The treatment of Rheumatoid Arthritis (RA) has changed dramatically in recent years, especially with the use of disease modifying drugs (DMARDs). Data on the management of this disease in clinical trials are abundant, but not so in real life. The aim of our study is to describe the management of an early RA cohort in daily clinical practice, especially DMARD discontinuations and reasons.
MethodsA retrospective observational study of patients with RA diagnosed between 01/07 and 12/14 followed up to 01/17, using >1 DMARD ≥ 3 months. Variables: sociodemographic, clinical, treatment, DMARD discontinuation and reason. Descriptive analysis of sociodemographic, clinical and treatment characteristics. Discontinuation incidence rate (DIR) due to survival techniques, expressed in 100 patients*year with 95% confidence interval.
Results814 patients were included with 2388 courses of treatment, 77% women, mean age 57.5 years. First course: monotherapy (92.75%), especially Methotrexate (56.06%). In later courses there was increased combined therapy and use of biologicals (mainly Etanercept). There were 1094 discontinuations (29.5 [27.8–31.3]). The DIR was higher for adverse events (15.9 [14.7–17.3]), biologicals (49.6 [43.1–57.2]) and combined therapy. The DMAR with the lowest DIR was MTX (25.8 [23.8–28.1]).
ConclusionMethotrexate was the most used drug, biologicals increased throughout the follow-up, the most frequent being Etanercept. The DMARD DIR was 29*100 patients per year, mainly due to adverse events. It seems to be higher in the therapies that include biologicals and combined therapies. MTX is the drug with the lowest DIR.
El tratamiento de la Artritis Reumatoide (AR) ha cambiado drásticamente en los últimos años, sobre todo con el uso de los fármacos modificadores de la enfermedad (FAME). Los datos sobre el manejo de esta enfermedad en ensayo clínico son abundantes, pero no en la vida real. El objetivo de nuestro estudio es describir el manejo de una cohorte de AR de inicio en práctica clínica diaria, especialmente las suspensiones de los FAME y sus causas.
MétodosEstudio observacional retrospectivo de pacientes con AR diagnosticados entre 01/07 y 12/14 seguidos hasta 01/17, que usaron >1 FAME ≥ 3 meses. Variables: sociodemográficas, clínicas, tratamiento, suspensión del FAME y causa. Análisis descriptivo de las características sociodemográficas, clínicas y de tratamiento. Incidencia de suspensión (IS) por técnicas de supervivencia, expresándose en 100 pacientes*año con intervalo de confianza 95%.
ResultadosSe incluyen 814 pacientes con 2388 cursos de tratamiento, 77% mujeres, edad media 57,5 años. Primer curso: monoterapia (92,75%), especialmente Metotrexate (56,06%). En posteriores cursos aumentan terapia combinada y uso de biológico (principalmente Etanercept). Se registraron 1094 suspensiones (29,5[27,8–31,3]). La IS fue mayor para evento adverso (15,9[14,7–17,3]), biológicos (49,6[43,1–57,2]) y terapia combinada. El FAME con menor IS fue MTX (25,8[23,8–28,1]).
ConclusiónEl Metotrexate fue el fármaco más utilizado, el biológico aumentó a lo largo del seguimiento, siendo el más frecuente Etanercept. La IS de los FAME fue 29*100 pacientes año, principalmente por evento adverso. Parece mayor en las terapias que incluyen biológicos y en las combinadas. El MTX es el fármaco con menor IS.
Rheumatoid arthritis (RA) is a chronic autoimmune disease which basically affects peripheral joints symmetrically, leading to inflammation, progressive destruction and bone erosion1; it is also associated with systemic complications.2 It also leads to deterioration of functional capacity, occupational disability, reduction of quality of life and increased mortality.3 Its prevalence ranges from between .5%-1% of the population.3,4
The appearance of joint damage is particularly fast at disease onset, suggesting that there is a “window of opportunity” at this stage, referring to the first few months since onset, when the patient is considered most likely to respond to treatment than in more advanced stages.5,6 Due to this, early diagnosis and treatment is essential, thereby increasing the possibility of controlling the inflammation and reducing damage. As a result, RA management and prognosis have drastically changed in recent years. The main objectives is to achieve complete remission or at least a low stable activity, improving functional prognosis of the patient by employing a treat to target strategy.7,8 Also, during management we must not forget pain control, and the reduction of corticosteroids or preferably their discontinuation.
Current therapeutic strategies include treatments aimed at fast pain and inflammation control and disease-modifying anti-rheumatic drugs (DMARDs) which may be synthetic (sDMARDs) or biologic (bDMARDs) (anti-TNF, other biologics and new targeted therapies). Their use should be introduced as soon as possible during the course of the disease, in full doses, alone or in combination so as to increase the therapeutic efficacy covered by the treat to target strategy.8,9
The lack of standardised forms of different treatment usage has lead to different combinations being used. These depend both on the physician and the availability of the drugs as well as the individual characteristics of each patient. The change in therapeutic objectives and the broadening of treatment options have determined multiple possibilities for patient management in daily clinical practice. Clinical trial data abound in the literature10,11 but not in real life. As a result, our aim was to obtain clinical practice information regarding recent onset RA. We will describe the clinical management and treatment, its persistence, and the reasons for discontinuation in a cohort of patients with recent onset RA in conditions of daily clinical practice followed up in an outpatient rheumatology unit consultation of a tertiary hospital.
