The prevalence of nutritional alterations in rheumatologic diseases ranges from 4 to 95%, depending on the detection method used. Formerly described as the single term rheumatoid cachexia, nutritional alterations can currently be grouped and subdivided based on the physiopathological mechanisms involved: chronic disease-related inflammatory conditions (cachexia), malnutrition associated to acute malnutrition inflammatory conditions (protein-caloric malnutrition) and starvation-related malnutrition. Clinical manifestations of malnutrition associated to rheumatic diseases vary from the patient with low weight or overweight and obesity; with lean body mass depletion as well as functional repercussions, and impact of quality of life as a common denominator. Additionally, the associated increase in body fat mass increases the risk for cardiovascular morbidity. A multidisciplinary approach towards rheumatic diseases should include aspects oriented towards prevention, early identification, diagnosis and correction of nutritional alterations.
Las prevalencia de las alteraciones nutricionales en pacientes con enfermedades reumatológicas varía entre un 4 y un 95%, dependiendo del método empleado para su detección. Inicialmente agrupadas bajo el término de caquexia reumatológica, en la actualidad es posible ampliar el concepto de desnutrición conforme los mecanismos fisiopatológicos que participan, sea desnutrición asociada a procesos inflamatorios crónicos (caquexia), desnutrición asociada a procesos inflamatorios agudos (desnutrición proteico-calórica) y desnutrición asociada a baja ingesta alimentaria. El espectro clínico de la desnutrición asociada a enfermedades reumatológicas varía desde el paciente con bajo peso hasta el paciente con sobrepeso u obesidad, con disminución en la cantidad de masa magra, repercusión funcional, en calidad de vida y pronóstico, como común denominador. Adicionalmente, el incremento asociado en masa grasa aumenta el riesgo para el desarrollo de enfermedad cardiovascular. El manejo integral de las enfermedades reumatológicas debe de incluir aspectos para la prevención, la identificación y el manejo oportunos de las alteraciones nutricionales.
The nutritional impact of rheumatologic diseases was first identified in 1873 by Sir James Paget and given the name rheumatoid cachexia.1,2 It is associated with inflammatory diseases1 and comprises the effects of nutritional alterations on motor and sensory functions, manifested by a combination of weakness, muscle atrophy and loss of functionality.1,3
Although some authors suggest that the altered nutritional state of patients with rheumatologic diseases is a marker for disease severity,3 there are still aspects that deserve more attention.
Relevance of the ProblemIn recent decades, the concept of rheumatoid cachexia has received more consideration2 based on the understanding of how nutritional alterations affect patient quality of life, evolution and the prognosis of rheumatologic conditions.3 Advances have also been made in our comprehension of the pathophysiological mechanisms that mediate the nutritional impact of inflammatory diseases (acute and/chronic).4,5 Meanwhile, the tools available for evaluating body composition have become more precise and can more adequately define the effects of inflammatory conditions on lean mass, fat mass and energy expenditure.6
From a pathophysiological standpoint, the importance of preventing the loss of lean mass stems from the fact that it is largely created by what is known as body cell mass and is the site where 95% of the metabolic activity of the body occurs.2,6–8
Lean mass is composed of muscle, functional organ tissue, the immune system and the skeletal system, with their related structural, metabolic, immunological, support and motor functions.2,6–8 Therefore, any alteration in lean mass would mean the loss or limitation of body functions and potential morbidities or mortality.6,8,9
It is well known that rheumatology patients with altered nutritional states have life expectancies that are up to 18 years shorter than patients with rheumatologic diseases without malnutrition; in addition, their risk for morbidity is 3–5 times higher than the general population.3 Mortality associated with malnutrition (infectious processes, cardiovascular and pulmonary diseases) even surpasses that of the morbidities associated with rheumatologic diseases.3,6
The concept of malnutrition in patients with chronic inflammatory conditions covers the clinical spectrum from underweight patients with reduced muscle mass to those patients who, according to their body mass index (BMI), are overweight or obese but also have decreased muscle mass associated with chronic inflammatory processes.4,5,8 At the same time, increased fat mass is a risk factor for the development of metabolic syndrome and cardiovascular diseases, whose prevalence is widespread in patients with rheumatologic diseases.2–10
PrevalenceThe incidence of rheumatoid cachexia is variable and depends directly on the sensitivity and specificity of the instruments used for its detection.11,12 The prevalence of nutritional alterations ranges from 4% to 26%–52%, particularly in populations with rheumatoid arthritis.11,13
A published study by Bravo-Ramírez et al.14 reported that 37.5% of the Mexican population with Systemic lupus erythematosus (SLE) had decreased lean body mass when they were evaluated with bioelectrical impedance vector analysis despite the fact that half of the sample had obese or overweight body mass indices.
