Vaccination coverage for seasonal influenza and pneumococcus in rheumatology patients receiving biological treatment. To identify variables that predict vaccination adherence.
Material and methodDescriptive cross-sectional study. The study involved rheumatology patients who initiated biological therapy between 01/01/2016 and 12/31/2016 in a regional referral hospital. Variables included sociodemographic information, diagnostic data, treating physician, referral to the vaccine unit and vaccination against pneumococcus with 13-valent pneumococcal conjugate vaccine (PCV13) and 23-valent pneumococcal polysaccharide vaccine (PPSV23), as well as seasonal influenza (2016/17). Univariate, bivariate (Chi-square) and multivariate analysis (logistic regression) were performed. The differences were considered significant (P<.05) and the PASW v.18 software package was used.
ResultsIn all, 222 patients were included. Vaccination coverage was: PCV13, 80.2%; PPSV23, 77.9%; influenza 2016/17, 78.8%; PCV13+PPSV23, 75.2%; PCV13+PPSV23+influenza 2016/17, 68.9%. Axial spondylitis had the highest coverage (>80%) for pneumococcal vaccination and combination of pneumococcal with influenza. Overall, 27% of the patients were not referred to the unit. The treating physician was associated with statistical significance in each vaccine alone or combined, but referral to the vaccine unit was independently associated with the highest vaccination coverage (P<.001) in all cases.
ConclusionsCompared to the scientific literature, we consider that the coverage of our patients against pneumococcus and influenza is high. Referral of these patients to the vaccine unit is the key to guarantee a correct immunization and to minimize some of the possible infectious adverse effects of biological therapies.
Conocer las coberturas de vacunación frente a gripe estacional y neumococo en pacientes reumatológicos con terapia biológica. Identificar las variables que predicen adherencia a la vacunación.
Material y métodoEstudio transversal. Se incluyeron los pacientes reumatológicos que iniciaron terapia biológica entre el 01/01/2016 y el 31/12/2016 en un hospital autonómico de referencia. Se recogieron variables sociodemográficas, relacionadas con el diagnóstico, médico prescriptor, derivación a la Unidad de Vacunas y vacunación frente a neumococo con vacuna conjugada de 13 serotipos (VNC13) y polisacárida de 23 serotipos (VNP23), así como gripe estacional (2016/17). Se realizó análisis univariante, bivariante (Chi-cuadrado) y multivariante (regresión logística). Se consideró significativa una p<0,05 y se utilizó el programa PASW v.18.
ResultadosSe incluyeron 222 pacientes. Las coberturas de vacunación fueron: VNC13, 80,2%; VNP23v, 77,9%; gripe 2016/17, 78,8%; VNC13+VNP23, 75,2%; VNC13+VNP23+gripe 2016/17, 68,9%. La espondilitis axial registró las coberturas más altas (>80%) para la vacunación antineumocócica y en combinación con la antigripal. El 27% de los pacientes no fueron derivados a la Unidad. El médico prescriptor se asoció de manera estadísticamente significativa con cada una de las vacunas y sus combinaciones, pero fue la derivación a la Unidad de Vacunas la que se asoció de manera independiente con las mayores coberturas de vacunación (p<0,001) en todos los casos.
ConclusionesComparando con la literatura científica, consideramos que las coberturas frente a neumococo y gripe en estos pacientes son elevadas. La derivación de estos pacientes a la Unidad de Vacunas resulta clave para garantizar una correcta inmunización y minimizar así algunos de los posibles efectos adversos infecciosos de las terapias biológicas.
The worldwide prevalence of musculoskeletal diseases has grown significantly in recent years. They affect the quality of life and functional capacity of individuals, and have major impact on health expenditure.1
The therapeutic approach of these disorders is sometimes complicated, therefore there are increasingly more patients who now require biological therapy. These drugs modulate immune response by targeting therapeutic targets of the immune cell line, inflammatory mediators or surface receptors.2,3 Moreover, it is known that they are not harmless since, according to the Spanish Registry for Adverse Events of Biological Therapy in Rheumatic Diseases (BIOBADASER), infections constitute 23.2% of serious, and 18.1% of fatal adverse events: upper and lower respiratory tract infections and pneumonia being among the most serious.4
Specialist assessment of these patients’ vaccination schedules in the vaccine units has become fundamental in the prevention of infection.5,6 Although there are many publications on vaccination in patients with rheumatoid arthritis,7–9 there are not so many, for example, that assess vaccination coverage in patients with rheumatological diseases in general, especially when they are receiving or are candidates for biological therapy. Furthermore, great variability of results can be observed among the existing research studies.10–13
All of the above, added to the fact that active immunisation is considered one of the most cost-effective tools within the preventive activities of the public health system, and that the 3 vaccinations studied are recommended and financed in the official regional calendar for these patients, means that vaccination coverage for these patients must be known and reinforced.