Material and methodsDesignA retrospective, longitudinal, observational study. It included patients diagnosed with RA between 01/01/07 and 31/12/14. Follow-up included up to the loss of follow-up or the end of the study (01/01/17).
Scope of the studyRheumatology Unit of the Hospital Clínico San Carlos, which is a tertiary public hospital with a catchment area serving approximately 600,000 people.
SubjectsPatients over 16 diagnosed with RA were included (ACR/EULAR 2010 criteria)12 less than one year prior to inclusion. To enter into the study it was necessary for the patient to sign the informed consent form, have completed at least 2 visits to the rheumatology unit and to have received at least one sDMARD (chloroquine, hydroxychloroquine, methotrexate, leflunomide, gold injections, sulfasalazine, cyclosporine, cyslophosphamide, azathioprine, mycophenolate mofetil) or a bDMARD (infliximab, adalimumab, etanercept, golimumab, certolizumab pegol, rituximab, abatacept, tocilizumab) during the inclusion period.
This study was approved by the ethics and institutional clinical trial committee of the Hospital Clínico San Carlos. Also, according to their indications, it referred to the Spanish Medicine Agency, which classified it as an observational study.
Data sourcesPatient data were collected through the departmental electronic clinical records of the rheumatology unit of the Hospital Clinic San Carlos where routine visits were recorded in “real time”. Furthermore, written clinical files were reviewed to complete data which was lacking.
Variables1) Sociodemographic: age, sex, civil status, level of education, category, employment situation and residential situation (alone, family/friends, institutionalised). 2) Clinical: a) date of diagnosis, b) smoker, c) baseline quality of life measured by the Rosser index, d) analytics: baseline sedimentation rate, rheumatoid factor, anti-citrullinated peptide antibodies, e) baseline disease activity measured through the HAQ and DAS 28. 3) Baseline comorbidity: high blood pressure, diabetes mellitus, hypercholesterolaemia, ischaemic cardiopathy, congestive heart failure, cerebrovascular pathology, peripheral artery disease, cancer and type of cancer, depression, gastroduodenal ulcer, chronic obstructive pulmonary disease, liver disease, osteoporosis, fractures, kidney failure, cognitive impairment and thyroid alterations. 4) sDMARDs: type (chloroquine, hydroxychloroquine, methotrexate, leflunomide, gold salts, sulfasalazine, cyclosporine, cyslophosphamide, azathioprine, mycophenolate mofetil), start and finish dates. 5) bDMARDs: type (infliximab, adalimumab, etanercept, golimumab, certolizumab pegol, rituximab, abatacept, tocilizumab), start and finish dates. 6) Concomitant treatments (if they have been used for at least 3 months since the beginning of each treatment course with sDMARDs): a) use of opioids and anti-inflammatory analgesics, b) mean corticoid dose. 7) reasons for suspension of sDMARDs: a) adverse event (AE): the type, severity and relationship with the drug were recorded b) inefficacy, c) patient’s decision, e) physician’s decision, f) remission or improvement defined by the criterion of the rheumatologist responsible for the patient, g) others (including the desire for children or the suspension of programmed surgery for over 3 months).
Statistical analysisA descriptive analysis was performed of the subjects with sociodemographic and clinical characteristics, treatments with tns DMARDs (first and second line), in monotherapy or combined therapies over time.
The reasons for discontinuation were described using frequencies, mean and standard deviation, medians or percentiles. The persistence of treatment and the incidence of suspension were assessed using survival techniques, expressed by100 patients/year with a 95% confidence interval.
All analysis was performed with the statistical programme Stata v.13 Special Edition–Statistics/Data Analysis. A two-tailed P value under .05 was considered statistically significant.
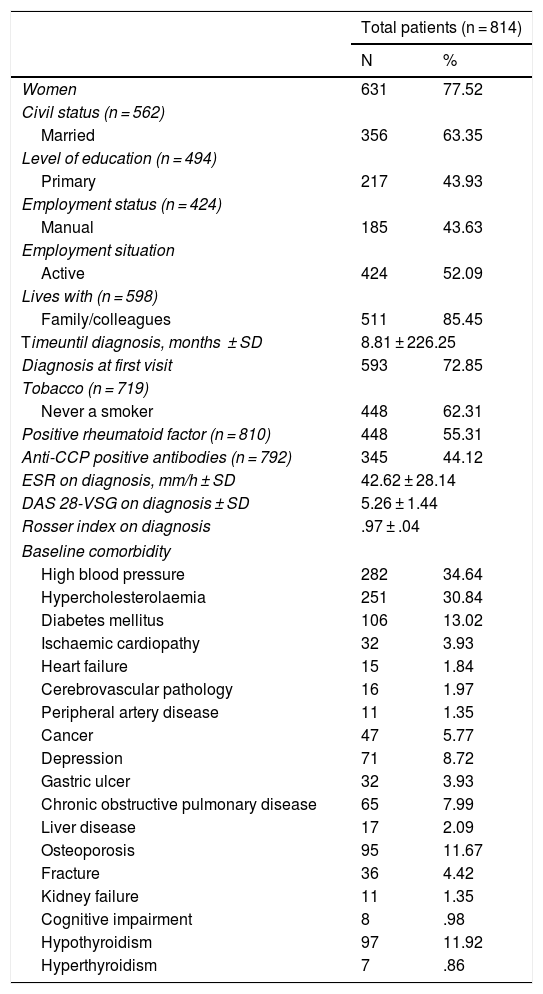
ResultsEight hundred and fourteen patients were included, with a mean follow-up of 5 years (median: 5.1 [p25−75: 2.97–7.12]). Table 1 contains the sociodemographic and baseline clinical characteristics. Over three quarters of the patients were women (77.52%) with a mean age at diagnosis of 57.53 (±15.50 years).
