To develop recommendations on the evaluation and management of patients with rheumatic autoimmune and inflammatory diseases during the reproductive age, pregnancy, post-partum and breastfeeding based on the best evidence and experience.
MethodsRecommendations were generated using nominal group and Delphi techniques. An expert panel of 12 rheumatologists was established. A systematic literature review and a narrative review (websites, clinical guidelines and other relevant documentation) were performed and presented to the panel in its 1st meeting to be discussed and to help define recommendations. A first draft of recommendations was generated and circulated for comments and wording refinement. A national survey analyzing different aspects of this topic was undertaken separately, followed by a Delphi process (2 rounds). Agreement with each recommendation was ranked on a scale of 1 (total disagreement) to 10 (total agreement), and was considered to be achieved if at least 70% voted ≥7. The level of evidence and grade of recommendation were assessed using the Oxford Centre for Evidence-based Medicine Levels of Evidence.
ResultsA total of 14 recommendations were generated for the preconception period (oral and hormonal contraception, reproductive techniques), pregnancy (planning, treatment and follow-up), and breastfeeding (treatment and follow-up). High-risk situations such as lupus or antiphospholipid syndrome were included. A consensus >90% was reached for all but one recommendation.
ConclusionsThese recommendations are intended to provide rheumatologists, patients, families and other stakeholders with a consensus on the evaluation and management of patients with autoimmune and inflammatory diseases during the reproductive age, pregnancy, postpartum and breastfeeding.
Desarrollar recomendaciones basadas en la mejor evidencia y experiencia sobre el manejo de pacientes con enfermedades reumáticas inflamatorias y autoinmunes durante la edad fértil, el embarazo, posparto y lactancia.
MétodosSe siguió la metodología de grupos nominales. Se seleccionó un grupo nominal de expertos (12 reumatólogos). Se realizó una actualización de una revisión sistemática de la literatura, una revisión literaria, así como una encuesta a nivel nacional sobre el manejo de estos pacientes. El grupo de expertos se encargó de definir el alcance, usuarios, apartados del manuscrito y posibles recomendaciones. El GA con las recomendaciones se votó siguiendo la metodología Delphi según una escala de 1 (total desacuerdo) a 10 (total acuerdo), definiéndose el acuerdo como una puntuación ≥ 7 por al menos el 70% de los participantes. El NE y GR se clasificaron según el modelo del Center for Evidence Based Medicine de Oxford. El documento completo inicial fue revisado por los expertos y el proyecto estuvo coordinado, en todo momento, por un metodólogo experto.
ResultadosSe generaron 14 recomendaciones sobre el periodo preconcepcional (anticoncepción, reproducción asistida), el embarazo (planificación, manejo farmacológico y seguimiento) y lactancia (manejo farmacológico y seguimiento). Incluye recomendaciones específicas sobre situaciones de especial riesgo como el lupus eritematoso sistémico y el síndrome antifosfolípido. Existió acuerdo > 90% con todas las recomendaciones menos en una de ellas.
ConclusionesEn los pacientes con enfermedades inflamatorias y autoinmunes estas actuaciones pueden mejorar los resultados y el pronóstico de los mismos.
Autoimmune and inflammatory rheumatic diseases particularly affect women of reproductive age.1,2 The traditional idea that these conditions are an insurmountable obstacle when it comes to considering pregnancy is currently obsolete, but it is true that pregnancy can, on certain occasions, become more complicated, with serious consequences (even death), for both the mother and the future child. The type of complications that can be expected and the proper manner of preventing them or, in the least favorable situation, of treating them, must be part of the core knowledge and skills of clinicians involved in the management of these diseases.3–5 Nevertheless, in routine clinical practice, there is wide variability in the management of fertility, pregnancy and breastfeeding in those patients, partly because there is little information concerning the appropriate way to deal with them.
Pregnancy entails physiological changes of which we should be aware.6 For example, the increase in blood and plasma volume leads to a reduction of the hematocrit and hemoglobin concentration, as well as a decrease in serum albumin. The erythrocyte sedimentation rate increases, especially during the third trimester.7 Creatinine and blood urea nitrogen decrease, whereas creatinine clearance increases by 30%. The alkaline phosphatase level nearly doubles, and cholesterol concentrations are also increased.8
It must be taken into account that the healthy population also has difficulties related to fertility and pregnancy. Between 15% and 30% of couples have problems with infertility,9 there are congenital malformations in 2–3%,10 and the rate of abortion is 13.5%, varying significantly depending on age (8.9% between 20–24 years and 74.7% after the age of 45 years)11 and lifestyle.12
The following recommendations have been drawn up for the purpose of improving the management of patients with autoimmune and inflammatory diseases during the reproductive age with regards to pregnancy, postpartum and breastfeeding, on the part of professionals customarily involved in diseases of these types, including rheumatologists, gynecologists, primary care physicians, internists, nephrologists and nurses.
MethodsTo prepare the recommendations we employed the Delphi technique.13 The entire process of writing the manuscript was carried out by distributing tasks and comments to all parts, with the aid of an update of a systematic review of the published literature14,15 and a narrative review, as well as advice from other health professionals related to the subject of the recommendations.
First, we established a nominal group of 12 rheumatologists with experience and interest in this issue; 3 of them acted as coordinators. Then, with the help of 2 methodologists, we defined the objectives, the scope and sections, which were distributed among the participants. We decided to deal with 3 large groups of questions: (a) reproductive age; (b) pregnancy and postpartum; and (c) breastfeeding. Subsequently, we held teleconferences with the panelists to discuss the most relevant aspects of each section and provisional recommendations were outlined. Moreover, we designed a survey to evaluate the knowledge and management of patients in the different aspects of the topics of this undertaking. It was sent to over 500 Spanish rheumatologists. At the same time, the systematic review of the literature was updated and the narrative review was performed.
The results of the survey were presented in a first meeting of the nominal group, at which we discussed each section with its provisional recommendations with the literature that endorsed each of them. The results were distributed to the group for commentaries.
The recommendations were voted in a first Delphi round to establish the level of agreement (LA) with each. This was done online and was anonymous. The LA was expressed by responses to a Likert scale from 1 (totally in disagreement) to 10 (totally in agreement). Agreement was achieved if at least 70% of the participants voted 7 or more. The recommendations with a LA less than 70% were reedited and voted in a second Delphi round. It was possible to include new recommendations in the first Delphi round.
With the aid of the fully developed material and the evidence provided by the reviews, we completed a first draft of a set of provisional recommendations, and of sections that were presented to the panel for the comments of the parts.
Next, the final article was written, with the definitive sections and recommendations. For each recommendation, and with methodological assistance, each was assigned a level of evidence (LE) and a grade of recommendation (GR) according to the recommendations for evidence-based medicine from the Centre for Evidence-Based Medicine of Oxford.16
Finally, the complete article was sent to the panelists for their last corrections and comments. This process was repeated several times until the final document was approved.
ResultsPreconception Period and PregnancyRecommendation 1. The panel recommends advising all patients of reproductive age—men and women—of the need to plan a pregnancy and offering preconception counseling whenever necessary (LE 2b, GR B, LA 100%).
The panel recommends regularly evaluating the thoughts of all patients of reproductive age about their feelings about pregnancy, especially women with autoimmune disorders and antiphospholipid syndrome (APS), but also those of patients, including men, with inflammatory joint diseases. In those cases in which a pregnancy is being contemplated, the patient should always be offered preconception counseling. Moreover, to the greatest possible extent, unnecessary delays in conception should be avoided.17,18
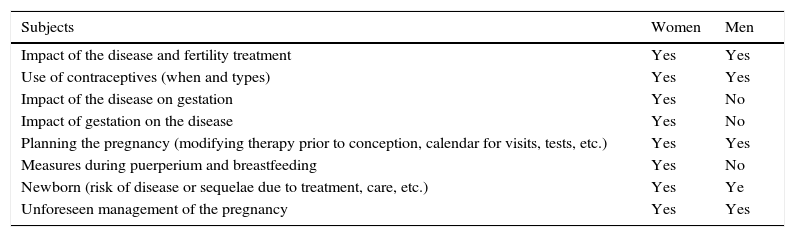
The characteristics of this preconception counseling (Table 1), which includes information on the impact of the disease on fertility and pregnancy, as well as on the attitude to adopt in the case of an unforeseen pregnancy, must be adjusted (age, disease, treatment, personal history, obstetric history, etc.) to our patients. This offer should also involve the patient's partner, whenever possible.
Aspects to Discuss During Preconception Counseling.
| Subjects | Women | Men |
|---|---|---|
| Impact of the disease and fertility treatment | Yes | Yes |
| Use of contraceptives (when and types) | Yes | Yes |
| Impact of the disease on gestation | Yes | No |
| Impact of gestation on the disease | Yes | No |
| Planning the pregnancy (modifying therapy prior to conception, calendar for visits, tests, etc.) | Yes | Yes |
| Measures during puerperium and breastfeeding | Yes | No |
| Newborn (risk of disease or sequelae due to treatment, care, etc.) | Yes | Ye |
| Unforeseen management of the pregnancy | Yes | Yes |
This should be done periodically, and it is necessary to insist in that planning a pregnancy is essential to reduce the maternal–fetal risks and ensure its success.
On the other hand, it is important to remember that rheumatic diseases (given their activity or established organic damage) and their treatment can be associated with fertility problems. It has been calculated that approximately 1.5% of the patients with systemic lupus erythematosus (SLE) may have difficulties with infertility,19 due to different causes like, for example, severe flares, moderate-to-severe renal failure with a low glomerular filtration rate (<60mL/min), etc.20 In other autoimmune diseases and vasculitis there is less experience and the results are variable.