The aim of this study, therefore, was to assess vaccination coverage against seasonal influenza and pneumococcal vaccination in rheumatological patients receiving biological therapy, and to identify the variables that predict these patients’ vaccination adherence.
Material and methodsType of studyA descriptive cross-sectional study was performed.
Participants: sample and selectionRheumatological patients in a regional referral hospital starting biological therapy for the first time between 1 January and 31 December 2016 were included in the study.
Data sourcesThe main source of information was the registry of biological therapies of the Hospital Pharmacy Service. The registry of biological therapies of the vaccine unit of the hospital's own preventive medicine and public health department was consulted, in addition to the primary care vaccination registry (OMI-AP) covering the entire autonomous region.
Study variablesSociodemographic variables and those relating to rheumatological diagnosis, biological treatment, treating physician and referral to the hospital's vaccine unit were taken into account. Pneumococcal coverage was evaluated with the 13-serotype conjugate vaccine (PCV13), and with the 23-serotype polysaccharide vaccine (PPSV23), and against seasonal influenza in the 2016/17 campaign. For this purpose, the abovementioned vaccination registries were checked. Two additional variables were created that covered combined pneumococcal vaccination (PCV13 and PPSV23), and combined vaccination with both pneumococcal vaccines, and seasonal influenza vaccine 2016/17.
Statistical analysisA descriptive analysis was performed of each variable (univariate analysis), expressing the absolute and relative frequencies of the qualitative variables investigated. The vaccination coverage was calculated for each of the values of the variables studied. A bivariate analysis was undertaken to identify whether each study variable was or was not associated with vaccination coverage, using the χ2 test. A multivariate analysis was performed using logistic regression to identify the predictive variables associated with vaccination adherence independently. In turn, it was assessed whether the rest of the study variables were associated with referral of the patient to the hospital's vaccine unit, by bivariate and multivariate analysis. A P value<.05 was considered statistically significant. PASW, version 18, (formally called SPSS) was used for the statistical analysis.
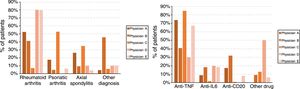
ResultsGeneral descriptionThe study sample comprised a total of 222 patients of whom 127 (57.2%) were females. The mean age was 52.68 years, and standard deviation±14.52 years. There were 5 rheumatology specialist biological therapy treating physicians. Of the treatments, 73% belonged to the anti-TNF family, followed by anti-IL6 (8.6%,) and anti-CD20 treatments (7.2%); the rest of the treatments (anti-IL12/23, anti-LT, antiIL17 and anti-PDE4) were grouped under the category other (11.4%). With regard to the main rheumatological diagnosis, rheumatoid arthritis recorded the greatest disease load (34.2%), ahead of psoriatic arthritis (31.5%) and axial spondylitis (23.4%); the remaining diagnoses (childhood idiopathic arthritis, undifferentiated arthritis, connective diseases and vasculitis) were grouped under the category other (10.8%). Of the patients, 73% had been referred to the hospital's vaccine unit for assessment and updating of their vaccination schedule.
