Polymyalgia rheumatica (PR) can be associated with large vessel vasculitis (LVV). We evaluate the diagnostic role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) and its impact on the treatment of LVV associated with PR.
Materials and methodsRetrospective study of patients diagnosed with PR. Data was collected from health records. Blood analysis included acute-phase reactants (APR), C-reactive protein (CRP) and erythrocyte sedimentation rate. An 18F-FDG PET/CT scan was performed in those patients whose symptoms persisted, in those with elevated APR, those who required higher doses of steroids or those who had atypical features of PR (low-grade fever, weight loss, among others).
ResultsTwenty-three were eligible; 48% (n=11) of the patients were diagnosed with LVV associated with PR. The site was heterogeneous, but mostly involved the aorta. In 80% of the patients with LVV, a disease-modifying antirheumatic drug was added to their treatment. Elevated CRP values were associated with the likelihood of presenting LVV.
ConclusionsLVV is not uncommon, clinical features and elevated CRP levels should raise suspicion of LVV associated with PR. 18F-FDG PET/CT is useful in identifying LVV associated with PR.
La polimialgia reumática (PMR) puede asociarse a vasculitis de grandes vasos (VGV). Este trabajo pretende evaluar el papel de 18F-FDG PET/TC en el diagnóstico de VGV asociado a PMR.
Materiales y métodoEstudio retrospectivo de pacientes con PMR. Se recogieron datos clínicos, analíticos, reactantes de fase aguda (RFA) (PCR, VSG), y a quienes tenían clínica persistente, elevación de RFA, precisaban dosis elevadas de corticoterapia o cuadros atípicos de PMR (febrícula, pérdida de peso, etc.) se realizó 18F-FDG PET/TC.
ResultadosVeintitrés pacientes se incluyeron; el 48% (11) de los cuales tuvieron VGV asociada a PMR. La localización fue heterogénea pero en su mayoría involucró a la aorta. En cuanto al tratamiento, se añadieron fármacos modificadores de la enfermedad a más del 80% de los pacientes con VGV. Los pacientes con VGV tenían niveles de PCR elevados comparado con aquellos con PMR aislada.
ConclusionesLa VGV en PMR no es infrecuente, tanto la clínica como los valores de PCR elevados deben hacer sospechar la posibilidad de VGV asociada. El estudio de imagen 18F-FDG PET/TC es una herramienta útil identificando VGV asociada a PMR.
Polymyalgia rheumatica (PMR) and giant cell arteritis (GCA) are common inflammatory diseases in Western countries,1 that generally affect people over the age of 50. PMR, more common than GCA is characterized by pain, stiffness and functional impotence in the scapular and pelvic girdle.2 GCA typically presents with temporary headache and fever, while the visual ischaemic events are its most severe manifestations.1 These 2 entities are closely related and can present simultaneously, polymyalgic symptoms are found in up to 50% of patients with GCA3 and GCA in up to 20% of those with PMR.4 Similarly, associations with other large vessel vasculitis (LVV) have been described, especially aortitis.5 Patients with isolated PMR, in general, are younger and do not have asthenia, anorexia or weight loss. Similarly, patients with LVV, tend to have higher levels of acute phase reactants (APR) and reduced haemoglobin levels.1 It is estimated that up to 15% of patients with PMR have an associated LVV, and can have serious complications, such as the formation of stenosis, aneurysms, and have a fatal outcome. It is important that it is detected early and appropriately treated.6 Positron emission tomography (PET) uses 18F-fluorodeoxyglucose, glucose analogue, marked with radioisotope 18fluoride. It has demonstrated great usefulness in Oncology, and for inflammatory processes because inflammatory cells can overexpress glucose transporters and accumulate it intracellularly.7 In diseases such as vasculatitis this technique coupled with tomography (CT) can detect metabolic changes even before structural changes.8
The objective of this study was to explore the diagnostic value of 18F PET-CT for the detection of LVV in patients with PMR.
Material and methodsExploratory retrospective study of patients with PMR diagnosed using the criteria of Chuang et al.,9 who underwent 18F PET-CT and blood tests including APR at the time of the imaging test, selected from 2011 to 2015 from the Rheumatology Department of HUP la Fe. The exclusion criteria were a diagnosis of active neoplasms and known previous vasculitis. Patients with symptoms suggestive of GCA (headache, claudication of the jaw) were also excluded.