In another study, also in Mexican patients with diagnosed rheumatoid arthritis (RA),13 48% of patients had decreased lean body mass, even when 94% had increased fat mass percentages. It is therefore evident that the prevalence of malnutrition, according to the criterion of decreased lean mass, is frequently underestimated.
The prevalence of malnutrition also varies depending on the gender of the population studied: in patients with RA, it can reach 52% in women and 30% in men.11
PresentationIn patients with malnutrition, the decrease in underlying lean mass is not always evident because, if there is preserved or increased fat mass, body weight is not modified.2,3,11,12 BMI can be within normal, overweight or even obese ranges, so patients are erroneously considered well nourished.2,11–14
In a population of Mexican women diagnosed with SLE evaluated by means of BMI, 20.5% and 29.2% were found within overweight and obese ranges, respectively. However, when an evaluation tool with greater sensitivity was used to estimate lean mass, such as bioelectrical impedance vector analysis, 37% of this population had decreased lean body mass and met criteria for malnutrition (cachexia). This condition would have gone undetected if only BMI had been evaluated.14
Thus, patients with chronic inflammatory diseases should be evaluated from a nutritional standpoint both systematically and periodically using a combination of instruments and tools with high sensitivity for the detection of alterations in body composition, even when patients show no or very slight changes in body weight.2,3,11,12 The resources available for assessing nutritional state in patients with rheumatologic diseases include nutritional screening scales, anthropometric measurements, dietary history, interpretation of biochemical parameters, functional evaluation and determination of body composition, the latter using bioelectric impedance, densitometry, computed tomography, magnetic resonance, muscle ultrasound, etc.6
In order to extend the diagnostic capacity of malnutrition in rheumatology patients, it is currently recommended to use resources with greater sensitivity than anthropometrics and BMI to detect nutritional alterations, particularly in lean mass and function,1–8 even when the BMI is within normal, overweight or obese ranges.4,6
PathophysiologyCurrently, malnutrition is classified into 3 clinical entities according to the pathophysiologic mechanisms involved in their development. This provides better understanding and integration of clinical-pathophysiologic aspects and the possibility to influence prevention, identification and management.4,5,8
Cachexia SyndromeThe term cachexia comes from the Greek kachexía (meaning poor condition), described as emaciation, wasting syndrome or consumption,4,5 refers to the malnutrition associated with chronic inflammatory processes, where the presence of inflammation, at varying degrees and extensions, is consistently present.9
In the case of chronic disorders, the sustained and long-term participation of inflammatory processes involves the activation in cascade of mediators like tumour necrosis factor-α, interleukin-1, interleukin-6, C-reactive protein, transforming growth factor-β, catecholamines, endogenous glucocorticoids, and activation of nuclear factor κβ, etc.8,15,16 Together, these exert deleterious effects on body composition and the metabolism of energy, resulting in increased baseline energy expenditure, muscle proteolysis and gluconeogenesis, mobilisation of muscle amino acids, insulin resistance, hyperglycaemia, muscle protein synthesis, increased mobilisation of adipose tissue free fatty acid, anaemia of chronic disease, bone demineralisation, endothelial dysfunction, platelet activation and aggregation, dyslipidaemia, atherogenesis, hyperfibrinogenaemia, hyperuricaemia, vasoconstriction, thrombogenesis and metabolic syndrome.2–5
In nutritional terms, the combined effect of inflammatory mediators leads to anorexia, unintentional weight loss (>5% over the last 6 months), decreased muscle mass (values below the 10th percentile)8 and adipose tissue, along with reduced strength and mobility, weakness, functional limitations, loss of autonomy, fatigue, immune dysfunction, greater susceptibility for infectious processes, altered healing processes and tissue repair, depression, poor quality of life and shorter life expectancy.2,3,8,10,17,18 To all these conditions, we can add the potential side effects of the medications used for the treatment of rheumatologic diseases (immunosuppressants and glucocorticoids) and their negative influence on body composition.19