Inflammatory diseases of the joints and rheumatoid arthritis (RA), in particular, are under-recognized causes of subfertility and infertility.21,22 Patients with RA have been found to have a lower birth rate, to take longer to become pregnant (more than 1 year in 25% versus 15% in the general population),23 and to recur more to the use of assisted reproduction techniques (9.8% versus 7.6% in the general population).23 In RA patients, the time to pregnancy has been associated with age, nulliparity, inflammatory activity and the use of >7.5mg of prednisone and nonsteroidal anti-inflammatory drugs (NSAID) prior to conception,24 but not to other factors such as having taken methotrexate (MTX) or the duration of the disease.
Some drugs prescribed in rheumatology can also have an influence on fertility. Because NSAID inhibit prostaglandins,22 they can affect implantation25 and ovulation26 in women. In men, on the other hand, there is a risk of oligo/azoospermia because of the use of sulfasalazine. Finally, the utilization of cyclophosphamide may require special management because it provokes potentially irreversible sterility in both sexes (measures for gonadal protection, in vitro fertilization, etc.).
Recommendation 2. The panel suggests evaluating and recommending an effective contraceptive method to be used to avoid an unforeseen pregnancy (LE 1b, GR A, LA 100%).
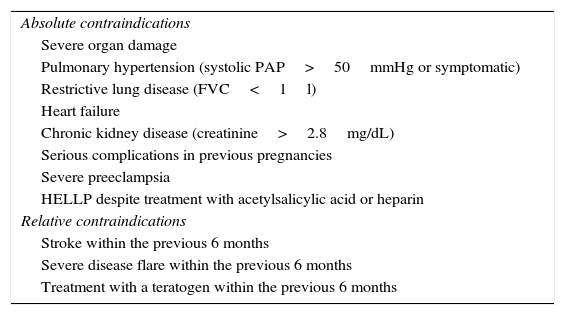
Aside from certain exceptions (Table 2), inflammatory diseases do not contraindicate pregnancy.18,27–31
Contraindications of Pregnancy in Patients With Inflammatory and Autoimmune Disease.
| Absolute contraindications |
| Severe organ damage |
| Pulmonary hypertension (systolic PAP>50mmHg or symptomatic) |
| Restrictive lung disease (FVC<1l) |
| Heart failure |
| Chronic kidney disease (creatinine>2.8mg/dL) |
| Serious complications in previous pregnancies |
| Severe preeclampsia |
| HELLP despite treatment with acetylsalicylic acid or heparin |
| Relative contraindications |
| Stroke within the previous 6 months |
| Severe disease flare within the previous 6 months |
| Treatment with a teratogen within the previous 6 months |
dL, deciliter; FVC, forced vital capacity; HELLP, hemolysis, elevated liver enzymes, low platelet count; mg, milligram; mmHg, millimeters of mercury; PAP, pulmonary arterial pressure.
We should recommend the utilization of effective contraceptive measures both for men and women, provided pregnancy is not being contemplated, is contraindicated or is being postponed (because of disease activity, initiation of the use of teratogens, etc.).
Combined hormonal contraception is the most efficacious method (Appendix A) (available online),17 especially for stable couples, unless there is a contraindication for the use of estrogens,5 in which case it would be necessary to utilize progestogens alone. However, the panel recommends that each case should be dealt with individually to suggest the contraceptive method that best adapts to the characteristics and circumstances of the patient/couple.
Moreover, in those patients with a high disease activity or significant residual damage (especially to internal organs), perhaps, on an individual basis, the idea of a pregnancy could be reconsidered and definitive methods of sterilization be assessed (Table 2).32–35
In mild-to-moderate flares, if the activity is controlled for at least 3 months with medication compatible with pregnancy, conception can be attempted. Severe flares (lupus nephritis, neuropsychiatric manifestations, severe vasculitis, pulmonary involvement in scleroderma) habitually require intensive treatment with teratogens like cyclophosphamide or mycophenolate mofetil. Thus, in those cases, pregnancy should be postponed at least 1 year until remission is achieved.18
Recommendation 3. The panel recommends that those patients who are candidates for assisted reproduction be provided with information on the available techniques (LE 4, GR D, LA 73%).
Patients with rheumatic diseases and fertility problems are increasingly interested in undergoing assisted reproduction techniques.
Although this subject is in the sphere of and is the responsibility of gynecologists, rheumatologists, as physicians responsible for these patients, should be familiar with the general aspects of these procedures to be able to provide any necessary information.
If there is no contraindication, it is essential not to delay referring patients to the infertility clinic of their center or health area, and to prepare complete reports for these units and become involved in the entire process. For this purpose, each professional must know how these clinics function and how they are managed.
In general, these techniques are safe, although it must be kept in mind that some of them, like ovarian stimulation, increase estrogen levels, which can be accompanied by flares and other complications in patients with SLE or APS. At the present time, we have little evidence on ovarian stimulation in patients with SLE, but it seems to be relatively safe.36–38 However, there is limited information on the thrombotic risk associated with this therapy. Therefore, we should pay special attention to those patients with a high risk of thrombotic events.39
Assisted reproduction techniques should only be considered at a time when the disease is inactive and that they be performed on an individual basis with close monitoring of the patient.
Recommendation 4. Planning a pregnancy should include an evaluation of the obstetric and rheumatic history of the patient, as well as analyses, immunological study, present status of the disease and treatments, comorbidities and possible contraindications for pregnancy (LE 5, GR D, LA 100%).
Recommendation 5. The panel recommends evaluating and reporting the maternal–fetal risk factors of pregnancy on an individual basis and on every aspect of their follow-up (LE 5, GR D, LA 100%).
Recommendation 6. The panel recommends that the patient achieve the best possible control of the inflammatory activity prior to attempting conception, with remission being the desired target, especially in the case of SLE (LE 3, GR C LA 100%).
Pregnancy can change the clinical activity of inflammatory rheumatic diseases and these, in turn, can be risk factors for the progression of the pregnancy. Controlling the clinical activity and the preconception use of agents that are low-risk in terms of the fetus make it possible to reduce maternal–fetal complications. For this reason, it is essential to plan the pregnancy.40,41
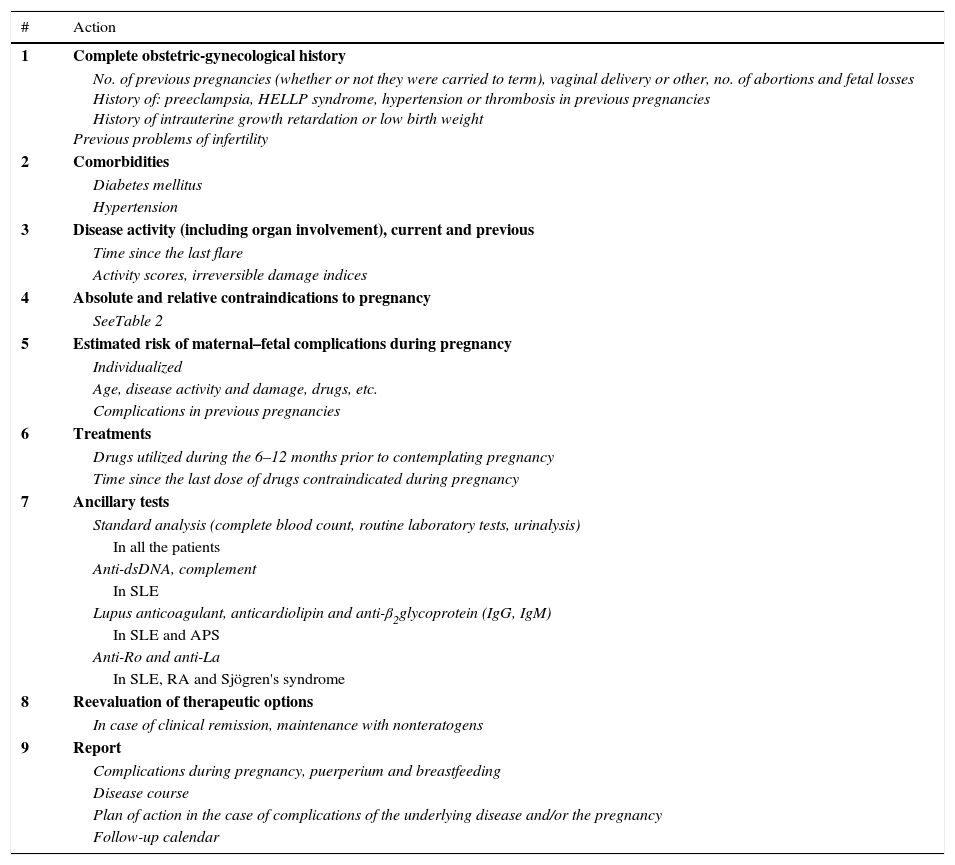
As a part of this planning (Table 3), an individual preconception evaluation should be performed, including an obstetric-gynecological history, contraindications of the pregnancy and the risk (and associated factors) of maternal–fetal complications.41–43 These depend on factors related to the mother (age, obstetric history), her disease (clinical activity at the time of conception, certain drugs, existence of irreversible damage, comorbidities or presence of certain autoantibodies) and with the medications utilized prior to and during the pregnancy (Table 4).18,40,41,44,45
Pregnancy Planning.