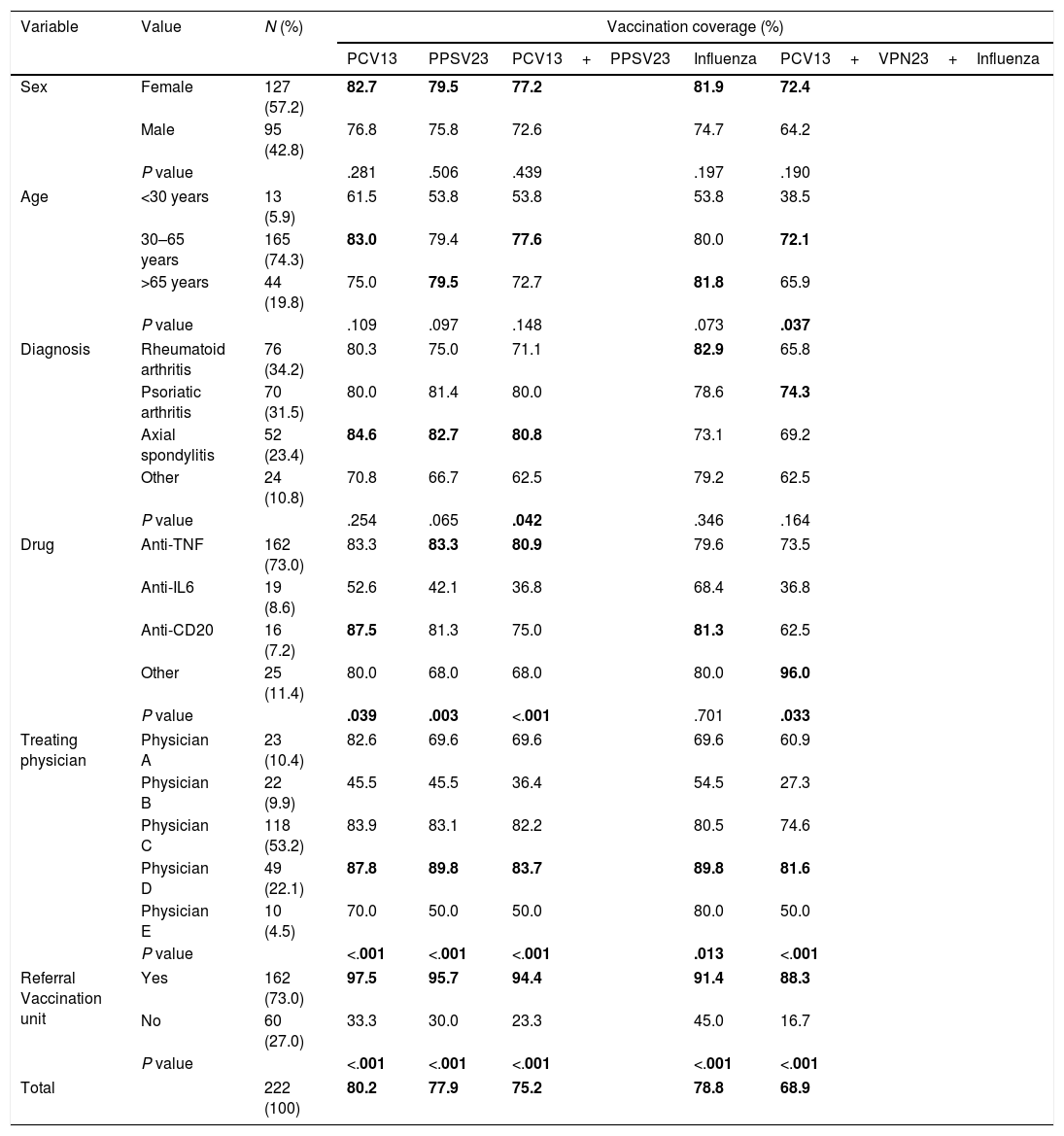
Vaccination coverageOf the patients assessed, 80.2% had a registered PCV13 vaccination, and 77.9% a PPSV23 vaccination. The combined pneumococcal vaccination was observed in 75.2% of the study sample. Furthermore, 78.8% of the patients had received the seasonal influenza vaccine in the 2016/17 campaign. Overall, 68.9% had correctly received the 3 prescribed vaccines (PCV3+PPSV23+influenza 2016/17). Table 1 shows the vaccination coverage according to the different study variables (sex, age, diagnosis, drug, treating physician and referral to the vaccine unit, and the statistical significance variables of the bivariate analysis.
Vaccination coverage according to the study variables: sex, age, diagnosis, drug, treating physician and referral to the vaccine unit (bivariate analysis).