Baseline sociodemographic and clinical characteristics.
| Total patients (n = 814) | ||
|---|---|---|
| N | % | |
| Women | 631 | 77.52 |
| Civil status (n = 562) | ||
| Married | 356 | 63.35 |
| Level of education (n = 494) | ||
| Primary | 217 | 43.93 |
| Employment status (n = 424) | ||
| Manual | 185 | 43.63 |
| Employment situation | ||
| Active | 424 | 52.09 |
| Lives with (n = 598) | ||
| Family/colleagues | 511 | 85.45 |
| Timeuntil diagnosis, months ± SD | 8.81 ± 226.25 | |
| Diagnosis at first visit | 593 | 72.85 |
| Tobacco (n = 719) | ||
| Never a smoker | 448 | 62.31 |
| Positive rheumatoid factor (n = 810) | 448 | 55.31 |
| Anti-CCP positive antibodies (n = 792) | 345 | 44.12 |
| ESR on diagnosis, mm/h ± SD | 42.62 ± 28.14 | |
| DAS 28-VSG on diagnosis ± SD | 5.26 ± 1.44 | |
| Rosser index on diagnosis | .97 ± .04 | |
| Baseline comorbidity | ||
| High blood pressure | 282 | 34.64 |
| Hypercholesterolaemia | 251 | 30.84 |
| Diabetes mellitus | 106 | 13.02 |
| Ischaemic cardiopathy | 32 | 3.93 |
| Heart failure | 15 | 1.84 |
| Cerebrovascular pathology | 16 | 1.97 |
| Peripheral artery disease | 11 | 1.35 |
| Cancer | 47 | 5.77 |
| Depression | 71 | 8.72 |
| Gastric ulcer | 32 | 3.93 |
| Chronic obstructive pulmonary disease | 65 | 7.99 |
| Liver disease | 17 | 2.09 |
| Osteoporosis | 95 | 11.67 |
| Fracture | 36 | 4.42 |
| Kidney failure | 11 | 1.35 |
| Cognitive impairment | 8 | .98 |
| Hypothyroidism | 97 | 11.92 |
| Hyperthyroidism | 7 | .86 |
Anti-CCP: anti-citrullinated peptide; DAS: disease activity score; ESR: erythrocyte sedimentation rate; SD: standard deviation.
Mean time up to diagnosis was 8.81 months (±226.25), achieving a diagnosis during the first visit in 72.85% of patients. A higher percentage of patients were recorded who had never smoked compared with active or ex-smokers. Approximately half of the patents presented with seropositive RA. Regarding disease activity on diagnosis, the median erythrocyte sedimentation rate was 42.62 (±28.14) mm/h and DAS 28 mean was 5.26 ± 1.44. Regarding measurement of quality of life using the Rosser index n diagnosis, a mean of .97 ± .04 was obtained. Regarding comorbidity, both blood pressure and hypercholesterolaemia were the most prevalent pathologies to be detected in over a third of patients on diagnosis.
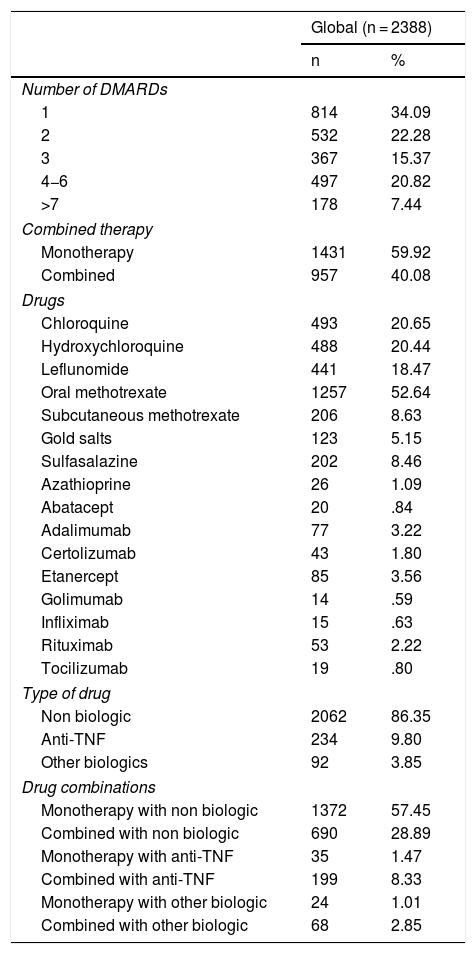
Two thousand, three hundred and eighty eight courses of treatment with DMARDs were registered in the 814 patients, with mean time up until the first line of treatment at 21 [0–43] days. The most commonly used drug was oral methotrexate followed by anti-malarial drugs (see Table 2). Combined therapy in our cohort was used in 40.08% (dual therapy in 31.87%; ≥3 drugs in 8.25%) and biologics were used in 13.65% of treatments, both as monotherapy and combined. Regarding the way in which the DMARDs were used, the most common was immunotherapy of sDMARDs (57.45%) followed by combined therapy of sDMARDs (28.89%).