The decrease in lean mass associated with cachexia is not completely reversible, even with optimal caloric and protein intake. It is the result of the multisystem effect of the inflammatory mediators and not able to be completely reverted with the regularisation or optimisation of the intake of proteins, calories and micronutrients.4,5,18 The possibility has been explored to modulate the extension of the inflammation with the selective use of certain nutrients (omega-3 fatty acids, β-hydroxymethylbutyrate, leucine), with regulatory properties over the inflammatory and immune response, which could modify its course towards more favourable and less deleterious profiles for the nutritional state.4,18
One clinical-pathological entity that frequently coexists with malnutrition associated with chronic inflammatory processes, particularly in senior populations, is the presence of sarcopenia,8 which is associated with the ageing process. Caused by multiple factors, sarcopenia is characterised by loss of muscle mass (<2 standard deviations from the reference value)8 and muscle strength (measured by dynamometry or walking speed and reference values for the population studied),8 increased adipose tissue and fatty infiltration of the muscles,6 with repercussions in terms of loss of muscle strength, weakness, prostration, loss of autonomy and eventually disability.8
The presence of sarcopenia and increased fat mass is known as sarcopenic obesity syndrome,6 which has a metabolic and nutritional behaviour characteristic of malnourished patients, overweight and obese BMI ranges, and higher risk for cardiovascular morbidity and mortality. The latter causes an important number of deaths in rheumatologic patients, some of which are premature.20–25
The association of increased body fat mass and malnutrition is prevalent. In a Mexican population of patients with RA, 35% had central obesity and morbidities included malnutrition, cardiovascular diseases and metabolic syndrome.13
Malnutrition Associated With Acute Inflammatory ProcessesThe characteristic of malnutrition associated with acute inflammatory processes (also known as protein-energy malnutrition or kwashiorkor)4,5 is a significant amplification (usually at supraphysiological intervals) of the inflammatory and immunological activation cascade.4,5,15,18 This leads to accelerated malnutrition with a significant loss of lean mass in a short period of time (days or weeks), accompanied by higher capillary permeability, water retention and formation of oedemas.4,5 Its presence in patients with rheumatologic diseases originates from exacerbation episodes of the baseline disease and/or the concurrence of another type of acute inflammatory processes, which are generally infectious.4,5,18
Without a quantified evaluation to determine the amount of lean mass and its functional translation, the diagnosis of malnutrition can be erroneously overlooked since the presence of oedema hides weight loss in its early stages.4,5,15,18
The effects of the rapidly progressive course of malnutrition secondary to acute inflammatory processes are added to the pre-existing and secondary effects of the chronic evolution of rheumatologic diseases. This promotes immune and muscle dysfunction, weakness, dysmetabolism, altered quality of life, long-term disability and prognosis, since, even if the acute inflammatory event is resolved, the limitations caused by the acute deterioration of the nutritional state do not always revert in the short term.2,3
Similar to chronic inflammatory processes, nutritional interventions in acute processes do not revert the entire underlying inflammatory cascade of effects on the nutritional state. Instead, they only contribute to avoiding any further deterioration in the patient's nutritional condition.2,18 The nutritional alterations associated with acute and chronic inflammatory processes are only reversible once the remission of the pathophysiological process is achieved. This is not always possible in most rheumatologic diseases where, even with total remission of the underlying inflammatory process, alterations to the metabolism and body composition persist over time.26
Malnutrition Associated With Inadequate Intake or NutritionDescribed with the term marasmus, the malnutrition associated with deficient energy intake or inadequate nutrition is a common entity, particularly in senior populations or those with disabilities. Its pathophysiology lies in the imbalance between energetic demand and nutritional consumption.4,5 When there is a confluence between malnutrition associated with acute and chronic inflammatory processes and malnutrition associated with inadequate calorie/protein intake, the impact on function is multiplied.4,5,8,26,27
Reduced intake of energetic substances leads to the implementation of adaptive mechanisms in order to temporarily preserve vital bodily functions. When this adaptive capability is surpassed, the pathophysiologic entity of malnutrition associated with low intake becomes established, with the resulting effects on function.4,5 Its co-participation in the scenario of chronic inflammatory processes is not rare, and its aetiology is generally multifactorial.4,5
