence of prematurity and LBW among infants of these patients.
During follow-up we found a single case of NNL. NNL classically has been associated with placental transfer of anti-Ro, anti-La and exceptionally, anti-RNP.16 In our sample we highlight the presence of a case of NNL associated with transplacental passage of anti-Sm antibodies, something not previously reported in the literature.4
Among the children of mothers with AID of our study we saw a 20% incidence of bronchial asthma. This rate is higher than that described in the general pediatric population, which is 12%.17 Although the sample size could imply a bias, the increased rate of asthma could be justified by an increased sensitivity to diseases of autoimmune or allergic nature. However, among our newborns we found no evidence of increased incidence of other childhood autoimmune diseases, such as diabetes, celiac disease or thyroid disease.6
Data collection revealed that blood tests had been conducted to determine the placental transfer of antibodies in 36% of newborns, and 33% of patients had undergone an ECG to rule out congenital AVB.
Since this was a retrospective study, there was no systematic collection of data, which limits its interpretation, yet the results are similar to those reported in the literature. For these reasons, we want to emphasize the importance of the establishment of multidisciplinary teams in which rheumatologists, obstetricians and neonatologists collaborate to plan pregnancies in patients with AID, implement monitoring protocols and anticipate possible complications that may affect the patients or their fetuses.
Ethical ResponsibilitiesProtection of People and AnimalsThe authors state that no experiments were performed on humans or animals.
Data ConfidentialityThe authors declare that they have followed the protocols of their workplace on the publication of data from patients and all patients included in the study have received sufficient information and gave their written informed consent to participate in this study.
Right to Privacy and Informed ConsentThe authors have obtained informed consent of patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Conflicts of InterestThe authors have no conflicts of interest to make.
Please cite this article as: Sanchez-Manubens J, et al. Recién nacidos de madre con enfermedad autoinmunitaria. Experiencia en un hospital comarcal. Reumatol Clin. 2012. http://dx.doi.org/10.1016/j.reuma.2012.08.002.