Before undertaking the 18F PET-CT the patient had to have fasted for a minimum of 6h, have baseline blood glucose levels under 140mg/dl, appropriate hydration and 24h rest beforehand. The images were obtained 90min post intravenous injection of the 18F-FDG. The images were reconstructed by iterative methods (OSEM) and processed in a Philips Extended Brilliance TMWorkspace Workstation (Philips Medical System). Analysis was performed through visual methods, assessing radiotracer uptake patterns in the aortic walls, and their location (aortic, thoracic or abdominal), distribution (lineal or segmentary) and uptake intensity (using hepatic activity as reference). Positive images were considered to be those with a lineal distribution with uptake intensity equal to or higher than the hepatic parenchyma.
Clinical, analytical and treatment data of the selected patients were reviewed. Analysis of results was performed using the R Foundation software with lineal regression models.
The study was approved by the ethics committee and was made by following the principles and recommendations of the Declaration of Helsinki. All personal and clinical data of the patients included were protected in keeping with current legislation.
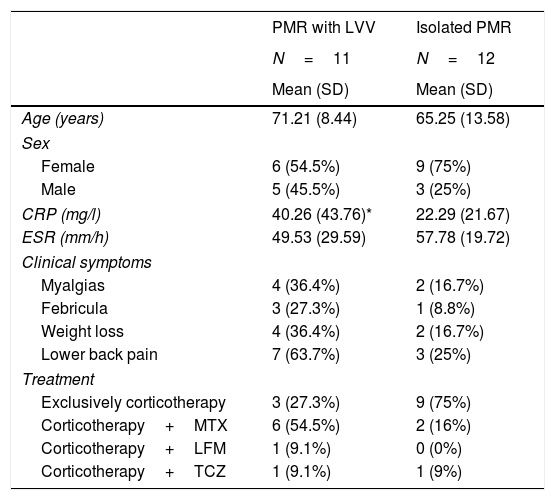
ResultsA total of 61 patients were reviewed, and 23 met with the study inclusion criteria. Sixty five per cent were women. The mean age of the 18F PET-CT was 68.29 years (11.21) and the mean duration of PMR symptoms prior to performing the 18F PET-CT was 18 weeks (2). Main patient characteristics are summarized in Table 1. Patients with PMR with an associated LVV presented with more non-specific clinical symptoms, such as myalgias, low-grade fever and weight loss, compared with those with isolated PMR.
Clinical and analytical features of the patients.
| PMR with LVV | Isolated PMR | |
|---|---|---|