| Variable | Value | N (%) | Vaccination coverage (%) | ||||
|---|---|---|---|---|---|---|---|
| PCV13 | PPSV23 | PCV13+PPSV23 | Influenza | PCV13+VPN23+Influenza | |||
| Sex | Female | 127 (57.2) | 82.7 | 79.5 | 77.2 | 81.9 | 72.4 |
| Male | 95 (42.8) | 76.8 | 75.8 | 72.6 | 74.7 | 64.2 | |
| P value | .281 | .506 | .439 | .197 | .190 | ||
| Age | <30 years | 13 (5.9) | 61.5 | 53.8 | 53.8 | 53.8 | 38.5 |
| 30–65 years | 165 (74.3) | 83.0 | 79.4 | 77.6 | 80.0 | 72.1 | |
| >65 years | 44 (19.8) | 75.0 | 79.5 | 72.7 | 81.8 | 65.9 | |
| P value | .109 | .097 | .148 | .073 | .037 | ||
| Diagnosis | Rheumatoid arthritis | 76 (34.2) | 80.3 | 75.0 | 71.1 | 82.9 | 65.8 |
| Psoriatic arthritis | 70 (31.5) | 80.0 | 81.4 | 80.0 | 78.6 | 74.3 | |
| Axial spondylitis | 52 (23.4) | 84.6 | 82.7 | 80.8 | 73.1 | 69.2 | |
| Other | 24 (10.8) | 70.8 | 66.7 | 62.5 | 79.2 | 62.5 | |
| P value | .254 | .065 | .042 | .346 | .164 | ||
| Drug | Anti-TNF | 162 (73.0) | 83.3 | 83.3 | 80.9 | 79.6 | 73.5 |
| Anti-IL6 | 19 (8.6) | 52.6 | 42.1 | 36.8 | 68.4 | 36.8 | |
| Anti-CD20 | 16 (7.2) | 87.5 | 81.3 | 75.0 | 81.3 | 62.5 | |
| Other | 25 (11.4) | 80.0 | 68.0 | 68.0 | 80.0 | 96.0 | |
| P value | .039 | .003 | <.001 | .701 | .033 | ||
| Treating physician | Physician A | 23 (10.4) | 82.6 | 69.6 | 69.6 | 69.6 | 60.9 |
| Physician B | 22 (9.9) | 45.5 | 45.5 | 36.4 | 54.5 | 27.3 | |
| Physician C | 118 (53.2) | 83.9 | 83.1 | 82.2 | 80.5 | 74.6 | |
| Physician D | 49 (22.1) | 87.8 | 89.8 | 83.7 | 89.8 | 81.6 | |
| Physician E | 10 (4.5) | 70.0 | 50.0 | 50.0 | 80.0 | 50.0 | |
| P value | <.001 | <.001 | <.001 | .013 | <.001 | ||
| Referral Vaccination unit | Yes | 162 (73.0) | 97.5 | 95.7 | 94.4 | 91.4 | 88.3 |
| No | 60 (27.0) | 33.3 | 30.0 | 23.3 | 45.0 | 16.7 | |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | ||
| Total | 222 (100) | 80.2 | 77.9 | 75.2 | 78.8 | 68.9 | |
The highest coverage for each of the vaccines in each category, and the statistically significant P values, are marked in bold.
Analysis of the vaccination coverage according to sex showed no statistically significant differences in coverage of the combined pneumococcal vaccine regimen (P=.439), or in the combination of pneumococcal and influenza vaccine (P=.190). With regard to age, the only significant differences were found in the combined vaccination against pneumococcus and influenza (P=.037), with the highest coverage in the group of 30–65 year olds.
Of the 3 most prevalent diseases in the study cohort (rheumatoid arthritis, psoriatic arthritis and axial spondylitis) it was observed that the latter registered the greatest coverage of the PCV13 (84.6%) and PPSV23 (82.7%) vaccinations, and the combined regimen of the two (80.8%); in the latter it was statistically significant with respect to the other diseases (P=.042). Influenza vaccination coverage was greater for rheumatoid arthritis (82.9%), whereas the highest coverage for the 3 vaccines was registered for psoriatic arthritis (74.3%), but the differences were not statistically significant.
Significant differences were also found on comparing the pneumococcal vaccination coverage according to drug type, except for the separate influenza vaccination, the lowest coverage was consistently associated with anti-IL6.
The treating physician was statistically significantly associated with each of the vaccines individually and in combination. It is worth mentioning, systematically, physician D registered the greatest coverage in every case, and it was always over 80%. By contrast, physician B obtained maximum coverage for influenza vaccination (54.5%), and minimum coverage for the combination of the 3 vaccines (27.3%).
Furthermore, referral to the vaccine unit was the variable that was systematically associated with greater vaccination coverage (P<.001)for all the vaccines, combined or separate.
In addition, a statistically significant association (P<.001) was confirmed between influenza vaccination and the sequential pneumococcal regimen, so that 90.4% of those who received the combined regimen were also vaccinated against influenza.
