Secondary amyloidosis can be found in some monogenic autoinflammatory diseases. In this study we present an 83-year-old man with no relevant medical history who presented with iron deficiency anaemia. In the study, a gastroscopy was performed with duodenum biopsy showing secondary AA-type amyloidosis.
Genetic analyses of monogenic autoinflammatory diseases revealed the heterozygous p.R92Q variant in the TNFRSF1A gene, with negative results in the complementary tests for other causes of amyloidosis.
In TRAPS, secondary amyloidosis has usually been associated with mutations affecting cysteine residues, but until now no association has been demonstrated with the p.RQ92 variant.
Secondary amyloidosis may be present in carriers of the p.RQ92 variant, therefore it is important to diagnose it to prevent possible complications.
La amiloidosis secundaria puede encontrarse en algunas enfermedades autoinflamatorias monogénicas. Presentamos el caso de un varón de 83 años sin antecedentes patológicos de interés. Tras detectarse una anaemia ferropénica, se realizó una gastroscopia y la biopsia duodenal evidenció amiloidosis secundaria de tipo AA. El estudio de enfermedades autoinflamatorias reveló la variante heterocigota p.R92Q en el gen TNFRSF1A, siendo negativas las pruebas complementarias para otras causas de amiloidosis. En el síndrome TRAPS la amiloidosis secundaria puede asociarse a mutaciones que afectan a residuos cisteína, no habiéndose evidenciado su asociación con la variante p.R92Q.
ConclusiónLa amiloidosis secundaria puede estar presente en individuos portadores de la variante p.RQ92, por lo que es importante su diagnóstico para intentar prevenir posibles complicaciones.
TNF receptor-associated periodic syndrome (TRAPS) is an autosomal dominant disease characterised by recurrent episodes of fever, migratory myalgias, skin rash, conjunctivitis, orbital oedema and abdominal or chest pain due to muscle inflammation,1,2 and even chronic refractory arthritis.3 Leukocytosis, neutrophilia, thrombocytosis and elevated acute phase reactants can occur. We present the case of an 83-year-old male with amyloidosis type AA and the p.R92Q variant in the TNFRSF1A gene.
Clinical caseAn 83-year-old male with a history of gastrointestinal bleeding and iron-deficiency anaemia due to gastritis and duodenal ulcer, benign prostatic hypertrophy, high blood pressure treated with tamsulosin (.4mg/24h), dutasteride (.5mg/24h) and omeprazole (20mg/24h).
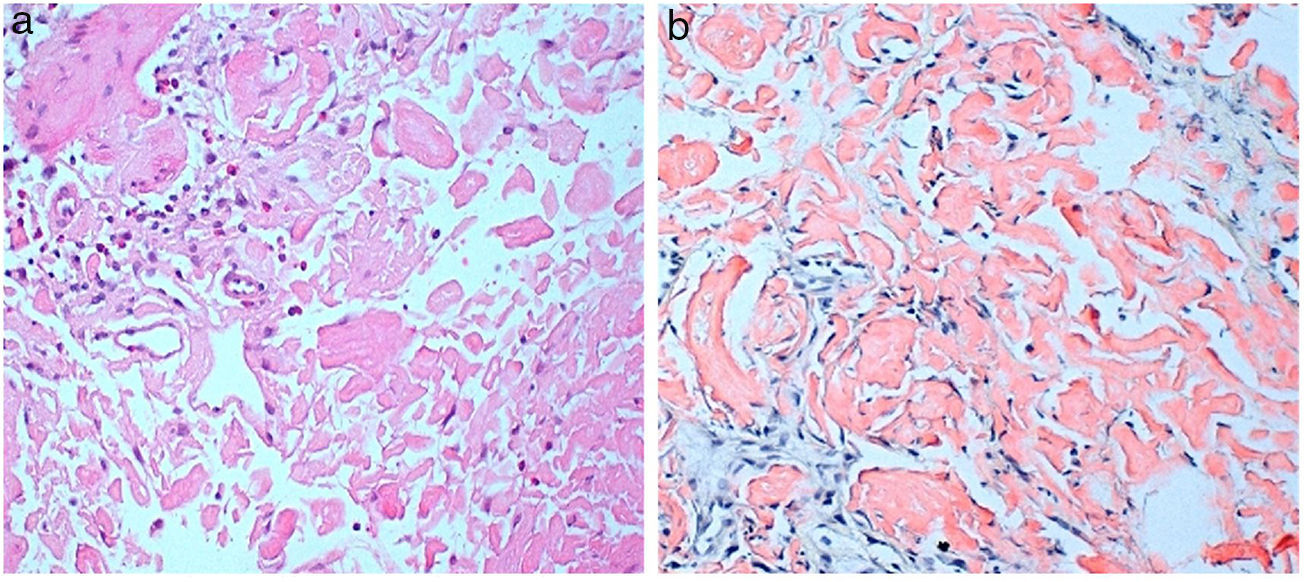
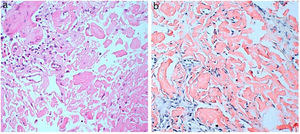
He consulted with tiredness and instability, with haemoglobin of 7.3g/dl. Gastroscopy revealed a duodenal ulcer. Gastric biopsy showed mild chronic gastritis. Duodenal biopsy showed focal ulceration of mucosa with preserved villi and lamina propria expanded by deposit of amorphous eosinophilic material (Fig. 1A); this deposit was also observed in the capillary walls and the submucosa, it was accompanied by minimal non-specific lymphocytic inflammatory infiltrate; Congo red stain was intensely positive and showed apple-green birefringence in polarised light (Fig. 1B). Immunohistochemical staining was also performed for AA protein, which was intensely positive.