The importance of the early and opportune identification of malnutrition associated with reduced dietary intake is because patients with rheumatologic disease frequently have inadequate eating patterns that do not cover basic nutritional requirements stemming from the pathophysiologic processes of rheumatologic diseases and the effects of the medicines used.13 Furthermore, this type of malnutrition is reversible with optimal nutrition.4,5
The aetiology of malnutrition associated with low dietary intake in patients with rheumatologic diseases arises from several factors, such as the negative impact of the disease on quality of life, independence, autonomy, self-sufficiency, functionality, adverse economic and social factors, depression, clinical course with exacerbations and remissions, frequent hospitalisations, side effects of medications, and the anorexigenic effects of the diverse inflammatory mediators that participate in rheumatologic diseases (particularly tumour necrosis factor α).13 Therefore, as an integral part of the management of patients with rheumatologic diseases, the food consumption history is an element with important predictive value for the prevention, identification, early detection and correction of nutritional alterations associated with this category.28
The prevalence of inadequate nutrition in patients with rheumatologic diseases is high. In one study done by Puente-Torres et al.13 in a Mexican population of patients with RA, it was demonstrated that 90% of the patients studied presented inconsistencies for meeting the criteria of a complete, balanced, sufficient and varied diet.
Nutritional Management of Patients With Rheumatologic DiseasesThe conceptual focus on the pathophysiologic processes that lead to malnutrition, and not just the degree of correspondence with cut-points established for anthropometric measurements or amounts of lean mass and fat mass, is currently considered an innovative proposal for the comprehension, prevention and the management of malnutrition.4,5,18,29–31 With these grounds, it is possible to implement specific strategies for the management of malnutrition, whose main objectives are:
- –
Nutritional management as an integral and interdisciplinary part of the medical management of patients with rheumatologic diseases.6,13
- –
Methods to evaluate body composition and provide a better definition of the effect that rheumatologic diseases have on lean mass and fat mass, thus increasing understanding, knowledge, prevention and management of nutritional alterations in order to reduce the impact on the course and prognosis of the disease, quality of life and funcionality.6
- –
Preservation of body composition, particularly with the conservation of lean mass and related functionality.2,9,10,13,26
- –
Prevention of increased body mass, excess weight gain, obesity and metabolic syndrome and their participation as risk factors for the development of cardiovascular diseases.13,20–25
- –
Reduction of the incidence and prevalence of nutritional alterations associated with the pathophysiological basis of rheumatologic diseases by optimally controlling the baseline disease; prevention, identification and management of acute concomitant processes, as well as minimisation of potential side effects of treatment on nutritional state.2,18
- –
Preservation of functionality, independence, autonomy, self-sufficiency and quality of life, with improved prognosis and life expectancy.6,9,10,26
- –
Potential use of pharmaconutrition to selectively modulate the underlying inflammatory response and influence the prognosis of the rheumatologic disease, even when this method of management is still under debate.28,32
To this end, proposed nutritional interventions include:
- –
Nutritional screening and evaluation of patients with rheumatologic diseases for the presentation, identification, diagnosis and management of the nutritional alterations associated with rheumatologic diseases.33
- –
Dietary prescription, monitoring and follow-up to maintain adequate macro and micronutrients that cover basic nutritional requirements as well as any requirements of the chronic disease, acute concomitant processes and those associated with the potential side effects of the drugs used for the management of rheumatologic diseases.13,33
- –
Rehabilitation programmes to preserve bone density, muscle mass and strength in order to maintain functionality, independence, autonomy, prevent disability and improve the quality of life of patients with rheumatologic diseases.34–38
- –
Selective modulation of inflammatory processes to attenuate the metabolic response that accompanies rheumatologic diseases and its effects on nutritional state and body composition.39–43
The patient is a 47-year-old woman with type 2 diabetes, hypertension, hypercholesterolaemia and hypertriglyceridaemia. She had been diagnosed with RA 6 years ago and currently showed evidence of articular inflammatory activity with phlogosis of the elbows and knees. The patient was admitted to the hospital with a diagnosis of community-acquired pneumonia.