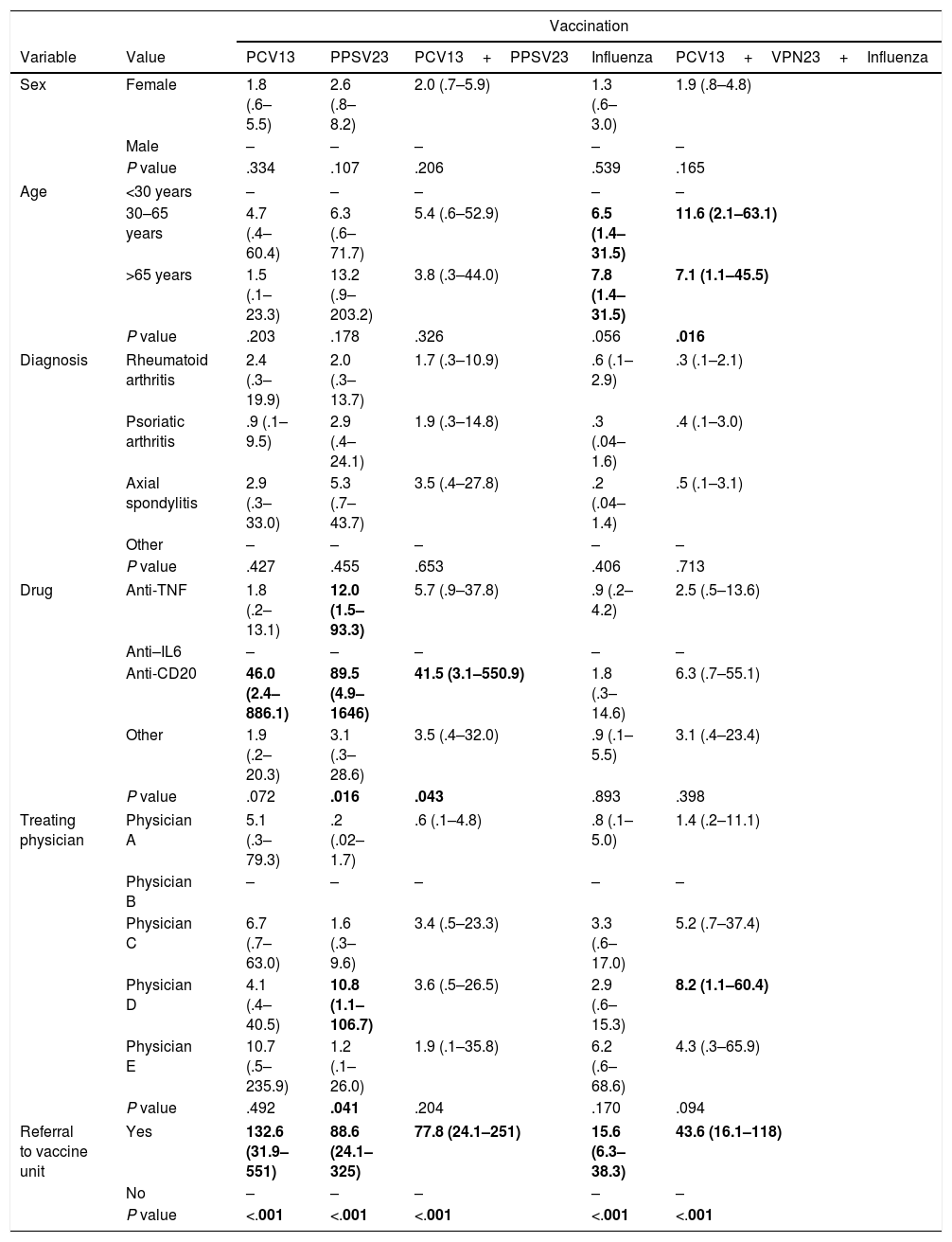
In the multivariate analysis (Table 2), the only variable associated statistically with better vaccine coverage irrespective of the rest, was referral to the vaccine unit (P<.001), with consistently very high OR for the separate vaccines as well as for the combined vaccination. With regard to the treating physician, statistically higher vaccination coverage was only maintained by physician D with respect to the PPSV23 vaccine, and for the combination of the 3 vaccines. In turn, it was found that the drug used was significantly associated with pneumococcal vaccination, separately and in combination, with higher coverage for the anti-CD20 users and, although only for the PPSV23 vaccine, the anti-TNF users. No statistical association was found with sex or diagnosis, but one was found with age, with greater likelihood of influenza vaccination, and for the 3 vaccines combined, in the older groups compared to those aged under 30.