Description of course of treatment.
| Global (n = 2388) | ||
|---|---|---|
| n | % | |
| Number of DMARDs | ||
| 1 | 814 | 34.09 |
| 2 | 532 | 22.28 |
| 3 | 367 | 15.37 |
| 4−6 | 497 | 20.82 |
| >7 | 178 | 7.44 |
| Combined therapy | ||
| Monotherapy | 1431 | 59.92 |
| Combined | 957 | 40.08 |
| Drugs | ||
| Chloroquine | 493 | 20.65 |
| Hydroxychloroquine | 488 | 20.44 |
| Leflunomide | 441 | 18.47 |
| Oral methotrexate | 1257 | 52.64 |
| Subcutaneous methotrexate | 206 | 8.63 |
| Gold salts | 123 | 5.15 |
| Sulfasalazine | 202 | 8.46 |
| Azathioprine | 26 | 1.09 |
| Abatacept | 20 | .84 |
| Adalimumab | 77 | 3.22 |
| Certolizumab | 43 | 1.80 |
| Etanercept | 85 | 3.56 |
| Golimumab | 14 | .59 |
| Infliximab | 15 | .63 |
| Rituximab | 53 | 2.22 |
| Tocilizumab | 19 | .80 |
| Type of drug | ||
| Non biologic | 2062 | 86.35 |
| Anti-TNF | 234 | 9.80 |
| Other biologics | 92 | 3.85 |
| Drug combinations | ||
| Monotherapy with non biologic | 1372 | 57.45 |
| Combined with non biologic | 690 | 28.89 |
| Monotherapy with anti-TNF | 35 | 1.47 |
| Combined with anti-TNF | 199 | 8.33 |
| Monotherapy with other biologic | 24 | 1.01 |
| Combined with other biologic | 68 | 2.85 |
DMARDs: disease-modifying ant rheumatic drugs; TNF: tumoral necrosis factor.
Concomitant treatments used were also collected. In 83.75% of treatments these were associated with corticoids with a mean dose of 1.20 (± .77) mg. In 36.06% of treatments the concomitant use of non-steroid anti-inflammatory was recorded whilst that of opioids was much lower (minor opioids 6.74% of courses; major opioids 5.65% of courses).
An analysis of drug combinations most commonly used was performed and we observed that the most used sDMARDs in monotherapy was methotrexate and in bDMARDs was etanercept; regarding combined therapy, in dual therapy the preferred combination of sDMARDs was methotrexate and hydroxychloroquine and that of sDMARDs with bDMARDs was methotrexate and adalimumab. Regarding triple therapy, in synthetics the preferred was methotrexate with hydroxychloroquine and leflunomide and in sDMARDs with bDMARDs was methotrexate with leflunomide and etanercept.