| # | Action |
|---|---|
| 1 | Complete obstetric-gynecological history |
| No. of previous pregnancies (whether or not they were carried to term), vaginal delivery or other, no. of abortions and fetal losses History of: preeclampsia, HELLP syndrome, hypertension or thrombosis in previous pregnancies History of intrauterine growth retardation or low birth weight Previous problems of infertility | |
| 2 | Comorbidities |
| Diabetes mellitus | |
| Hypertension | |
| 3 | Disease activity (including organ involvement), current and previous |
| Time since the last flare | |
| Activity scores, irreversible damage indices | |
| 4 | Absolute and relative contraindications to pregnancy |
| SeeTable 2 | |
| 5 | Estimated risk of maternal–fetal complications during pregnancy |
| Individualized | |
| Age, disease activity and damage, drugs, etc. | |
| Complications in previous pregnancies | |
| 6 | Treatments |
| Drugs utilized during the 6–12 months prior to contemplating pregnancy | |
| Time since the last dose of drugs contraindicated during pregnancy | |
| 7 | Ancillary tests |
| Standard analysis (complete blood count, routine laboratory tests, urinalysis) | |
| In all the patients | |
| Anti-dsDNA, complement | |
| In SLE | |
| Lupus anticoagulant, anticardiolipin and anti-β2glycoprotein (IgG, IgM) | |
| In SLE and APS | |
| Anti-Ro and anti-La | |
| In SLE, RA and Sjögren's syndrome | |
| 8 | Reevaluation of therapeutic options |
| In case of clinical remission, maintenance with nonteratogens | |
| 9 | Report |
| Complications during pregnancy, puerperium and breastfeeding | |
| Disease course | |
| Plan of action in the case of complications of the underlying disease and/or the pregnancy | |
| Follow-up calendar |
APS, antiphospholipid syndrome; ds, double-strand; HELLP, hemolysis, elevated liver enzymes, low platelet count; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Risk Factors for Maternal -fetal Complications in Patients With Inflammatory and/or Autoimmune Diseases.
| Previous adverse obstetric history |
| Severe preeclampsia |
| HELLP syndrome |
| History of previous thrombotic events |
| Thrombosis within the preceding 6 months |
| Risk factors for preeclampsia |
| Age>40 years |
| Family history of preeclampsia |
| Personal history of preeclampsia, multiple pregnancies, nulliparity or diabetes |
| Obesity or hypertension at the beginning of pregnancy |
| Active SLE |
| History of lupus nephritis class iii or iv |
| Presence of antiphospholipid antibodies |
| Chronic kidney disease |
| Creatinine>2.8mg/dL |
| Evidence of APS |
| High frequency of abortions, neonatal deaths or premature or low birth weight infants |
| Avoid pregnancy in patients with severe pulmonary hypertension or thrombosis within the preceding 6 months |
| SLE |
| High risk of SLE flares |
| High frequency of abortions, neonatal deaths or premature or low birth weight infants |
| Avoid pregnancy in patients with severe pulmonary hypertension, interstitial lung disease, heart failure, or with stroke or severe relapse of the disease in the preceding 6 months |
| RA |
| High risk of preeclampsia, need for cesarean section and low birth weight infants |
| Exacerbation/flare during the postpartum period |
| Spondyloarthropathies |
| Increased number of cesarean sections due to sequelae of the disease |
| Vasculitis |
| Premature delivery |
| Systemic sclerosis |
| Premature delivery |
| Intrauterine growth retardation |
| Low birth weight |
| Exposure to teratogens |
| Between 6 and 12 months before conception |
APS, antiphospholipid syndrome; dL, deciliter; HELLP, hemolysis, elevated liver enzymes, low platelet count; mg, milligram; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
In pregnant SLE patients, there is a greater risk of exacerbation of the disease,46–53 including nephropathy, thrombotic events, infections and thrombocytopenia,54–56 as well as preeclampsia,57 eclampsia and hemolysis, elevated liver enzymes and low platelets (HELLP syndrome).56,58 The presence of activity during the 6 months prior to conception increase the risk of flares during the pregnancy and maternal–fetal complications.59–62
There are higher incidences of abortion, neonatal death and premature or low birth weight infants in both in SLE and APS.47,57,59,63
In contrast, some RA patients improve in terms of clinical activity during pregnancy and become worse during the puerperium.29,30 However, the presence of activity at the start of pregnancy has been associated with persistence of activity throughout gestation and an increase in the risk of flares during the puerperium.64 In RA there is also a higher risk of preeclampsia, cesarean section and low birth weight infants,65–68 risks that diminish if disease activity is controlled or is low.69
In general, spondyloarthritis does not negatively interfere with pregnancy, the fetus or breastfeeding. However, a higher rate of cesarean sections, often caused by the disease itself, has been reported.70,71
Patients with systemic sclerosis may have an increased risk of preterm delivery, intrauterine growth restriction and low birth weight.72 Vasculitis does not usually affect the development of pregnancies, but there is an increase in the risk of preterm birth.73
The planning of a pregnancy in patients with SLE and APS should also include a general analysis and testing for lupus anticoagulant and anticardiolipin, anti-β2 glycoprotein, anti-Ro, anti-La and anti-double-strand (ds) DNA antibodies, with components of the complement system.32,74
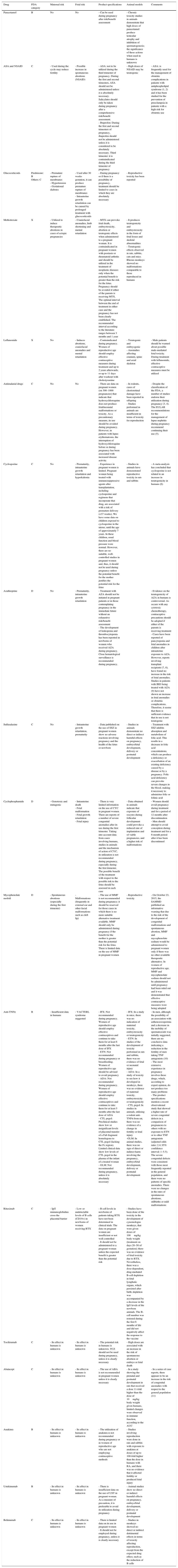
Another fundamental aspect of planning is for the patient to achieve remission (or, otherwise, on an individual basis, the minimal disease activity possible) with nonteratogens (Table 5) at least 6–12 months before conception.41 For this, treatment must be reevaluated. Agents that are contraindicated during pregnancy (MTX, leflunomide) or considered to be of high risk (cyclophosphamide) should be discontinued and replaced by safe drugs and be maintained for 3–4 months with the patient in clinical remission prior to conception.43 In cases of severe disease, a waiting period of at least 1 year in remission is recommended before pregnancy.18 If the patient is taking an anti-tumor necrosis factor α (TNFα) agent, the possibility of maintaining it until the pregnancy is confirmed or until the end of the second trimester (depending on the patient) can be evaluated. In certain cases, the option of continuing to take it during the entire pregnancy is a decision that must be discussed and agreed upon because of a lack of data.75,76 The level of placental transfer of etanercept and certolizumab pegol is low (see subsequent sections).77,78 If the patient is being treated with rituximab, it must be taken into account that the half-life can be as long as several months and, thus, it should be discontinued between 6 and 12 months prior to conception. For other biological agents, there is very little information, and the need to suspend them or replace them with other medications prior to conception or on the confirmation of the pregnancy is a decision that must made on an individual basis.
Use of Drugs During Pregnancy.