Enteroscopy demonstrated from the distal bulb, and continuously the length of the path explored, intestinal mucosa with superficial ulcerations and of infiltrative appearance, with areas of spontaneous bleeding on passing the endoscope. The most significant findings were located in the distal bulb and the second and third duodenal portions, with totally denuded and ulcerated mucosal areas. Colonoscopy was normal. Rectal biopsy showed no amyloid material.
Thoracic CT was normal, while abdominal CT revealed discrete thickening with dilation of the duodenal wall and first jejunal loops (up to 3.2cm) with involvement of the adjacent fat, of probable inflammatory origin; ureteral and bladder lithiasis. Abdominal CT-angiography showed no valuable findings.
Aetiological study of secondary amyloidosis was performed. Liver and kidney function, proteinogram, and serum amyloid A protein were normal. Antinuclear antibodies, rheumatoid factor, citrullinated peptide, neutrophil antibody and serum beta-2-microglobulin were negative. C-reactive protein (CRP) was normal on admission, but intermittent elevations of around 50mg/l (normal .0-6-0) were evidenced in some of the routine checks; there were no associated symptoms. Abdominal fat biopsy showed no amyloid material. The 24-h urine protein test was negative.
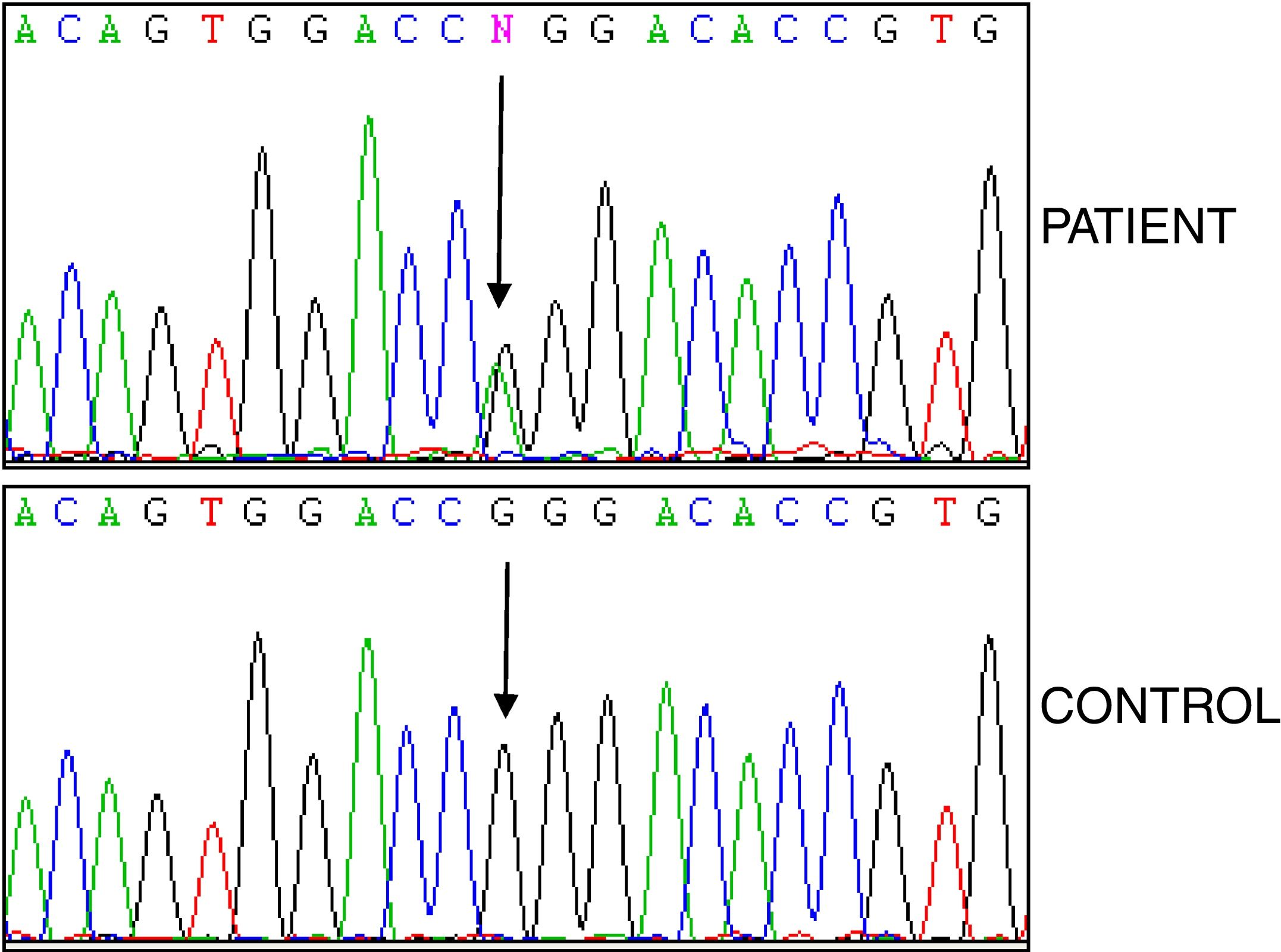
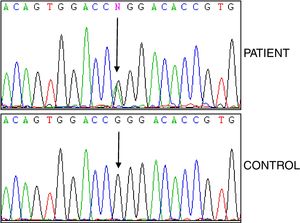
Studies of genes for familial Mediterranean fever (FMF) and familial amyloid polyneuropathy (FAP) were negative, while analysis of the TNFRSF1A gene revealed the heterozygous variant p.R92Q (Fig. 2). The electrocardiogram was normal, and the echocardiogram showed a left ventricle that was neither dilated nor hypertrophied, preserved ejection fraction with impaired relaxation. Electromyography showed slight signs of chronic radicular involvement bilateral L4, L5 and S1, there were no conclusive signs of polyneuropathy. Lumbar-sacral magnetic resonance showed osteodegenerative changes in the interapophyseal joints L4-L5-S1.