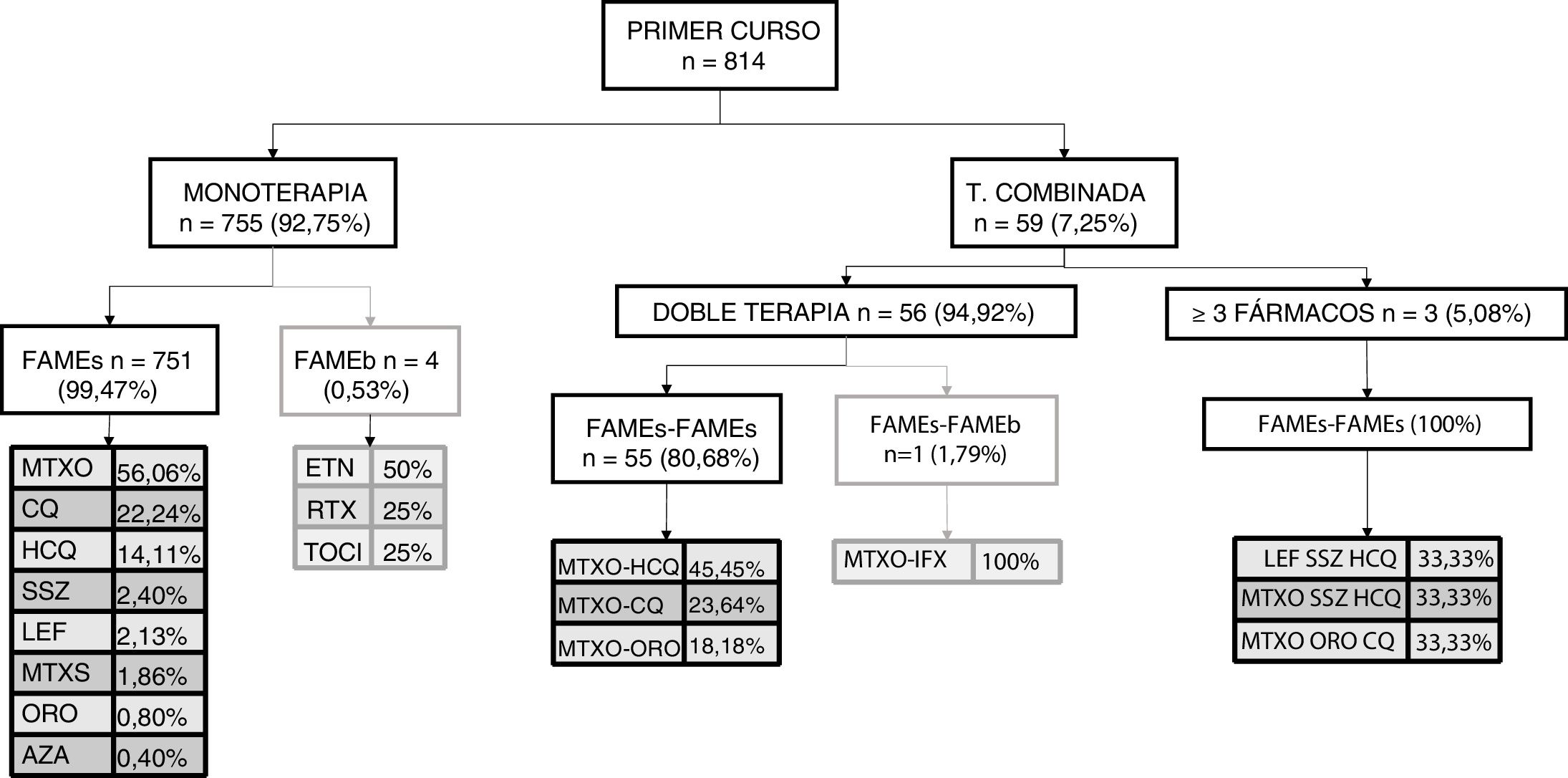
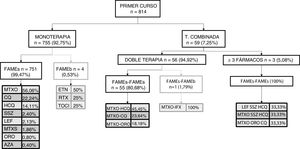
This same analysis was repeated by course of treatment. Fig. 1 shows the first course of treatment. During the first course, monotherapy was most commonly used, and within it, monotherapy with sDMARDs, especially methotrexate; 4 cases of monotherapy with bDMARDs were recorded. Regarding combined therapy, in the first course of treatment, its use was very low and was essentially given as dual, or occasionally as triple therapy. sDMARDs were almost always used; a single case of a combination of sDMARDs and bDMARDs as the first course of treatment was recorded.
In later courses of treatment we were able to appreciate several differences. Regarding monotherapy, the most used sDMARD was also methotrexate (41.22%) but there were differences in the bDMARDs, since etanercept and adalimumab (20% both) were used equally. Combined therapy was used with greater frequency (57.05%), and within it dual therapy was particularly used (78.51%). In dual therapy essentially sDMARDs were used with methotrexate-hydroxychloroquine being the most used combination (22.18%) followed by methotrexate-chloroquine (20.93%); within dual therapy with biologics there was a more frequent use of methotrexate-adalimumab (13.70%) followed by leflunomide-etanercept (9.59%). Regarding triple therapy (21.49%), which was most frequently used as a combination of bDMARDs (62.18%), the most used was methotrexate-leflunomide-etanercept (6.67%); when triple therapy with sDMARDs was chosen, the most used was the combination of methotrexate-leflunomide-hydroxychloroquine (15.07%).