| Drug | FDA category | Maternal risk | Fetal risk | Product specifications | Animal models | Comments |
|---|---|---|---|---|---|---|
| Paracetamol | B | No | No | - Can be used during pregnancy after risk/benefit assessment | - Chronic toxicity studies in animals demonstrate that high doses of paracetamol produce testicular atrophy and inhibition of spermatogenesis; the significance of these actions when used in humans is unknown | |
| ASA and NSAID | C | - Used during the cycle may reduce fertility | - Possible increase in spontaneous abortions (NSAID) | - ASA: not to be utilized during the third trimester of pregnancy. During the first and second trimesters, ASA should not be administered unless it is absolutely necessary. Salicylates should only be taken during pregnancy after a comprehensive risk/benefit assessment. - Ibuprofen: During the first and second trimesters of pregnancy, ibuprofen should not be administered unless it is considered to be absolutely necessary. Third trimester: it is contraindicated during the third trimester of pregnancy | - High doses of NSAID may be teratogenic | - ASA: is frequently used for the management of obstetric complications in patients with antiphospholipid syndrome (1, 2) and it has been studied for the prevention of preeclampsia in patients with a high risk for obstetric use |
| Glucocorticoids | Prednisone: B Others: C | - Premature rupture of membranes - Hypertension - Gestational diabetes | - Used after 30 weeks gestation, it can produce premature rupture of membranes - Intrauterine growth retardation can be caused by prolonged treatment with glucocorticoids | - During pregnancy or if there is a possibility of pregnancy, treatment should be limited to cases in which they are absolutely necessary | - Reproductive toxicity has been reported | |
| Methotrexate | X | - Utilized to induce therapeutic abortions in cases of ectopic pregnancies | - Craniofacial anomalies, limb shortening and mental retardation | - MTX can provoke fetal death, embryotoxicity, abortion or teratogenic effects when administered to a pregnant woman. It is contraindicated in pregnant women with psoriasis or rheumatoid arthritis and should be utilized in the treatment of neoplastic diseases only when the potential benefit is greater than the risk for the fetus. Pregnancy should be avoided if either of the parents is receiving MTX. The optimal interval between the end of treatment in either case and the pregnancy has not been clearly established. The recommended interval according to the literature ranges between 3 months and 1 year | - It produces teratogenicity and embryotoxicity in the form of fetal losses and skeletal abnormalities - Teratogenic effects observed in rats, rabbits, cats and mice. Rhesus monkeys showed no malformations comparable to those reproduced in humans | |
| Leflunomide | X | No | - Induces abortions, craniofacial anomalies and mental retardation | - Contraindicated during pregnancy. Women of reproductive age should employ effective contraceptive measures during treatment and up to 2 years afterwards, or up to 11 days after washout with cholestyramine | - Teratogenic and embryogenic - Anomalies affecting cranium, spine and axial skeleton | - Male patients should be warned about possible male-mediated fetal toxicity. During treatment with leflunomide, effective contraceptive measures must be utilized |
| Antimalarial drugs | C | No | No | - There are data on pregnant women (on 300–1000 pregnancies) that indicate that hydroxychloroquine does not produce fetal/neonatal malformations or toxicity. As a precautionary measure, its use should be avoided during pregnancy. However, in patients with lupus erythematosus, the interruption of hydroxychloroquine before or during pregnancy has been associated with increased disease activity. | - In rodents, cases of chorioretinal damage have been reported in offspring - Studies performed in animals are insufficient in terms of toxicity for reproduction | - Despite the classification of the FDA, a number of studies endorse their utilization during pregnancy (3, 4). The EULAR recommendations for the management of lupus nephritis during pregnancy recommend continuing their use (5). |
| Cyclosporine | C | No | - Prematurity, intrauterine growth retardation and hyperkalemia | - Experience in pregnant women is limited. Pregnant women being treated with immunosuppressive agents after transplantation, including cyclosporine and regimens that incorporate that drug, are associated with a risk of premature delivery (<37 weeks). We have some data on children exposed to cyclosporine in the uterus, until the age of approximately 7 years. In these children, renal function and blood pressure were normal. However, there are no suitable, well-controlled studies in pregnant women and, thus, it should not be used during pregnancy unless the potential benefit for the mother justifies the potential risk for the fetus | - Studies in animals have demonstrated reproductive toxicity in rats and rabbits | - A meta-analysis has concluded that cyclosporine is not related to an increase in teratogenicity in humans (6) |
| Azathioprine | D | No | - Prematurity, intrauterine growth retardation | - Treatment with AZA should not be initiated in pregnant patients or in those contemplating pregnancy in the immediate future without an exhaustive risk/benefit assessment - The development of leukopenia and thrombocytopenia has been reported in newborns of women who received AZA during pregnancy. Close hematological surveillance is recommended during pregnancy. | - Evidence on the teratogenicity of AZA in humans is controversial. As occurs with any cytotoxic chemotherapy, contraceptive precautions should be adopted if either of the parents is receiving treatment - Cases have been reported of pancytopenia and fetal anomalies in children after intrauterine exposure to AZA. However, reports involving transplant recipients (7, 8), have found no increase in the risk of fetal anomalies. Studies in patients with IBD being treated with AZA (9) have not shown an increase in fetal anomalies or obstetric complications. Therefore, it seems that there is sufficient evidence that its use is not teratogenic | |
| Sulfasalazine | C | No | - Intrauterine growth retardation, prematurity | - Data published on the use of SSZ in pregnant women show no adverse reactions involving pregnancy and the health of the fetus or newborn | - Studies in animals demonstrate no direct or indirect harmful effects on pregnancy, embryo/fetal development, delivery or postnatal development | - Treatment with SSZ inhibits absorption and metabolism of folic acid. This results in a decrease in folic acid concentrations, which can produce a deficiency or exacerbation of an existing deficiency caused by a disease or by a pregnancy. Folic acid deficiency can provoke severe changes in the blood, making it necessary to administer folic or folinic acid |
| Cyclophosphamide | D | - Genotoxic and mutagenic | - Intrauterine death - Fetal malformation - Fetal growth retardation - Fetal injury | - There is very limited information on the use of CYC in pregnant women. There are reports of a number of severe congenital anomalies after its use during the first trimester. Taking into account data from cases involving humans, studies in animals and the mechanism of action of CYC, its utilization is not recommended during pregnancy, especially during the first trimester. The possible benefit of the treatment with respect to the possible risk to the fetus should be assessed in each case | - Data obtained in animals indicate that exposure of oocytes during follicular development could provoke a reduced rate of implantation and of viable pregnancies, and a higher risk of malformations | - Women should avoid pregnancy during treatment and for a period of 12 months after discontinuation - Men should attempt to avoid conception during treatment and for a 6-month period after it has been discontinued |
| Mycophenolate mofetil | D | - Spontaneous abortions (especially during the first trimester) | - Malformations (frequently in external ear and other facial malformations such as cleft lip) | - The use of MMF is not recommended during pregnancy; it should be reserved for those cases in which there is no more suitable alternative treatment available. MMF should only be administered during pregnancy if the benefit for the mother is greater than the potential risk for the fetus. There is limited data on the use of MMF in pregnant women | - Reproductive toxicity | - On October 23, 2015, the SAMMD published an informative warning that due to the risk of the development of congenital malformations and spontaneous abortion, MMF and mycophenolate sodium would be administered to pregnant women only if there was no other available therapeutic alternative. In women of reproductive age, MMF and mycophenolate sodium should not be administered until pregnancy had been ruled out and it was demonstrated that effective contraceptive measures were being adopted |
| Anti-TNFα | B | - Insufficient data in humans | - VACTERL syndrome suggested | - IFX: Not recommended during pregnancy. Women of reproductive age should employ effective contraceptives and continue to take them for at least 6 months after the last dose of IFX - ETN: Not recommended during pregnancy or breastfeeding. Women of reproductive age should be advised to avoid pregnancy - ADA: Not recommended during pregnancy. Women of reproductive age should employ effective contraceptives and continue to take them for at least 5 months after the last dose of ADA - CTL pegol: Preclinical studies show low or insignificant levels of placental transfer of a Fab fragment homologous to CTL pegol (lacking the Fc region). Limited clinical data show low levels of CTL pegol in the plasma of the infant of a treated woman - GLM: Not recommended during pregnancy, unless it is absolutely necessary | - IFX: In a study in mice, there was no indication of maternal toxicity, embryotoxicity or teratogenicity - ETN: In studies of the development of toxicity performed in rats and rabbits, there was no evidence of fetal or neonatal injury - ADA: In a study of toxicity to see how it developed in monkeys, there was no evidence of maternal toxicity, embryotoxicity or teratogenicity - CTL pegol: In studies in animals, utilizing a rodent anti-TNFα from rat, there was no evidence of a change in fertility or fetal injury -GLM: In animal studies, there was no sign of direct or indirect harm involving the pregnancy, embryo/fetal development, delivery or postnatal development | - In men, although the possibility of an association with asthenozoospermia and a decrease in the mobility of spermatozoids was initially suggested, there are no conclusive data indicating a reduction in the fertility of men taking TNF antagonists (10) - The most extensive experience in pregnancy involves these drugs, which, according to expert opinion, do not produce too many problems - The product specifications mention a recent observational study that showed a higher rate of severe congenital defects in a comparison of pregnancies to others with no exposure to ETN or to other TNF antagonists (adjusted odds ratio: 2.4; 95% confidence interval: 1–5.5). The severe congenital defects were consistent with those most frequently reported in the general population, and there were no patterns of specific anomalies. There were no changes in the rates of spontaneous abortions, stillbirths or mild malformations |
| Rituximab | C | - IgG immunoglobulins cross the placental barrier | - Low or undetectable levels of B cells (CD19+) in newborns of women receiving RTX | - B-cell levels in newborns of patients taking RTX have not been determined in clinical trials. The data on pregnant women are insufficient or not well controlled - It should not be administered to a pregnant woman unless the expected benefit is greater than the potential risk | - Studies have been done of the toxicity in the development of cynomologus monkeys, that were given doses of 100mg/kg body weight (treatment on days 20–50 of gestation); there was no evidence of fetal toxicity due to RTX. Nevertheless, there was a dose-dependent, drug-mediated B-cell depletion in fetal lymphatic organs, which persisted after birth; depletion was accompanied by a decrease in the IgG levels of the newborn animals. The B-cell number was restored during the first 6 months of life and did not negatively affect the response to the vaccine | |
| Tocilizumab | C | - Its effect in humans is unknown | - Its effect in humans is unknown | - The potential risk in humans is unknown. TCZ should not be used during pregnancy, unless it is clearly necessary | - High doses are associated with an increase in the risk of spontaneous abortion or embryo or fetal death | |
| Abatacept | C | - Its effect in humans is unknown | - Its effect in humans is unknown | - The use of ABA is not recommended in pregnant women unless it is clearly necessary | - In a study showing the prenatal and postnatal development in rats that received a dose 11-fold higher than the dose of 10mg/kg body weight given humans, limited changes were observed in immune function, according to the AUC | - In a series of case reports, there appears to be an increase in the risk of congenital anomalies with respect to the general population (11) |
| Anakinra | B | - Its effect in humans is unknown | - Its effect in humans is unknown | - The utilization of anakinra is not recommended during pregnancy or in women of reproductive age who are not employing contraceptive methods | - Studies involving reproduction were done in rats and rabbits with exposure to anakinra at doses of up to 100-fold higher than the dose in humans with RA, and there was no evidence that it affected fertility or produced fetal injury | |
| Ustekinumab | B | - Its effect in humans is unknown | - Its effect in humans is unknown | - There is insufficient data on the use of UST in pregnant woman. As a measure of precaution, it is preferable to avoid its utilization during pregnancy | - Animal studies show no direct or indirect harmful effects on pregnancy, embryo/fetal development, delivery or postnatal development | |
| Belimumab | C | - Its effect in humans is unknown | - Its effect in humans is unknown | - There is limited data on its use in pregnant women - It should not be employed during pregnancy, unless it is clearly necessary | - Studies in monkeys showed no direct or indirect detrimental effects in terms of toxicity affecting reproduction, except from the expected drug effect, such as the reduction of B cells |
| Safety of drugs during pregnancy. Classification of the United States Food and Drug Administration (FDA) | ||
|---|---|---|
| Category | Safety | Description |
| A | Controlled studies have shown no risk. Remote risk of fetal injury | Studies in pregnant women have shown no evidence of risk for the fetus during the first trimester of gestation, nor is there evidence during the rest of the pregnancy |
| B | There are no risks reported in humans. Their use is accepted during pregnancy | Animal studies have not indicated any risk, but there are no appropriate studies in pregnant women, or there are animal studies in which it is possible to detect adverse effects, but they have not been confirmed in pregnant women |
| C | Fetal risk has not been ruled out. Risk/benefit assessment should be performed before they are prescribed | Studies in animals have demonstrated adverse effects but there are no studies in pregnant women, or there are no studies either in pregnant women or in animals |
| D | There are indications of fetal risk. They should be used only in cases in which there are no alternatives | Studies in pregnant women have demonstrated the risk of adverse effects, but there are occasions in which the benefits may be greater than those risks |
| X | Contraindicated during pregnancy | Studies in pregnant women and in animals have demonstrated that the potential risks are clearly greater than the possible benefits |
ABA, abatacept; ADA, adalimumab; ASA, acetylsalicylic acid; AUC, area under the curve; AZA, azathioprine; CTL pegol, certolizumab pegol; CYC, cyclophosphamide; ETN, etanercept; EULAR, European League Against Rheumatism; FDA, United States Food and Drug Administration; GLM, golimumab; IBD, inflammatory bowel disease; IFX, infliximab; MMF, mycophenolate mofetil; MTX, methotrexate; NSAID, nonsteroidal anti-inflammatory drugs; RTX, rituximab; SAMMD, Spanish Agency of Medicines and Medical Devices; SSZ, sulfasalazine; TCZ, tocilizumab; TNF, tumor necrosis factor; UST, ustekinumab; VACTERL, vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, limb abnormalities.