Multivariate analysis (logistic regression) of the factors associated with vaccination coverage for each vaccine or combination of vaccines.
| Vaccination | ||||||
|---|---|---|---|---|---|---|
| Variable | Value | PCV13 | PPSV23 | PCV13+PPSV23 | Influenza | PCV13+VPN23+Influenza |
| Sex | Female | 1.8 (.6–5.5) | 2.6 (.8–8.2) | 2.0 (.7–5.9) | 1.3 (.6–3.0) | 1.9 (.8–4.8) |
| Male | – | – | – | – | – | |
| P value | .334 | .107 | .206 | .539 | .165 | |
| Age | <30 years | – | – | – | – | – |
| 30–65 years | 4.7 (.4–60.4) | 6.3 (.6–71.7) | 5.4 (.6–52.9) | 6.5 (1.4–31.5) | 11.6 (2.1–63.1) | |
| >65 years | 1.5 (.1–23.3) | 13.2 (.9–203.2) | 3.8 (.3–44.0) | 7.8 (1.4–31.5) | 7.1 (1.1–45.5) | |
| P value | .203 | .178 | .326 | .056 | .016 | |
| Diagnosis | Rheumatoid arthritis | 2.4 (.3–19.9) | 2.0 (.3–13.7) | 1.7 (.3–10.9) | .6 (.1–2.9) | .3 (.1–2.1) |
| Psoriatic arthritis | .9 (.1–9.5) | 2.9 (.4–24.1) | 1.9 (.3–14.8) | .3 (.04–1.6) | .4 (.1–3.0) | |
| Axial spondylitis | 2.9 (.3–33.0) | 5.3 (.7–43.7) | 3.5 (.4–27.8) | .2 (.04–1.4) | .5 (.1–3.1) | |
| Other | – | – | – | – | – | |
| P value | .427 | .455 | .653 | .406 | .713 | |
| Drug | Anti-TNF | 1.8 (.2–13.1) | 12.0 (1.5–93.3) | 5.7 (.9–37.8) | .9 (.2–4.2) | 2.5 (.5–13.6) |
| Anti–IL6 | – | – | – | – | – | |
| Anti-CD20 | 46.0 (2.4–886.1) | 89.5 (4.9–1646) | 41.5 (3.1–550.9) | 1.8 (.3–14.6) | 6.3 (.7–55.1) | |
| Other | 1.9 (.2–20.3) | 3.1 (.3–28.6) | 3.5 (.4–32.0) | .9 (.1–5.5) | 3.1 (.4–23.4) | |
| P value | .072 | .016 | .043 | .893 | .398 | |
| Treating physician | Physician A | 5.1 (.3–79.3) | .2 (.02–1.7) | .6 (.1–4.8) | .8 (.1–5.0) | 1.4 (.2–11.1) |
| Physician B | – | – | – | – | – | |
| Physician C | 6.7 (.7–63.0) | 1.6 (.3–9.6) | 3.4 (.5–23.3) | 3.3 (.6–17.0) | 5.2 (.7–37.4) | |
| Physician D | 4.1 (.4–40.5) | 10.8 (1.1–106.7) | 3.6 (.5–26.5) | 2.9 (.6–15.3) | 8.2 (1.1–60.4) | |
| Physician E | 10.7 (.5–235.9) | 1.2 (.1–26.0) | 1.9 (.1–35.8) | 6.2 (.6–68.6) | 4.3 (.3–65.9) | |
| P value | .492 | .041 | .204 | .170 | .094 | |
| Referral to vaccine unit | Yes | 132.6 (31.9–551) | 88.6 (24.1–325) | 77.8 (24.1–251) | 15.6 (6.3–38.3) | 43.6 (16.1–118) |
| No | – | – | – | – | – | |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | |
Odds ratio and 95% confidence interval; the empty rows indicate the benchmark value.
The statistically significant values are marked in bold.