Discontinuations of 1094 of the 2388 courses of treatment were recorded in 361 patients, 150 due to inefficacy in 110 patients, 124 due to the physician’s decision in 102 patients, 66 due to improvement in 61 patients, 116 due to the patient’s decision in 103 patients and 54 due to other causes in 51 patients.
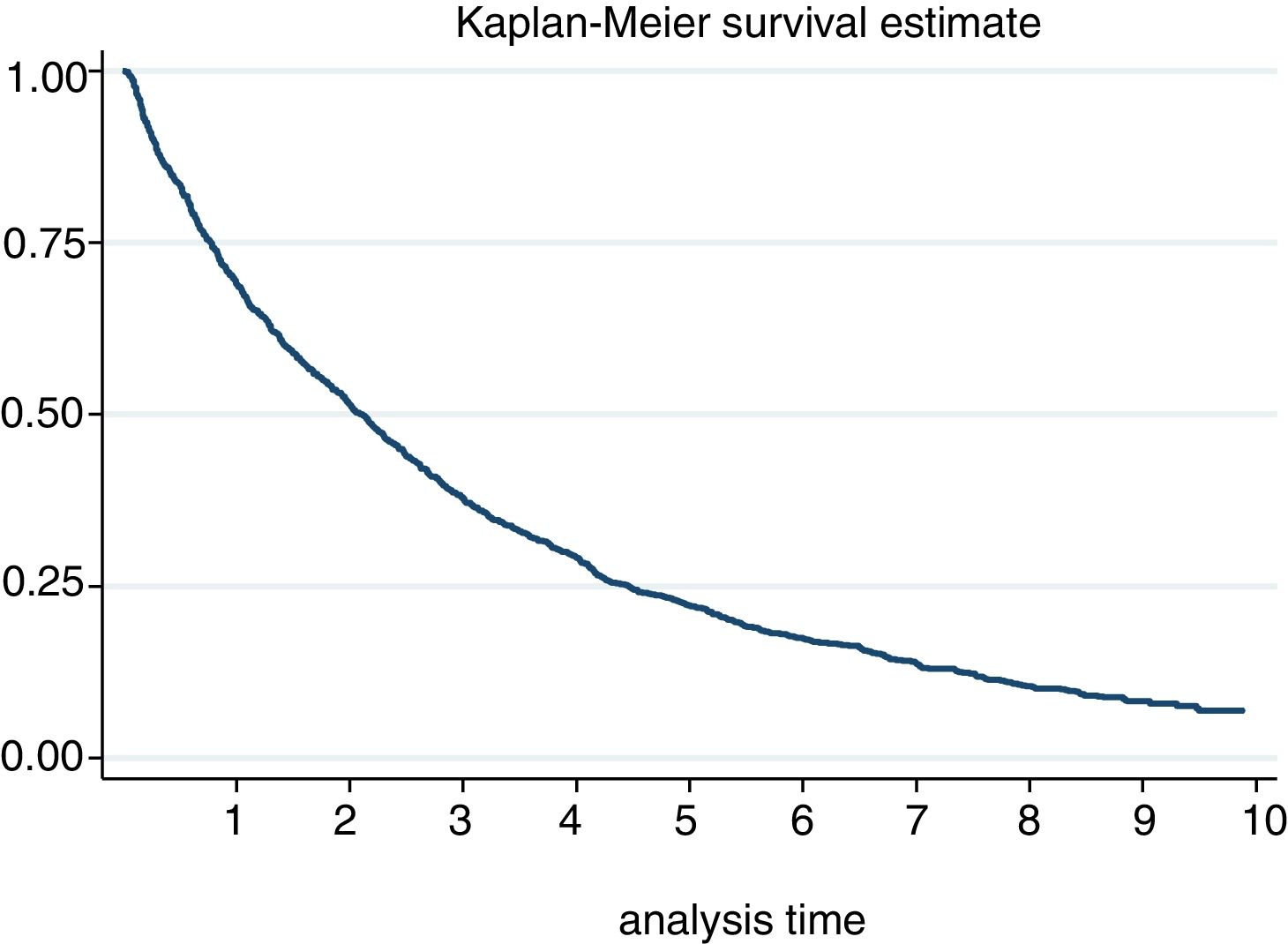
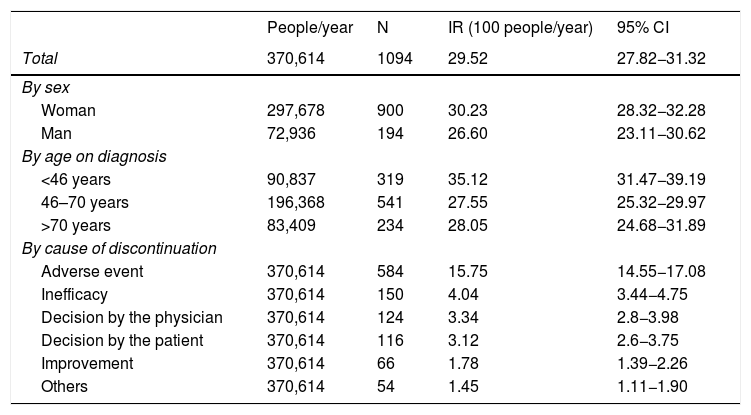
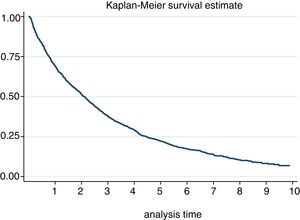
The incidence of discontinuation in our cohort was estimated at 29.52 [27.82−31.32]. After one year and after 5 years, 30% and 78% respectively discontinued treatment (Fig. 2). In fact, 50% of patients discontinued treatment at 2. [1,9–2,3] years from the start of the same. According to the causes of discontinuation, the highest incidence was AE, followed by inefficacy, the patient’s decision and the physician’s decision. With regard to the sociodemographic characteristics, the incidence was higher in women and in patients under 46 (Table 3). A 20% loss of follow-up was recorded.
Rates of global discontinuations, by age and ex, by cause of discontinuation.
| People/year | N | IR (100 people/year) | 95% CI | |
|---|---|---|---|---|
| Total | 370,614 | 1094 | 29.52 | 27.82−31.32 |
| By sex | ||||
| Woman | 297,678 | 900 | 30.23 | 28.32−32.28 |
| Man | 72,936 | 194 | 26.60 | 23.11−30.62 |
| By age on diagnosis | ||||
| <46 years | 90,837 | 319 | 35.12 | 31.47−39.19 |
| 46–70 years | 196,368 | 541 | 27.55 | 25.32−29.97 |
| >70 years | 83,409 | 234 | 28.05 | 24.68−31.89 |
| By cause of discontinuation | ||||
| Adverse event | 370,614 | 584 | 15.75 | 14.55−17.08 |
| Inefficacy | 370,614 | 150 | 4.04 | 3.44−4.75 |
| Decision by the physician | 370,614 | 124 | 3.34 | 2.8−3.98 |
| Decision by the patient | 370,614 | 116 | 3.12 | 2.6−3.75 |
| Improvement | 370,614 | 66 | 1.78 | 1.39−2.26 |
| Others | 370,614 | 54 | 1.45 | 1.11−1.90 |
CI: Confidence interval; IR: incidence rate.