1. Cowchock FS, Reece EA, Balaban D, Branch DW, Plouffe L. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol. 1992;166:1318–23 [Online publication 01.05.92].
2. Villa PM, Kajantie E, Raikkonen K, Pesonen AK, Hamalainen E, Vainio M, et al. Aspirin in the prevention of pre-eclampsia in high-risk women: a randomized placebo-controlled PREDO Trial and a meta-analysis of randomized trials. BJOG. 2013;120:64–74 [Online publication 07.11.12].
3. Motta M, Tincani A, Faden D, Zinzini E, Lojacono A, Marchesi A, et al. Follow-up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation. J Perinatol. 2005;25:86–9 [Online publication 22.10.04].
4. Osadchy A, Ratnapalan T, Koren G. Ocular toxicity in children exposed in utero to antimalarial drugs: review of the literature. J Rheumatol. 2011;38:2504–8 [Online publication 18.10.11].
5. Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and pediatric lupus nephritis. Ann Rheum Dis. 2012;71:1771–82 [Online publication 02.08.12].
6. Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051–5 [Online publication 26.05.01].
7. Haugen G, Fauchald P, Sodal G, Leivestad T, Moe N. Pregnancy outcome in renal allograft recipients in Norway. The importance of immunosuppressive drug regimen and health status before pregnancy. Acta Obstet Gynecol Scand. 1994;73:541–6 [Online publication 01.08.94].
8. Radomski JS, Ahlswede BA, Jarrell BE, Mannion J, Cater J, Moritz MJ, et al. Outcomes of 500 pregnancies in 335 female kidney, liver, and heart transplant recipients. Transplant Proc. 1995;27:1089–90 [Online publication 01.02.95].
9. Casanova MJ, Chaparro M, Domenech E, Barreiro-de Acosta M, Bermejo F, Iglesias E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:433–40 [Online publication 16.02.13].
10. Gomez Reino J, Loza E, Andreu JL, Balsa A, Batlle E, Canete JD, et al. Consenso SER sobre la gestion de riesgo del tratamiento con terapias biologicas en pacientes con enfermedades reumaticas [Consensus statement of the Spanish Society of Rheumatology on risk management of biologic therapy in rheumatic patients]. Reumatol Clin. 2011;7:284–98 [Online publication 20.09.11].
11. Kumar M, Ray L, Vemuri S, Simon TA. Pregnancy outcomes following exposure to abatacept during pregnancy. Semin Arthritis Rheum. 2015 [Online publication 27.07.15].
In SLE patients, hydroxychloroquine (HCQ) reduces the risk of exacerbations during pregnancy79 and, thus, its use is recommended if the patient is considering pregnancy, because it does not need to be discontinued after conception.79–81 Likewise, some SLE and APS patients should continue to take antiplatelet and/or anticoagulant therapy, even prior to conception (see subsequent sections).44,57,60,63,82–84
Patients must be aware of the treatment they can take during pregnancy and even agree on what to do in the case of flares or serious complications that can put their lives at risk or cause fetal loss. They should also be informed of the risks during the postpartum period and while breastfeeding,18 and what will be involved in follow-up throughout their pregnancy.
Given that a relatively high percentage of pregnancies are not planned,85,86 during the reproductive age, any patient with a rheumatic disease should have preferential access to all of this information.
Recommendation 7. Patients with a high risk—that is, with APS and connective tissue diseases, or with arthritis or spondyloarthritis with moderate-to-high activity or who require corticosteroids or biological agents to control their disease—should be attended to in high-risk obstetric units once pregnancy has been confirmed (LE 5, GR D, LA 100%).
Pregnancy in women with SLE or APS is always a “high-risk” pregnancy because of the increase in maternal–fetal morbidity and mortality.32,42,43,56 In other autoimmune rheumatic diseases, a pregnancy is considered to be “high risk” because of the inflammatory activity and fetal toxicity of many of the treatments employed.18 All of those cases should be referred to high-risk units.
However, in a highly specific group of patients, those who have mild involvement or are under very good medical control, follow-up in the usual consultations can be contemplated.
Recommendation 8. The panel recommends looking for a medical profile as effective and safe as possible for pregnancy, that is individualized for each patient (LE 3, GR B-C, LA 100%).
Knowledge on the safety of medications is one of the cornerstones of an effective and safe obstetric and medical care. Still, we do not have access to enough evidence on all of the drugs to be able to issue robust and explicit recommendations.87 Thus, the prescription of medications during pregnancy should be done with precaution, taking into account a detailed analysis of the risk-benefit in each patient on an individual basis. Table 5 shows the major agents utilized in rheumatology.
Any NSAID should be discontinued upon reaching the third trimester of gestation given the risk of preterm delivery and early closure of the ductus arteriosus.87 The data on the use of cyclooxygenase-2 is very limited, but a recent study seems to show that its utilization increases the risk of fetal malformations, and thus it should be avoided during pregnancy.88 Nonfluorinated corticosteroids (prednisone and prednisolone) can be utilized with caution during pregnancy at the lowest possible doses,89 whereas fluorinated corticosteroids (dexamethasone) are able to cross the placental barrier and, thus, should be administered only at the end of gestation, to favor lung maturation, in the case of risk of preterm birth.87 With respect to synthetic disease-modifying drugs, the United States Food and Drug Administration (FDA) and the Spanish Agency of Medicines and Medical Devices (SAMMD) contraindicate the use of MTX, leflunomide and mycophenolate mofetil during pregnancy. In the case of MTX in men, it has been seen that it can produce azoospermia, although its effects on embryogenesis are now being questioned.90,91
With regard to biological therapies, IgG antibodies are able to cross the placenta from the second trimester on, an occurrence that takes place due to neonatal constant fragment (Fc fragment) receptors.92 We should point out that both etanercept (which has a lower affinity for binding to placental receptors than other anti-TNFα drugs)77 and, especially, certolizumab pegol (which lacks Fc),78 have a low level of placental transfer, a fact that could confer them a better safety profile.
In pregnant women being treated with infliximab and adalimumab, these agents have been detected in umbilical cord blood in the second and third trimester and in the infant until up to 6 weeks after being born.75,76 Thus, in those patients in whom they were maintained during pregnancy, it is recommended that they be discontinued at the end of the second trimester, although at the present time there is no data (in relation to fetal complications) that advise against their use during this entire period.93 Therefore, on an individual basis and in highly justifiable cases (marked disease activity), an agreement can be reached in terms of maintaining them until the end of the pregnancy.
Anti-TNFα agents taken by men at the time of conception have no influence on the fertility or the pregnancy of their partners.94
With respect to rituximab, its use during conception or pregnancy has been associated with preterm births and spontaneous abortions; however, in those studies, the influence of the severity of the underlying disease cannot be ruled out in those cases.95–97 On the other hand, the drug can cross the placental barrier during the second and third trimester and provoke a transient depletion of B cells in the fetus or infant, increasing the risk of infection. However, the long-term effects on the immune system of newborns are unknown. If it had not been discontinued prior to conception, it is advisable to do so as soon as the pregnancy is confirmed.
Finally, we have little data on anakinra, abatacept, tocilizumab, ustekinumab, secukinumab and belimumab.96 Thus, it is recommended that these agents be discontinued as soon as the pregnancy is confirmed, if they had not been prior to conception.
Recommendation 9. The panel recommends close follow-up and control of patients with rheumatic diseases who become pregnant (LE 2a, GR B, LA 100%).