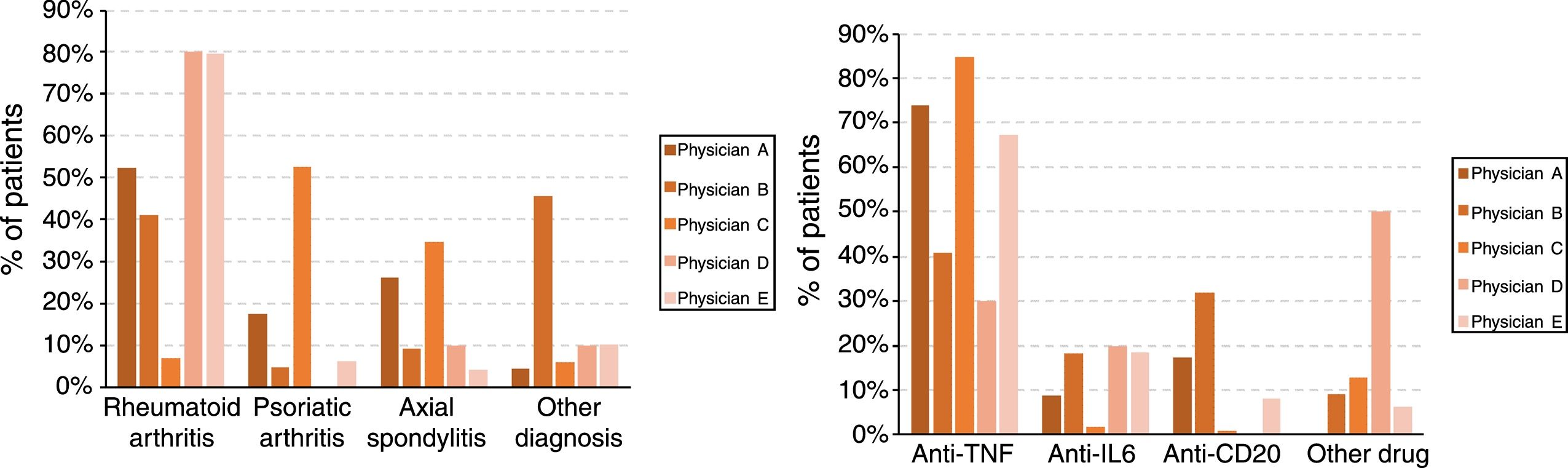
Finally, the treating physicians had different profiles, with statistically significant differences, both in the patients’ diagnoses (P<.001) and the drugs used (P<.001) which, in turn, were associated with each other (P<.001). Fig. 1 shows the diagnosis and drug profiles according to the treating physician.
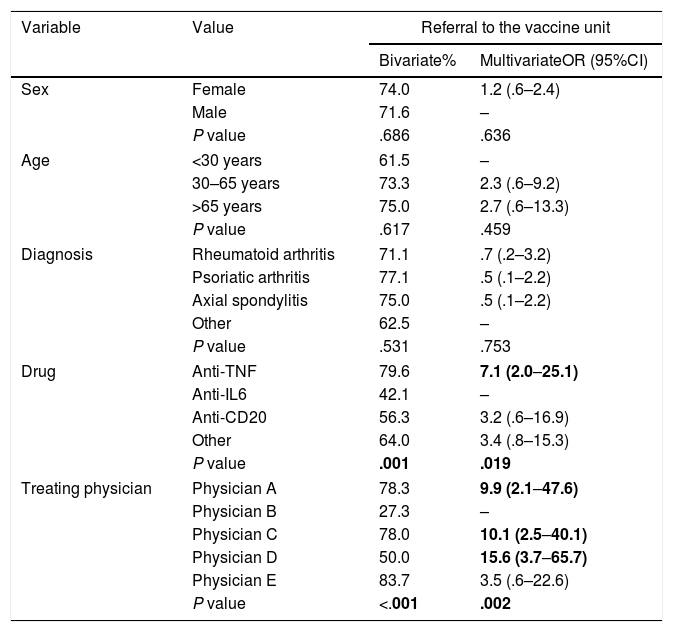
Referral to the vaccine unitStatistically significant differences were observed in referral to the vaccine unit (Table 3) according to the treating physician, both in the bivariate (P<.001) and the multivariate analysis (P=.002), where one had significantly lower referral percentages. In turn, a significant association was found with the drug used (P=.001 for the bivariate and P=.019 for the multivariate analysis), with higher percentages of referral to the vaccine unit in the anti-TNF users. There was no relationship with age, sex or diagnosis and referral to the vaccine unit.
Bivariate analysis (chi-squared) and multivariate (logistical regression) of the factors associated with referral of the patient to the hospital's vaccine unit.