| N=11 | N=12 | |
| Mean (SD) | Mean (SD) | |
| Age (years) | 71.21 (8.44) | 65.25 (13.58) |
| Sex | ||
| Female | 6 (54.5%) | 9 (75%) |
| Male | 5 (45.5%) | 3 (25%) |
| CRP (mg/l) | 40.26 (43.76)* | 22.29 (21.67) |
| ESR (mm/h) | 49.53 (29.59) | 57.78 (19.72) |
| Clinical symptoms | ||
| Myalgias | 4 (36.4%) | 2 (16.7%) |
| Febricula | 3 (27.3%) | 1 (8.8%) |
| Weight loss | 4 (36.4%) | 2 (16.7%) |
| Lower back pain | 7 (63.7%) | 3 (25%) |
| Treatment | ||
| Exclusively corticotherapy | 3 (27.3%) | 9 (75%) |
| Corticotherapy+MTX | 6 (54.5%) | 2 (16%) |
| Corticotherapy+LFM | 1 (9.1%) | 0 (0%) |
| Corticotherapy+TCZ | 1 (9.1%) | 1 (9%) |
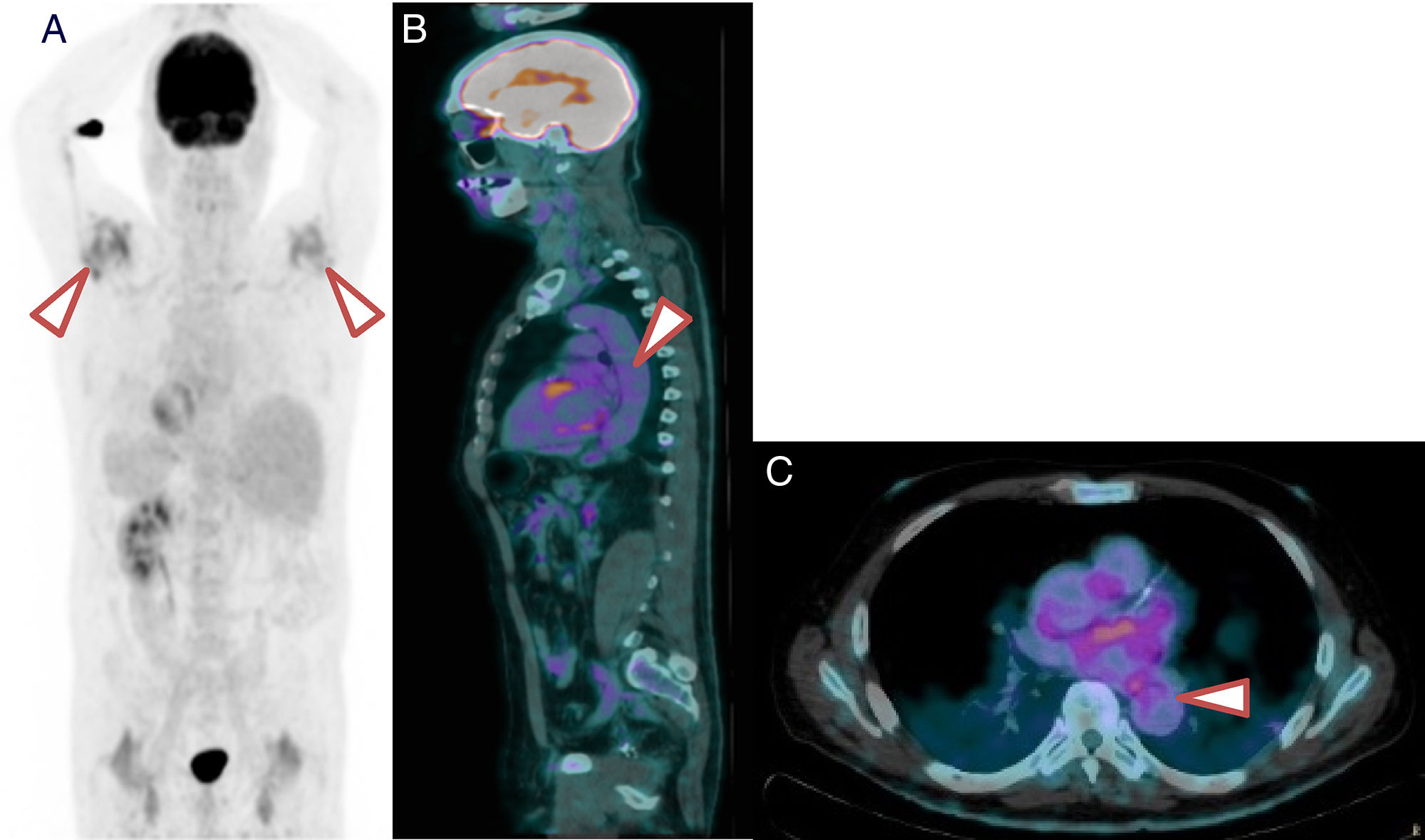
In 48% of patients PMR with an associated LVV was observed. Despite the heterogeneity of its location, the majority involved the aorta and its large branches, as shown in Fig. 1.
18F-FDG PET/TC of a patient with PMR and LVV. (A) 18F-FDG PET/TC imaging showing major inflammation of the shoulders. (B) Saggital slice. Note the grade 2 metabolic activity of lineal distribution in the aorta. (C) Axial slice. Note the grade 2 metabolic activity of lineal distribution in the thoracic aorta.
Of the patients with LVV-PMR, 8 required the addition of disease modifying anti-rheumatic drugs (DMARD's) (6 methotrexate, one tocilizumab and one leflunomide). The reason for the combination with DMARD's was mostly inappropriate response (65%) and in 35% of cases as a means of sparing corticoids. The other 52% of patients without LVV were mainly treated exclusively with corticotherapy and only in one case were DMARD's required for corticoid sparing.
Patients with LVV-PMR presented with higher values of CRP than those who did not present with LVV (40.26mg/l vs 22.29mg/l; P=.03). No statistically significant association was observed between the raising of ESR and the determination of LVV.