Patients who are pregnant should undergo rigorous obstetric monitoring, as well as a strict control of their underlying disease, preferably in multidisciplinary units that combine the presence of gynecologists with experience in high-risk pregnancies and rheumatologists with broad experience in the planning and control of gestation.
The regularity of visits to their physicians will depend on one hand on the obstetric evaluation and, on the other, on the underlying disease, its activity and the development of flares. In general terms, in a patient with low or virtually no activity, who is stable in clinical terms, the regularity can be every 4–6 weeks during the first 2 trimesters, and be scheduled every 2 weeks starting from 32 to 36 weeks gestation. In the case of disease flares or the development of obstetric complications, the regularity would be determined by the attending physicians. In SLE (depending on the case), check-ups were recommended approximately every 4 weeks by the obstetrician and every 4–6 weeks by the rheumatologist until the 20th week of gestation, every 2 weeks until the 28th week and every week until the end of the pregnancy.50,98–100 Patients with anti-Ro/La antibodies should receive special mention because it is recommended they undergo fetal echocardiography each week between 16 and 26 weeks gestation with measurement of the PR interval and fetal heart rate.56
The evaluation of the patients should be systematic. It ought to include monitoring arterial blood pressure and body weight and a basic physical examination. Analyses should involve a complete blood count with erythrocyte sedimentation rate, routine laboratory tests (glucose, renal and liver function), C-reactive protein (CRP) and urinalysis. In a stable patient, analyses can be performed every 8–12 weeks and more frequently in the case of a flare. The only exception is the urine analysis, which should be carried out in all the check-ups. It is recommended that, in SLE patients, components of the complement system and anti-dsDNA be determined at each visit.74 Usually, in the remainder of the diseases, it is not considered necessary to repeat other autoantibodies. The attempt should be made so that the analyses requested coincide with the timing of the tests routinely called for in obstetrics.
The activity should be evaluated in accordance with routine clinical practice, although taking into consideration that the pregnancy may influence certain parameters used in the evaluation. In the case of RA, the use of the 28-joint Disease Activity Score (DAS28)-CRP is recommended, without the overall patient-reported health status assessment component; in the case of SLE, there are scores adapted for pregnancy.101
The rheumatic diseases most frequently treated in multidisciplinary clinics are RA, spondyloarthritis, APS and SLE. The major recommendations concerning follow-up and monitoring involve these diseases rather than other processes that, because of their incidence or epidemiology, are substantially less common, for example scleroderma, Sjögren's syndrome or systemic vasculitis.14,15
Recommendation 10. In the case of activity or complication, the panel recommends treating each patient on an individual basis, taking into account the type and seriousness of the event, the underlying disease, the trimester of the pregnancy and the available therapeutic options (LE 5, GR D, LA 100%).
In each case, the therapeutic approach should be individualized and agreed upon by the patient and, if necessary, with both the gynecologist and obstetrician.
If a flare occurs in a patient with arthritis, intra-articular injection is safe and effective. The use of NSAID can be considered except in the third trimester. If systemic corticosteroid therapy is required, it is recommended that prednisone, prednisolone and methylprednisolone be administered at the minimum effective doses. If the inflammatory activity persists and it is necessary to add a disease-modifying drug, possible alternatives are HCQ, azathioprine and sulfasalazine.18,102 If the activity persists and the use of biological therapy is proposed, the crossing of the placental barrier must be taken into account; etanercept has a low level of placental transfer, which is minimum in the case of certolizumab pegol18,77,78,102,103 (see preceding sections). It is also necessary to keep in mind the vaccination calendar.
In patients with SLE, it may be necessary to consider the differential diagnosis involving a situation of preeclampsia and a nephritis flare. The presence of other clinical or analytical data on the activity of SLE (extrarenal manifestations of lupus, active urinary sediment, hypocomplementemia and anti-dsDNA antibodies) and normouricemia (versus hyperuricemia in preeclampsia) point to a diagnosis of nephritis.104 In this case, the drugs that should be employed are prednisone and azathioprine.105
If a pregnant woman has cutaneous manifestations of SLE, it will be necessary to decide on topical treatment or local injection of corticosteroids or calcineurin inhibitors, as well as the use of an antimalarial agent, preferably HCQ.
If we detect thrombocytopenia in a patient with SLE, it is essential to rule out preeclampsia, HELLP syndrome or another cause, and it must be confirmed that the low values are a consequence of the underlying disease. In this case, corticosteroids or immunosuppressive agents like azathioprine should be administered. If thrombocytopenia persists or there is evidence of bleeding, intravenous immunoglobulins can be utilized.18,102 If an antimalarial drug or acetylsalicylic acid (ASA) has been prescribed, it should be maintained at low doses.
On the other hand, if the patient tests positive for antiphospholipid antibodies, but does not have symptoms, the option is low-dose ASA or no treatment, provided there are no other additional risk factors. If the patient has a history of repeated abortions (<10 weeks gestation) or of fetal loss (>10 weeks gestation), it is recommended that she receive low-dose ASA together with low-molecular weight heparin (LMWH) at prophylactic doses, since the combination of the 2 drugs is more effective in reducing losses than the administration of ASA alone.106,107 If the patient is receiving anticoagulant therapy with dicoumarol, the latter should be replaced by LMWH at therapeutic doses.108,109 This change should be made before the sixth week of gestation (or even before conception), given that the highest risk of malformations associated with dicoumarol coincides with weeks 6–12.110
In the case, that the patient has SLE or APS and is receiving anticoagulant therapy, the change to oral anticoagulants should be done to heparin prior to conception or, at least, within the 2 weeks after the first missed period, as oral anticoagulants cross the placenta and are associated with embryopathy, congenital malformations and a higher risk of intracranial hemorrhage.111
During pregnancy, the recommended anticoagulant therapy is unfractionated heparin or LMWH, although the latter has the advantage of having a longer plasma half-life and a more predictable dose response, which favors its administration in a single daily dose.111 At the time of delivery, the last dose of LMWH should be administered 24h earlier, and then the patient should receive half of the daily dose. Therapeutic doses of LMWH should be reinitiated 24–72h after delivery, depending on the obstetric procedure and any complications.112
In case the pregnant patient with a rheumatic disease has an infection that requires the utilization of an antibiotic, it is necessary to choose one with a good safety profile regarding pregnancy (Appendix A) (available on the web).113
If the pregnant patient has HELLP syndrome, the best treatment would be to finalize the pregnancy if it is discovered after the 34th week of gestation or if there is deterioration of the maternal or fetal status. Conservative treatment is controversial but should be considered in certain cases prior to 34 weeks gestation.114
Again, if the pregnant patient was previously receiving antiplatelet or anticoagulant therapy due to APS, the decision as to when to discontinue those treatments prior to delivery and reinitiated them afterwards is usually made by the anesthesiologist responsible for the patient. It seems that preoperative antiplatelet therapy does not increase the incidence of major or mild bleeding complications associated with the epidural anesthesia.115 Thus, treatment with low-dose ASA in women with APS could be maintained until the time of delivery or, at least, until a week earlier and reinitiated 24h afterwards.112
With regards to patients receiving LMWH during pregnancy, it is usually recommended that epidural anesthesia be programmed 10–12h after the last dose (if it was prophylactic) or at least 24h after the last dose.112
In the case the patient has not received proper preconception counseling or simply was pregnant when she came to the clinic, the recommendations mentioned above should be adjusted to the situation of the patient and her pregnancy.
Recommendation 11. The panel recommends promoting collaboration among specialties (rheumatology, obstetrics, hematology, etc.) (LE 5, GR D, LA 100%).
The primary care physician should establish direct contact with the rheumatologist in the undesired case in which the pregnancy occurs without the previous knowledge on the part of the latter. It is also recommendable that there be good coordination and communication between the rheumatologist and the obstetrician to carry out the follow-up up of the patient during the pregnancy, as well as with other specialists involved (hematologist, etc.). Aside from the personal relationship among all of the specialists mentioned, the use of the phone for consultation, teleconsultation or e-mail is of great advantage to discuss doubts or solve incidents, all of which is facilitated by the utilization of the electronic health record, which is becoming increasingly widespread.
Whenever possible, it is recommended that the possibility of introducing multidisciplinary units or preferential referral circuits and good coordination among specialties.
Breastfeeding and PostpartumRecommendation 12. Having an inflammatory or autoimmune rheumatic disease does not contradict breastfeeding (LE 1, GR A, LA 100%).
For the most part, breast milk from patients with inflammatory or autoimmune diseases is not contraindicated. If the mother wants to nurse her child, care must be taken to use treatments that are not contraindicated during that period.116
On the other hand, patients must be informed during the postpartum that there is a risk of a flare of the disease activity (especially in RA), as well as a risk of thrombosis in other diseases, like SLE.117 These situations may implicate a reevaluation of the therapy.