Irregular pharmacological management for baseline diseases had included metformin, enalapril, prednisone, methotrexate and chloroquine. Her usual weight was 78.5kg and current weight was 65.5kg; weight loss of 13kg had occurred over the last 12 months. BMI was 26.2kg/m2 (overweight). Ankle oedema was observed in both legs.
The evaluation of her daily eating habits identified high consumption of complex carbohydrates and low intake of protein.
Anthropometric assessment showed: mid-arm circumference 26.8cm (below 10th percentile44); abdominal circumference 97cm (higher than the reference point for the Spanish population45).
Body composition was analysed by vector impedance, which revealed lean mass (10th percentile46) and high fat mass (95th percentile46).
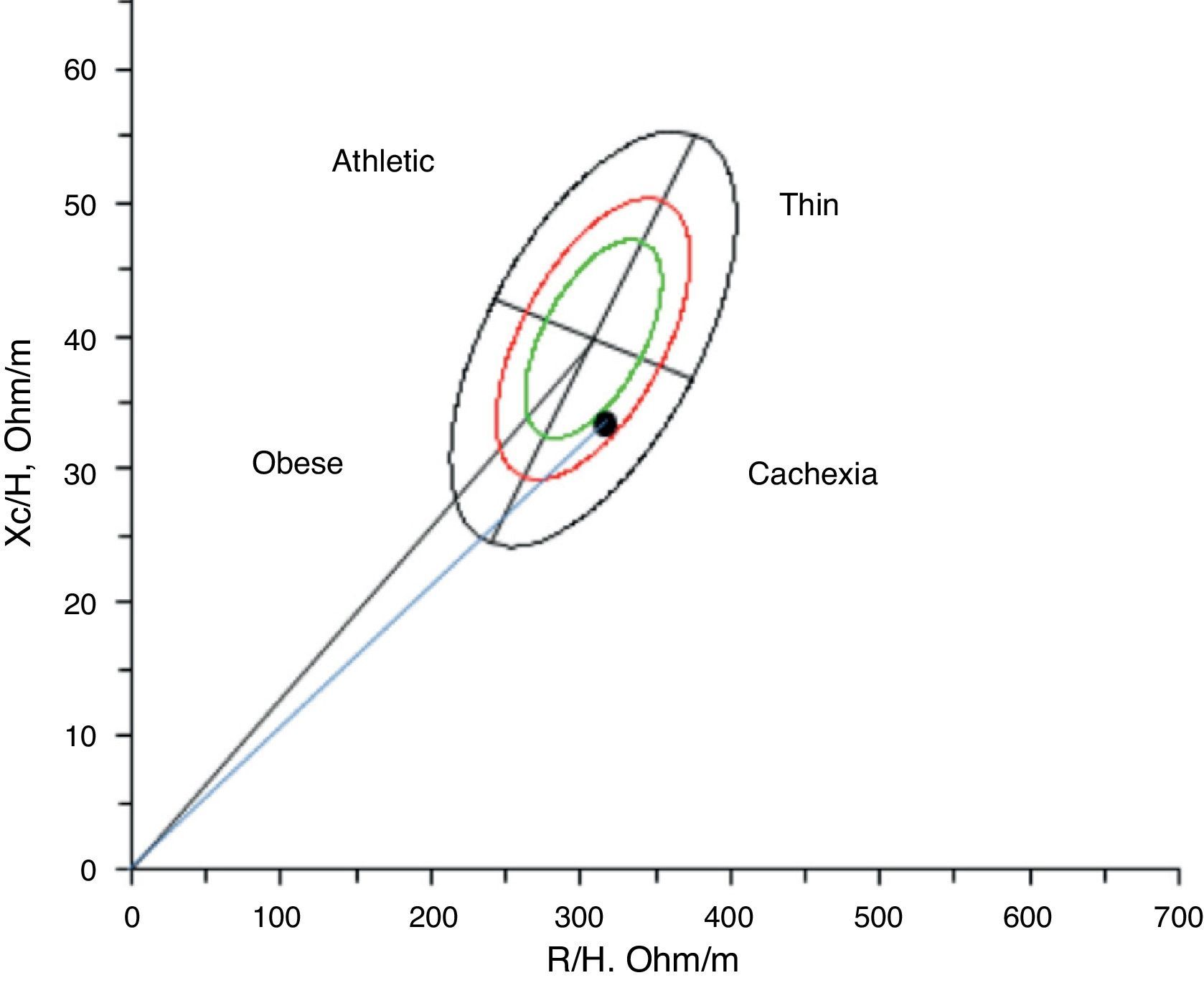
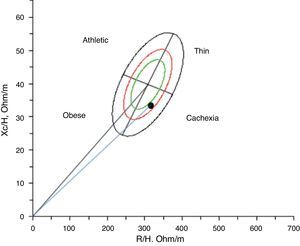
Given this assessment of lean mass using bioelectrical impedance vector analysis of the body composition, the patient was cachectic47 (Fig. 1), with the additional presence of sarcopenic obesity (increased fat mass and reduced fat mass).6,48
Functional evaluation with dynamometry showed a muscle strength of 14kg (10th percentile49).