DiscussionTRAPS syndrome is a monogenic auto-inflammatory disease that can affect different ethnic groups. The mutations that cause this syndrome are located in the TNFRSF1A gene, and about 114 variants have been described. Among them, the p.R92Q variant stands out in terms of frequency, currently classified as a variant of uncertain significance, and also presenting a low incidence of secondary amyloidosis.4
In TRAPS syndrome, symptoms usually appear during the first decade of life. In the case of adult onset, it is common to identify low-penetrance variants.5 Prolonged febrile episodes (between 1 and 4 weeks) are most frequent manifestation of the syndrome, which recur at irregular intervals of weeks or months, accompanied by myalgias and migratory skin exanthema. There may be triggers such as infections, trauma, stress, etc.6
It is the prolonged elevation of acute phase reactants, and especially serum amyloid protein, that increases the risk of type AA amyloidosis. The patient we describe presented elevated CRP in some of the routine tests without any symptoms. Concentric ventricular hypertrophy was not observed, nor was amyloid deposition evidenced in the abdominal fat or rectal biopsies. Duodenal biopsy ruled out Crohn's disease (CD), since in CD there may be ulceration and fissures, but with predominance of severe/moderate lymphoplasmocellular inflammatory infiltration, granulomas and acute glandular inflammation, which were not present in this case.
Thoracic and abdominal CT, gastroscopy, enteroscopy and colonoscopy did not reveal neoplasms, and the proteinogram did not show monoclonal bands.
Amyloidosis can occur in TRAPS,7,8 but to date its association with the p.RQ92 variant, has not been demonstrated.2
In conclusion, amyloidosis may be present in carriers of the p.RQ92 variant, and therefore its diagnosis is important to avoid possible complications.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Nicolás-Sánchez FJ, Aróstegui-Gorospe JI, Pallarés-Quixal J, Nicolás-Sarrat FJ, Sarrat-Nuevo RM, Nogue Bou RM, et al. Mutación p.RQ92 asociada a amiloidosis. Reumatol Clin. 2021;17:46–48.