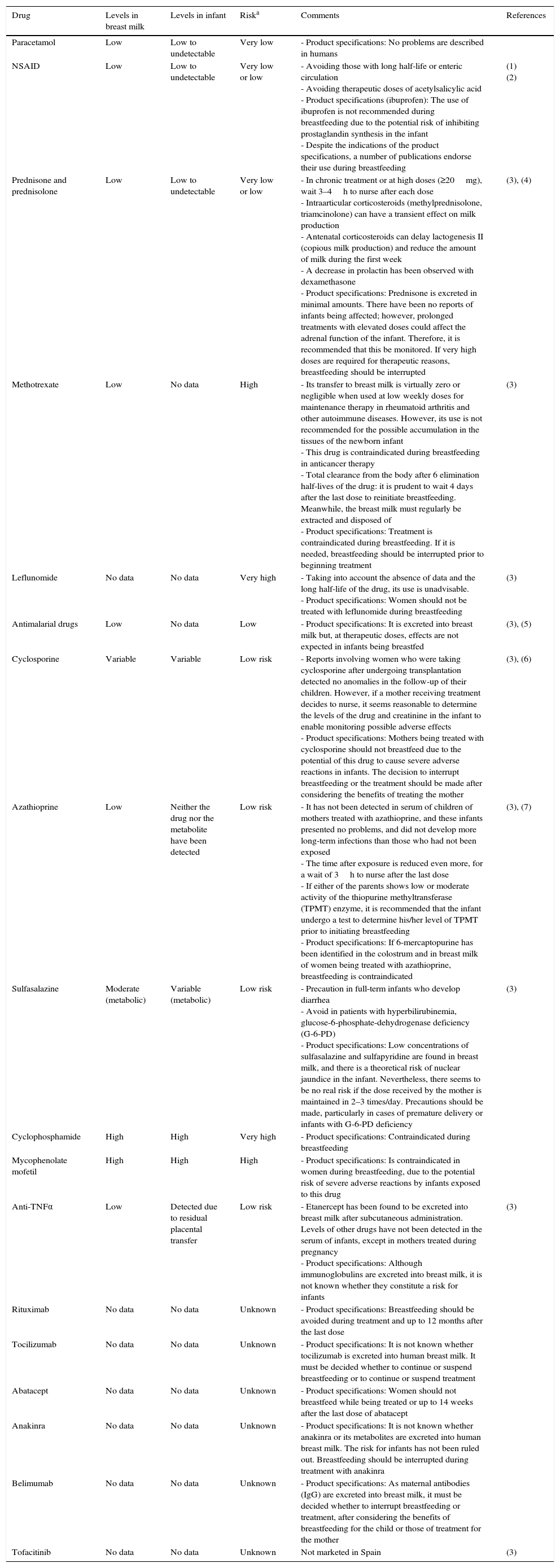
There is little available evidence on pharmacological safety during breastfeeding (Table 6). Fortunately, not all drugs reach breast milk in significant quantities and the presence of a minimum amount of a drug does not always place the infant at risk.116
Drugs During Breastfeeding.
| Drug | Levels in breast milk | Levels in infant | Riska | Comments | References |
|---|---|---|---|---|---|
| Paracetamol | Low | Low to undetectable | Very low | - Product specifications: No problems are described in humans | |
| NSAID | Low | Low to undetectable | Very low or low | - Avoiding those with long half-life or enteric circulation - Avoiding therapeutic doses of acetylsalicylic acid - Product specifications (ibuprofen): The use of ibuprofen is not recommended during breastfeeding due to the potential risk of inhibiting prostaglandin synthesis in the infant - Despite the indications of the product specifications, a number of publications endorse their use during breastfeeding | (1) (2) |
| Prednisone and prednisolone | Low | Low to undetectable | Very low or low | - In chronic treatment or at high doses (≥20mg), wait 3–4h to nurse after each dose - Intraarticular corticosteroids (methylprednisolone, triamcinolone) can have a transient effect on milk production - Antenatal corticosteroids can delay lactogenesis II (copious milk production) and reduce the amount of milk during the first week - A decrease in prolactin has been observed with dexamethasone - Product specifications: Prednisone is excreted in minimal amounts. There have been no reports of infants being affected; however, prolonged treatments with elevated doses could affect the adrenal function of the infant. Therefore, it is recommended that this be monitored. If very high doses are required for therapeutic reasons, breastfeeding should be interrupted | (3), (4) |
| Methotrexate | Low | No data | High | - Its transfer to breast milk is virtually zero or negligible when used at low weekly doses for maintenance therapy in rheumatoid arthritis and other autoimmune diseases. However, its use is not recommended for the possible accumulation in the tissues of the newborn infant - This drug is contraindicated during breastfeeding in anticancer therapy - Total clearance from the body after 6 elimination half-lives of the drug: it is prudent to wait 4 days after the last dose to reinitiate breastfeeding. Meanwhile, the breast milk must regularly be extracted and disposed of - Product specifications: Treatment is contraindicated during breastfeeding. If it is needed, breastfeeding should be interrupted prior to beginning treatment | (3) |
| Leflunomide | No data | No data | Very high | - Taking into account the absence of data and the long half-life of the drug, its use is unadvisable. - Product specifications: Women should not be treated with leflunomide during breastfeeding | (3) |
| Antimalarial drugs | Low | No data | Low | - Product specifications: It is excreted into breast milk but, at therapeutic doses, effects are not expected in infants being breastfed | (3), (5) |
| Cyclosporine | Variable | Variable | Low risk | - Reports involving women who were taking cyclosporine after undergoing transplantation detected no anomalies in the follow-up of their children. However, if a mother receiving treatment decides to nurse, it seems reasonable to determine the levels of the drug and creatinine in the infant to enable monitoring possible adverse effects - Product specifications: Mothers being treated with cyclosporine should not breastfeed due to the potential of this drug to cause severe adverse reactions in infants. The decision to interrupt breastfeeding or the treatment should be made after considering the benefits of treating the mother | (3), (6) |
| Azathioprine | Low | Neither the drug nor the metabolite have been detected | Low risk | - It has not been detected in serum of children of mothers treated with azathioprine, and these infants presented no problems, and did not develop more long-term infections than those who had not been exposed - The time after exposure is reduced even more, for a wait of 3h to nurse after the last dose - If either of the parents shows low or moderate activity of the thiopurine methyltransferase (TPMT) enzyme, it is recommended that the infant undergo a test to determine his/her level of TPMT prior to initiating breastfeeding - Product specifications: If 6-mercaptopurine has been identified in the colostrum and in breast milk of women being treated with azathioprine, breastfeeding is contraindicated | (3), (7) |
| Sulfasalazine | Moderate (metabolic) | Variable (metabolic) | Low risk | - Precaution in full-term infants who develop diarrhea - Avoid in patients with hyperbilirubinemia, glucose-6-phosphate-dehydrogenase deficiency (G-6-PD) - Product specifications: Low concentrations of sulfasalazine and sulfapyridine are found in breast milk, and there is a theoretical risk of nuclear jaundice in the infant. Nevertheless, there seems to be no real risk if the dose received by the mother is maintained in 2–3 times/day. Precautions should be made, particularly in cases of premature delivery or infants with G-6-PD deficiency | (3) |
| Cyclophosphamide | High | High | Very high | - Product specifications: Contraindicated during breastfeeding | |
| Mycophenolate mofetil | High | High | High | - Product specifications: Is contraindicated in women during breastfeeding, due to the potential risk of severe adverse reactions by infants exposed to this drug | |
| Anti-TNFα | Low | Detected due to residual placental transfer | Low risk | - Etanercept has been found to be excreted into breast milk after subcutaneous administration. Levels of other drugs have not been detected in the serum of infants, except in mothers treated during pregnancy - Product specifications: Although immunoglobulins are excreted into breast milk, it is not known whether they constitute a risk for infants | (3) |
| Rituximab | No data | No data | Unknown | - Product specifications: Breastfeeding should be avoided during treatment and up to 12 months after the last dose | |
| Tocilizumab | No data | No data | Unknown | - Product specifications: It is not known whether tocilizumab is excreted into human breast milk. It must be decided whether to continue or suspend breastfeeding or to continue or suspend treatment | |
| Abatacept | No data | No data | Unknown | - Product specifications: Women should not breastfeed while being treated or up to 14 weeks after the last dose of abatacept | |
| Anakinra | No data | No data | Unknown | - Product specifications: It is not known whether anakinra or its metabolites are excreted into human breast milk. The risk for infants has not been ruled out. Breastfeeding should be interrupted during treatment with anakinra | |
| Belimumab | No data | No data | Unknown | - Product specifications: As maternal antibodies (IgG) are excreted into breast milk, it must be decided whether to interrupt breastfeeding or treatment, after considering the benefits of breastfeeding for the child or those of treatment for the mother | |
| Tofacitinib | No data | No data | Unknown | Not marketed in Spain | (3) |
Very low: safe; with no risk for breastfeeding or for the infant. Low: quite safe; risk is slight or improbable. High: not very safe; careful assessment; avoid or employ a safer alternative. Very high: use an alternative or interrupt breastfeeding.
Source: Based on a publication by Sammaritano and Bermas.116
1. Sachs HC. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132:e796–809 [Online publication 28.08.13].
2. Montgomery A, Hale TW. ABM clinical protocol #15: analgesia and anesthesia for the breastfeeding mother, revised 2012. Breastfeed Med. 2012;7:547–53 [Online publication 12.12.12].
3. Sammaritano LR, Bermas BL. Rheumatoid arthritis medications and lactation. Curr Opin Rheumatol. 2014;26:354–60 [Online publication 13.03.14].
4. Ost L, Wettrell G, Bjorkhem I, Rane A. Prednisolone excretion in human milk. J Pediatr. 1985;106:1008–11 [Online publication 01.06.85].
5. Ostensen M, Motta M. Therapy insight: The use of antirheumatic drugs during nursing. Nat Clin Pract Rheumatol. 2007;3:400–6 [Online publication 30.06.07].
6. Nyberg G, Haljamae U, Frisenette-Fich C, Wennergren M, Kjellmer I. Breast-feeding during treatment with cyclosporine. Transplantation. 1998;65:253–5 [Online publication 11.02.98].
7. Habal FM, Huang VW. Review article: a decision-making algorithm for the management of pregnancy in the inflammatory bowel disease patient. Aliment Pharmacol Ther. 2012;35:501–15 [Online publication 10.10.12].