Nutritional considerations:
- 1.
Patient with morbidities associated with malnutrition, chronic inflammatory state, use of glucocorticoids, metabolic syndrome and acute infectious inflammatory process.
- 2.
Despite having a BMI in the overweight range, the patient was malnourished as she has lost 16.5% of her usual weight over the course of the last 12 months.
- 3.
There was a loss of lean muscle (mid-arm circumference in 10th percentile44 and fat-free mass in 10th percentile46).
- 4.
Given the body composition defined by bioelectrical impedance vector analysis, the patient was cachectic (decreased lean mass and overhydration).47
- 5.
Functional effects of decreased lean mass with loss of grip strength (dynapenia).49
- 6.
Along with the loss of lean mass, fat mass was increased (body mass index in 95th percentile46), which added the entity of sarcopenic obesity.6,48
- 7.
Fat mass was concentrated in the abdominal area, and abdominal circumference was higher than normal for women in this population group.45
- 8.
The aetiology of the malnutrition was multifactorial2,8,9:
- –
Malnutrition associated with chronic inflammatory process (rheumatologic cachexia): RA and metabolic syndrome, and possible additional effects of the drugs used (glucocorticoids).
- –
Malnutrition associated with acute inflammatory processes, exacerbation of baseline disease, with inflammatory activity of the joints and inflammatory/infectious pulmonary process.
- –
Malnutrition associated with low protein intake and increased caloric intake from carbohydrates.
- –
- 9.
Patient with morbidity associated with malnutrition as well as increased cardiovascular risk due to a high level of body fat, particularly in the abdominal area, and metabolic syndrome.45
- 10.
Proposed medical/nutritional management strategy:
- –
Resolution of the acute infectious inflammatory process.
- –
Optimal pharmacological management of the baseline rheumatologic disease, metabolic control (diabetes mellitus and dyslipidaemia) and hypertensive treatment.
- –
Prescribed diet for adequate nutrition.
- –
In summary, nutritional alterations are prevalent in patients with rheumatologic diseases. Their diagnosis depends on the nutritional assessment tools used. We suggest the preferable use of instruments that provide the objective evaluation of body composition, particularly those that estimate or measure lean body mass.
The aetiology of nutritional alterations in rheumatologic diseases is multifactorial. A combination of variable degrees of malnutrition may be observed in association with chronic inflammatory processes (rheumatoid cachexia), malnutrition associated with inflammatory processes and malnutrition associated with inadequate nutrition.
The presence of malnutrition in patients with rheumatologic diseases has an impact on prognosis, quality of life, autonomy, independence and even mortality.
The coexistence of malnutrition with a reduction in lean mass coinciding with increased fat mass (sarcopenic obesity) is frequent, which at the same time increases the risk for cardiovascular disease.
Interdisciplinary strategies are required for the prevention, identification, diagnosis and management of the nutritional alterations associated with rheumatologic diseases.
A focus on nutrition should form part of the integral management of patients with rheumatologic diseases.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflict of InterestsNone have been declared.
Please cite this article as: Hurtado-Torres GF, González-Baranda LL, Abud-Mendoza C. Caquexia reumatológica y otras alteraciones nutricionales en las enfermedades reumatológicas. Reumatol Clin. 2015;11:316–321.