DiscussionBoth PMR and LVV are inflammatory rheumatic diseases closely related to one another. Cases of PMR associated with LVV should not go unnoticed because of their prognostic implications and because they may require a change in treatment.6 in general, patients with PMR respond quickly and efficiently to a 10–20mg daily dose of prednisone whilst patients with GCA usually required higher doses of prednisone, between 40 and 60mg, to avoid visual ischaemic events. Mean doses of corticoids improve polymialgic symptoms but do not lower the risk of permanent blindness in GCA patients.1 Another complication of LVV is the formation of aneurysms and their possible ruptures. Patients with LVV generally usually present more frequently with non-specific clinical signs such as myalgias, weight loss, low-grade fever and raised APR levels.10
The 18F PET-TC is a non-invasive technique with an ability to determine inflammation in almost all the main arteries of the body and may be a valuable tool for the diagnosis of LVV in these patients. The 18F PET-CT provides a functional metabolic image of inflammation of the blood vessel walls prior to the existence of structural changes, and is therefore useful in LVV presentations, especially in aortitis.11 This imaging technique, apart from demonstrating the presence of vasculitis, is able to demonstrate cervical bursitis and of the lumbar spine, confirming the PMR diagnosis.12 However, it is necessary to highlight that because of the dimensions and anatomical location of the cranial arteries, it is not possible to demonstrate inflammation of these with this technique. Therefore, if there is a suspicion of GCA, a Doppler ultrasound scan is recommended of the temporal arteries or axillaries and biopsy of the temporal arteries.13 Patients with GCA who also present with LVV, are generally younger than those with classical GCA and their diagnosis is generally delayed.11
In this study the usefulness of 18F PET-CT for the diagnosis of PMR with associated LVV was assessed and it was observed that a major percentage, close to half of the cases, presented with LVV. It is therefore important in patients with isolated PMR to identify signs of ischaemia.12 There are markers to determine PMR with associated LVV, such as raised APR values, despite appropriate treatment, which is an indication of the persistence of inflammation.2 These findings coincide with recent studies which have demonstrated that almost a third of patients with PMR, with no vasculiti symptoms, may have LVV.12
A salient point is that for patients in corticotherapy, 18F PET-CT may have limitations since inflammation could be masked. However, in most cases it is useful for diagnosing LVV.8
As previously mentioned, isolated PMR usually responds quickly to intermediate doses of corticotherapy.1 However, in cases where complete remission is not achieved, due either to the persistence of clinical symptoms, such as lumbar pain, pain in lower limbs, constitutional symptoms (asthenia, low-grade fever, anorexia, weight loss of over 5%),11,12 or due to raised parameters of analysis, an associated vasculitis should be suspected.14 In one patient series with non-infectious aortitis the most common clinical feature was PMR.11 In fact, in this series it was confirmed that the patients who presented with PMR associated with LVV did not always have associated symptoms and mostly required changes in treatment. Moreover, previous studies have reported normalization of the blood vessel walls in 18F PET-CT after treatment,10 and this technique could also be useful during follow-up.
Our study has limitations because it was a retrospective study with a low number of patients and this limits generalizations being made about our results and conclusions. The high percentage of patients with LVV detected compared to other series may be due to a selection bias, because 18F PET-CT was performed on those patients with a poor response to treatment with corticoids, and the probability of an associated LVV was higher. Although we only found significant differences in the CRP between the groups with LVV detected with 18F PET-CT, it is true that differences were observed regarding sex, and clinical manifestations but these were of no significance, probably due to the low number of patients included in the study.
Another limitation is lower sensitivity to the 18F PET-CT in patients treated with corticoids, and non-detection of vasculitis in smaller arteries which are sometimes the only ones affected in GCA. In fact 6% of patients with negative 18F PET-CT required the addition of methotrexate and 9%, tocilizumab. Some of these patients probably had false negatives.
To conclude, performing of an imaging analysis with 18F PET-CT, in patients with poor initial treatment response, especially if the CRP is persistently high, may be helpful in determining cases of patients with LVV associated to PMR.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Arévalo Ruales K, Negueroles Albuixech R, Loaiza Gongora J, Grau García E, Ivorra Cortés J, Román Ivorra JA. 18F-FDG PET/TC en pacientes con polimialgia reumática: despistando vasculitis. Reumatol Clin. 2020;16:38–41.