Breast milk does not usually interfere with the administration of most of the vaccines required by the mother despite the presence of maternal antibodies in the milk.118 Moreover, it has been observed that breastfeeding increases the effect of certain vaccines like those preventing pneumococcal diseases and Haemophilus influenzae type b (Hib)119 and reduces the incidence of fever after immunization.120
The administration of vaccines to the mother does not generally involve a risk for the infant despite the fact that the production of antibodies is excreted into breast milk. This includes inactivated and even attenuated vaccines since, the virus is not transmitted through breast milk. Should this occur, the concentrations would be very small and would not be associated with infections and, thus, the majority of the vaccines do not suppose a risk for the newborn. An exception are the smallpox and yellow fever vaccines, which have been involved in serious conditions in infants, such as encephalitis, and are therefore absolutely contraindicated.121,122
On the basis of the above information, any decision made during breastfeeding must be discussed with the patient.123
Recommendation 13. Once the pregnancy is over, the usual follow-up of the patient should be undertaken as soon as possible, especially if there have been factors like those mentioned above have been observed, which could indicate a poor prognosis (LE 3, GR C, LA 100%).
It is important that patients with rheumatic diseases return to follow-up as soon after delivery as possible, among other reasons because there is a considerable risk of flares at that time, especially in those patients who have had activity throughout the pregnancy, particularly during the first trimester. Other factors associated with flares are preterm delivery and a low birth weight newborn.124 The frequency of visits will depend on the situation of each patient. The patients should also undergo the usual gynecological and pediatric check-ups.
The evaluation of the disease must be individualized. It should be taken into account that in the immediate postpartum period, aside from the physical changes, patients may have elevated acute-phase reactants. Thus, in the utilization of indicators of activity it is recommendable to employ the CRP, rather than the erythrocyte sedimentation rate.68 All other tests (laboratory analyses, etc.) will be done as usual.
The possible reintroductions or changes in medication will be done depending on the patient's course. This means, for example, that an RA patient in remission whose therapy was interrupted because of the pregnancy, will not need to receive it while she is still in remission.
On the other hand, patients with APS will take LMWH as prophylaxis. If there is a history of thrombosis, therapy will consist of acenocoumarol and/or warfarin, as these drugs are safe during breastfeeding.
Finally, the panel considers that the rheumatologist should be fully involved in the follow-up, regardless of whether the patient had been followed in a high-risk unit or in the usual offices.
Recommendation 14. The newborn does not require special care unless the mother is positive for anti-Ro or anti-La antibodies, has been exposed to biological therapies during the pregnancy or the infant was born with a low birth weight (LE 2b, GR B-C, LA 91%).
Neonatal lupus is the process developed in newborns of mothers with anti-Ro and/or anti-La antibodies, as a consequence of their crossing the placenta, regardless of whether or not there is evidence of SLE, RA or underlying Sjögren's syndrome, or even in the absence of a diagnosed disease.125 Its prevalence can reach 20% and it encompasses manifestations like the skin rash, thrombocytopenia or congenital heart block (CHB). The risk of it developing is related to the antibody titer and not only to their being present.
The risk of CHB is 5% in patients with moderate-to-high titers of anti-Ro antibodies, and is nearly nonexistent with low titers.126 This risk is multiplied approximately 10-fold in the case of women who have previously had a child with neonatal lupus in any of its manifestations.127 Except for CHB, most of the signs of neonatal lupus disappear after clearance of maternal antibodies at the age of 3 to 6 months of life of the newborn. The risk of mortality associated with CHB is approximately 20%, generally due to hydrops fetalis and to myocarditis. The majority of the children who survive require a pacemaker.128
The current recommendations on the management of patients with a high risk include performing weekly fetal echocardiography between 16 and 26 weeks gestation, and every 2 weeks thereafter until week 34.
If there is evidence of progressive CHB and fetal heart failure, the decision should be made to plan the best time for delivery and finalize the pregnancy. No therapeutic option has been found to be efficient in the case of established block. Owing to the low incidence of this complication, it has not been possible to conduct randomized controlled trials.
The administration of fluorinated corticosteroids, which cross the placenta, continues to be recommended in the case of atrioventricular block, pericarditis or another form of myocardial inflammation, although the response is controversial. At the present time, the efficacy of intravenous immunoglobulin therapy has not been demonstrated.129 Recently, 2 important articles reported that the use of HCQ significantly reduces the risk of CHB in anti-Ro or anti-La-positive patients.130,131 More studies on the use of HCQ during pregnancy are needed to determine the possible “protective” effect on these patients.
Infants who have been exposed to anti-TNFα during pregnancy may have a slight increase of mild infections during the first 2 years. Vaccination with non-live vaccines should be carried out in accordance with the established calendar.132 However, due to the reported case of disseminated tuberculosis in a newborn after exposure to an anti-TNFα agent during pregnancy after having been vaccinated with Bacillus Calmette-Guérin vaccine,133 it is recommended that these infants not be vaccinated with live vaccines until they are 6 months old. It is also recommended that analyses be performed during the first weeks to check for the possible development of neutropenia.
DiscussionWhen a woman with systemic autoimmune or chronic inflammatory diseases is planning a pregnancy, it is necessary to take into account a number of aspects. In the first place, chronic inflammatory diseases like RA or spondyloarthritis do not always have detrimental effects on pregnancy and/or fetal health, although they indeed have been reported, whereas in other systemic diseases like SLE, vasculitis or systemic sclerosis, this risk may be greater. This signifies that the therapeutic approach will be different. Generally, all of these patients are concerned about maintaining treatment during pregnancy. Its sudden interruption means that the disease becomes active again. Thus, the best moment of planning conception and the manner of adjusting treatment are key aspects that must be discussed in preconception counseling.
Overall, the pregnancy should be planned for it to take place when the diseases are not active and the patients are receiving safe medication from the point of view of the pregnancy and the fetus.
The evaluation of patients with rheumatic diseases who wish to have a child should follow the same general pattern, regardless of the specific diagnosis. First, the disease activity must be determined; if it is active at that time, it would not be a suitable moment for conception, and the pregnancy should be postponed until the episode is resolved and the patient can be treated aggressively. Once the disease has been controlled for at least 6 months, the situation can be reevaluated taking into account not only the activity but also the possible development of chronic lesions that contraindicate pregnancy (for example, severe cardiomyopathy or valve disease, pulmonary hypertension, interstitial lung disease, neurological disorder or chronic kidney disease).
Then, there must be an assessment of the autoantibodies presented by the patients since some of them will require specific monitoring, fetal echocardiograms or additional therapy, as in the case of antiphospholipid antibodies. With all of this, accurate and complete information will be prepared for the couple who is planning the pregnancy.
The probability of successful conception will also be greater when combined with the multidisciplinary evaluation of the rheumatologist and an obstetrician with experience in high-risk pregnancies, working as a team, with the identification and understanding of the possible specific risks for an individual patient.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was financed by an independent grant from UCB.
Conflicts of InterestFrancisco Javier López Longo has received fees for presentations from Abbvie, Roche Farma, Bristol-Myers Squibb, Pfizer, UCB, MSD and Actelion and funding for research projects from Abbvie and GSK.
M. Luz García Vivar has received fees for presentations from UCB, Roche, Janssen, Glaxo, Abbvie, Pfizer, Bristol-Myers Squibb and MSD, and worked as a consultant for Roche, Janssen and Cellgene. She has also received funding for research projects from Roche, Novartis, Janssen, Glaxo, Abbvie, Pfizer, MSD and Lilly and financing to attend courses and meetings from Roche, Pfizer, Novartis, Abbvie, Janssen and Bristol-Myers Squibb.
Víctor Martínez-Taboada has been awarded grants from the Spanish Health Research Fund, the Fundación Marqués de Valdecilla-IFIMAV, Instituto de Salud Carlos III, Spanish Rheumatology Foundation, Foundation for Medical Research «Mutua Madrileña Automovilista», Schering-Plough, Wyeth-Pharma, and Roche. He has received fees for presentations from the Spanish Society of Rheumatology (SER), Pharmacia-Pfizer, Schering-Plough, Lilly, Zambon, Wyeth-Pharma, Abbott, Almiral, Brystol-Myers Squibb, Roche and UCB-Pharma, and for the organization of and participation in Continuing Medical Education (SER), Abbott and Brystol-Myers Squibb. He has worked as a consultant for UCB-Pharma, Brystol-Myers Squibb, Roche, Cellerix, Pfizer, Sobi, Boehringer Ingelheim, Servier and Hospira, and has participated in clinical trials for Novartis, Schering-Plough, Centocor, Wyeth-Pharma, Abbott, Brystol-Myers Squibb, Roche, Serono, Amgen, GlaxoSmithKline, Pfizer and Servier.
Paloma Vela has received fees as a consultant for Novartis, and for presentations and development of material for educational purposes for Abbvie, Roche, MSD, UCB and BMS. She has received financing for travel and hotel accommodations to attend scientific congresses and meetings from Roche, Abbvie, MSD, UCB, Pfizer and BMS.
Juan Antonio Martínez-López has received fees as a consultant for UCB and funding for presentations from Roche, BMS, Pfizer, Lilly and UCB.
The remaining authors declare they have no conflicts of interest.
All of the rheumatologists who participated in the survey carried out prior to drafting the recommendations.
Please cite this article as: Martínez López JA, García Vivar ML, Cáliz R, Freire M, Galindo M, Hernández MV, et al. Recomendaciones sobre actuaciones a seguir durante la edad fértil, el embarazo, posparto y lactancia en pacientes con enfermedades reumáticas inflamatorias y autoinmunes. Reumatol Clin. 2017;13:264–281.