| Variable | Value | Referral to the vaccine unit | |
|---|---|---|---|
| Bivariate% | MultivariateOR (95%CI) | ||
| Sex | Female | 74.0 | 1.2 (.6–2.4) |
| Male | 71.6 | – | |
| P value | .686 | .636 | |
| Age | <30 years | 61.5 | – |
| 30–65 years | 73.3 | 2.3 (.6–9.2) | |
| >65 years | 75.0 | 2.7 (.6–13.3) | |
| P value | .617 | .459 | |
| Diagnosis | Rheumatoid arthritis | 71.1 | .7 (.2–3.2) |
| Psoriatic arthritis | 77.1 | .5 (.1–2.2) | |
| Axial spondylitis | 75.0 | .5 (.1–2.2) | |
| Other | 62.5 | – | |
| P value | .531 | .753 | |
| Drug | Anti-TNF | 79.6 | 7.1 (2.0–25.1) |
| Anti-IL6 | 42.1 | – | |
| Anti-CD20 | 56.3 | 3.2 (.6–16.9) | |
| Other | 64.0 | 3.4 (.8–15.3) | |
| P value | .001 | .019 | |
| Treating physician | Physician A | 78.3 | 9.9 (2.1–47.6) |
| Physician B | 27.3 | – | |
| Physician C | 78.0 | 10.1 (2.5–40.1) | |
| Physician D | 50.0 | 15.6 (3.7–65.7) | |
| Physician E | 83.7 | 3.5 (.6–22.6) | |
| P value | <.001 | .002 | |
Bivariate: % of referral to the vaccine unit; multivariate: odds ratio and 95% confidence interval; the empty rows indicate the benchmark value.
The statistically significant values are marked in bold.
With the results obtained using the methodology outlined we believe we have met the objectives set. The study was performed in a regional referral hospital that covers 3 of the 8 health areas and attends, approximately, 40% of the autonomous regions’ rheumatological population.
With regard to immunosuppressed patients and although, at the moment, the importance of vaccination in this risk group6 is not disputed, it is known that there is an urgent need to improve vaccine coverage. Research studies including more than 22,000 immunosuppressed people from the United States showed that only 5.2% of solid organ transplant patients had received the pneumococcal vaccination, and the greatest coverage is registered for the group with HIV (31%).14 Moreover, when dermatological patients receiving biological therapy were assessed, only 17% had received the pneumococcal vaccine and 38% the influenza vaccine, despite more than 25% of cases having other comorbidities with an indication for vaccination.15
There is currently great variability among authors regarding vaccine coverage in patients receiving biological therapy. Therefore, some studies obtain coverage rates from 28%12 to 60%13 for the influenza vaccine. In the case of the pneumococcal vaccine, the most prominent research study recorded coverage of 58%,11 whereas it barely exceeds 12% in others.10 By contrast, this research study exceeds 80% PCV13 coverage, and reaches almost 78% for PPSV23, and 75.2% for combined vaccination coverage. In addition, influenza coverage is also greater in this cohort than that described by other authors.12
Furthermore, referral to the vaccine unit seems to play a very relevant role in adherence to immunisation schedules. It is logical to think that personalised organisation and follow-up of vaccination schedules in this type of unit is associated with better vaccine coverage, as this research study demonstrates. Similarly, the association of age with greater adherence to influenza vaccination is as expected since age itself is a risk factor for influenza virus complications.16
Vaccination according to sex has also been studied by other authors, especially in cohorts of patients with rheumatoid arthritis.7,8 Thus, in convergence with the literature, no statistically significant differences have been found between coverage in men and women despite the fact that, in this research study, the women systematically obtained greater coverage for all the vaccines and their combinations.
It is worth noting that, although some publications assess vaccination coverage as an indicator of the outcome of a targeted intervention,6,12,17,18 this research study is the first assessment of vaccination in rheumatological patients receiving biological therapy performed in our area. Likewise, we found no national studies with which to compare these results. Therefore, we interpreted the results observed positively because, although improvable, they are better than expected considering the international context. This is understood as the responsibility and active concern of rheumatologists in the comprehensive and multidisciplinary approach to patients receiving biological therapy, an issue that other authors have demonstrated in relation to influenza vaccination in people over 65 years of age, and vaccination against pertussis in pregnancy when it was found that high vaccination coverage was associated with direct and proactive recommendation by healthcare providers to patients.19–21
Finally we should point out that, despite the results obtained, this research study has its limitations. Principally, it is a single centre study which means that the results cannot be extrapolated to other contexts. Even so, and given the few national studies undertaken on the subject, we consider that the sample included is sufficient for a first approach to the topic.
In conclusion, influenza and pneumococcal vaccination in the cohort studied are higher than those observed by other authors. Referral of these patients by the rheumatologist to the vaccine unit is crucial to ensure correct immunisation, and thus minimise some of the possible infectious adverse effects of the biological therapies.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Fernández-Prada M, Brandy-García AM, Rodríguez-Fonseca OD, Huerta-González I, Fernández-Noval F, Martínez-Ortega C. Evaluación de las coberturas de vacunación antineumocócica y antigripal en pacientes reumatológicos con terapia biológica de un hospital autonómico de referencia. Reumatol Clin. 2020;16:97–102.