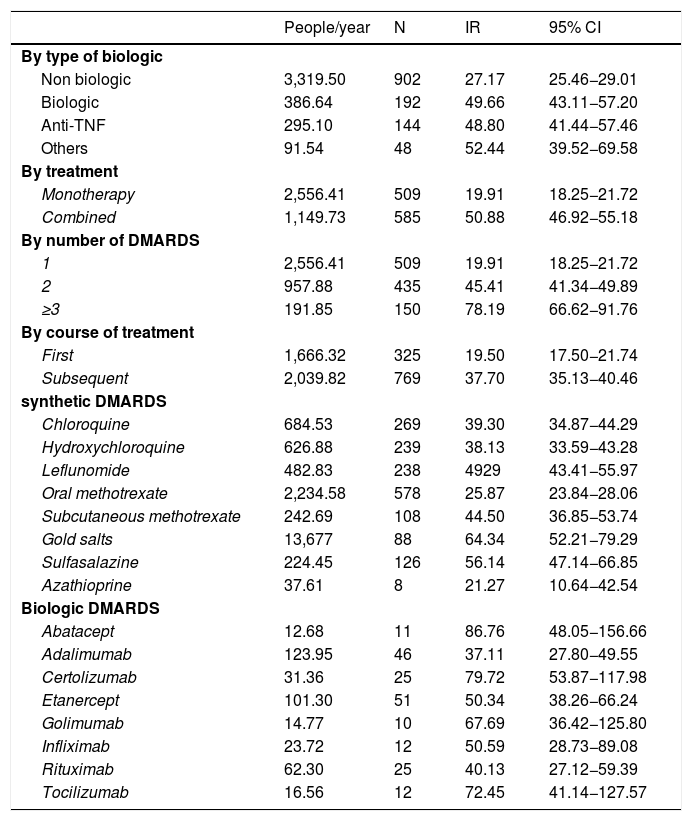
Table 4 presents the rates of discontinuation analysed according to treatment, and we can see that the bDMARDs rate of discontinuation is almost double that of the sDMARDs, with no major differences between the large groups of biologic agents (anti-TNF or other biologics). Regarding drug combinations, the rate of discontinuation is over double in combined therapies compared with monotherapy, especially when the combination is of 4 or more drugs. The discontinuations were analysed according to the course of treatment, with the posterior course being higher compared with the first one.
Rates of discontinuation according to treatment.
| People/year | N | IR | 95% CI | |
|---|---|---|---|---|
| By type of biologic | ||||
| Non biologic | 3,319.50 | 902 | 27.17 | 25.46−29.01 |
| Biologic | 386.64 | 192 | 49.66 | 43.11−57.20 |
| Anti-TNF | 295.10 | 144 | 48.80 | 41.44−57.46 |
| Others | 91.54 | 48 | 52.44 | 39.52−69.58 |
| By treatment | ||||
| Monotherapy | 2,556.41 | 509 | 19.91 | 18.25−21.72 |
| Combined | 1,149.73 | 585 | 50.88 | 46.92−55.18 |
| By number of DMARDS | ||||
| 1 | 2,556.41 | 509 | 19.91 | 18.25−21.72 |
| 2 | 957.88 | 435 | 45.41 | 41.34−49.89 |
| ≥3 | 191.85 | 150 | 78.19 | 66.62−91.76 |
| By course of treatment | ||||
| First | 1,666.32 | 325 | 19.50 | 17.50−21.74 |
| Subsequent | 2,039.82 | 769 | 37.70 | 35.13−40.46 |
| synthetic DMARDS | ||||
| Chloroquine | 684.53 | 269 | 39.30 | 34.87−44.29 |
| Hydroxychloroquine | 626.88 | 239 | 38.13 | 33.59−43.28 |
| Leflunomide | 482.83 | 238 | 4929 | 43.41−55.97 |
| Oral methotrexate | 2,234.58 | 578 | 25.87 | 23.84−28.06 |
| Subcutaneous methotrexate | 242.69 | 108 | 44.50 | 36.85−53.74 |
| Gold salts | 13,677 | 88 | 64.34 | 52.21−79.29 |
| Sulfasalazine | 224.45 | 126 | 56.14 | 47.14−66.85 |
| Azathioprine | 37.61 | 8 | 21.27 | 10.64−42.54 |
| Biologic DMARDS | ||||
| Abatacept | 12.68 | 11 | 86.76 | 48.05−156.66 |
| Adalimumab | 123.95 | 46 | 37.11 | 27.80−49.55 |
| Certolizumab | 31.36 | 25 | 79.72 | 53.87−117.98 |
| Etanercept | 101.30 | 51 | 50.34 | 38.26−66.24 |
| Golimumab | 14.77 | 10 | 67.69 | 36.42−125.80 |
| Infliximab | 23.72 | 12 | 50.59 | 28.73−89.08 |
| Rituximab | 62.30 | 25 | 40.13 | 27.12−59.39 |
| Tocilizumab | 16.56 | 12 | 72.45 | 41.14−127.57 |
CI: Confidence interval; DMARDs: disease-modifying antirheumatic drugs; IR: incidence rate; TNF: tumoral necrosis factor.
Lastly, discontinuation rates were presented for each drug individually. Among the sDMARDs gold salts, sulfasalazine and leflunomide stand out as those which show a higher rate of discontinuation; among the bDMARDs adalimumab followed by certolizumab were those presenting a higher rate of discontinuation.
DiscussionThis study helps to increase our knowledge about managing daily life conditions in recent onset RA patients, describing the causes of discontinuation of the different DMARDS used.
Our cohort includes 814 patients with recent onset RA and maximum follow-up o f10 years. Most patients were women with a mean age at disease onset of 57 years and over half of them were currently employed. The diagnosis was established in almost three quarters of patients at the first visit and over half presented with a seropositive RA with higher disuse activity. The most common comorbidities were high blood pressure and hypercholesterolemia. Our cohort may be considered representative of the early onset RA population, being comparable to other publications regarding sociodemographic and clinical characteristics. The Scottish SERA13 is analogous, although its patients had a higher frequency of seropositivity and lower disease activity at the beginning; the English is NORFOLK,14,15 was very similar to ours but seropositivity was lower as was disease activity and more time passed until the beginning of the first DMARD; and the Canadian multicentre CATCH16 and French ESPOIR17,18 were very similar to ours except their patients were slightly younger.
Regarding the management of our patients, treatment with DMARDs was initiated early, less than one month after the establishment of the diagnosis of RA. The start of treatment was made preferably in monotherapy with sDMARDs, with oral methotrexate being the most widely used drug both in mono and in combined therapy, and the most used of the bDMARDs being etanercept followed by adalimumab. Combined therapy was used in 40% of the courses of treatment, particularly dual treatment and bDMARDs were used in 13% of them. Regarding the most frequently used drug combinations overall, sDMARDs most used were methotrexate-hydroxychloroquine and sDMARDs with bDMARDs most used were methotrexate-adalimumab. In triple therapy, in synthetics the preferred was methotrexate-hydroxychloroquine-leflunomide and in sDMARDs with bDMARDs it was methotrexate-leflunomide-etanercept.
In the first course of treatment most patients received monotherapy with sDMARDs, and of these the most used was methotrexate. Combined therapy was used in under 10% of patients and when it was used it was essentially dual therapy with sDMARDs, mainly methotrexate-antimalarial. In a few cases therapy with bDMARDs was used in the first course and when this was done the most used drug was etanercept. In subsequent courses the most used drug in monotherapy was still methotrexate, but the use of combined therapy increased considerably reaching up to over half of patients, particularly in dual therapy. In combined therapy again methotrexate-anti-malarials were still the most common; with regard to bDMARDs the most common combination was methotrexate-adalimumab.
In all previously published cohorts, the most used DMARD is methotrexate, as in our cohort with a very similar use of bDMARDs to ours13–18 and maybe a little higher in the French one.17,18 Regarding combined therapy, the French cohort was outstanding17,18 where it was used with much greater frequency. Regarding biologics, the most used were also adalimumab and etanercept.16–18
One thousand and ninety four discontinuations were recorded in 2388 courses of treatment, with the main cause of discontinuation being the development of an AE, followed by inefficacy. These results coincide globally with previously published data. Of note are the reports of bDMARDs LORHEN19 and SCQM-RA20 and the single centre cohort studies of Flendrie et al.21 and León et al.,22 since in them over half of discontinuations were also due to AE with inefficacy ranking second. In the DANBIO and DREAM23,24 reports, the main cause of discontinuation is inefficacy followed by AE. It should be considered that these are national reports of patients with established RA who receive anti-TNF with results published early (the 6 first months of treatment in DANBIO23 and the first year in DREAM24). Lastly, the data published by the national registers of bDMARD in rheumatic diseases in Portugal,25 Spain26 and Italy27 were analysed. Analysis of the data referring to RA revealed that the main cause of discontinuation was inefficacy, followed by AE. Regarding non anti-TNF bDMARDS, there are fewer data available for these drugs since their marketing came after that of the anti-TNF drugs.
The main limitation of this study is its retrospective design, which entails the existence of lost data. Furthermore, it is of note that the number of patients who received the newest bDMARDs (tocilizumab or abatacept) was lower than that of those who received the other drugs, due to their later availability on the market. However, the great strength of this study is its inclusion of a wide number of recent onset RA patients who were followed up long term, and this provided fortitude to the results. Also, this study was conducted in real life conditions with patients who were not selected from a single centre.
To conclude, we describe a cohort representative of early onset RA, followed up for 10 years, in which disease management was made in keeping with standards, achieving a rapid diagnosis and establishing early and aggressive treatment with DMARDs. With regard to the persistence of the sDMARDs, we show that almost 30/100 patients per year discontinued one or some of these drugs, with mean administration of 2 years, and we corroborated that most discontinuation was due to AE.
These long-term, real life, longitudinal studies provide highly useful information for daily practice. It would be very interesting to complete these cohorts in a few years time with further details on the new biologics (rituximab, abatacept y tocilizumab) and the JAK-Kinase inhibitors.
FinancingThis study was conducted with support from the Instituto de Salud Carlos III, Ministry of Health [Miguel Servet research contract: CP11/00189 of Lydia Abásolo; health Investigation Fund: PI18/01188; Research Network in Inflammation and Rheumatic Diseases: RD16/0012/0014] and Sanofi-Aventis, S.A.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Rosales Rosado Z, Font Urgelles J, Hernández Rodríguez I, León Mateos L, Abásolo Alcázar L, Jover Jover JÁ. Manejo clínico y discontinuación de tratamiento en pacientes con artritis reumatoide de inicio en una consulta de Reumatología. Reumatol Clin. 2022;18:77–83.