To analyse the effect of secukinumab on self-reported variables of patients diagnosed with psoriatic arthritis and/or ankylosing spondylitis in relation to their health status, pain, fatigue, sleep and quality of life.
MethodsA six-month, observational, longitudinal, prospective, multicentre study was conducted with 39 patients who initiated treatment with secukinumab as therapy for psoriatic arthritis and/or spondylitis. The main variables were changes in patient-reported measures and they were evaluated by means of the questionnaires: FACIT-fatigue, Insomnia Severity Index, EuroQol-3L-5D and PsAQoL. In addition, depending on the type of disease (peripheral psoriasis or spondyloarthritis) the DAS28 with ESR or the BASDAI were calculated, respectively.
ResultsLevels of fatigue, moderate and severe insomnia significantly reduced after 6 months of treatment with secukinumab. At the same time, patient-reported quality of life increased significantly (P = .006). Data on pain and discomfort also show significant improvement after the treatment.
ConclusionsPatients with psoriatic arthritis and/or ankylosing spondylitis who start treatment with secukinumab show improvement at 6 months in all effect sizes of the treatment, particularly in sleep, fatigue and quality of life. Furthermore, patient-reported outcome measures are of additional clinical value and allow more accurate and closer assessment of their real status of health and well-being.
Analizar el efecto del secukinumab sobre las variables propias reportadas por el paciente diagnosticado de artritis psoriásica y/o espondilitis anquilosante en relación con su estado de salud, dolor, fatiga, sueño y calidad de vida.
MétodosSe realizó un estudio observacional, longitudinal, prospectivo y multicéntrico a 6 meses con 39 pacientes que iniciaron tratamiento con secukinumab para la terapia de artritis psoriásica y/o espondilitis. Las variables principales fueron los cambios en las medidas reportadas por el paciente, evaluándolas por medio de los cuestionarios FACIT-fatiga, Índice de Gravedad del Insomnio, EuroQol-3L-5D y PsAQoL. Adicionalmente, y dependiendo del tipo de enfermedad (psoriásica periférica o espondiloartritis), se recogió el DAS28 con velocidad o el BASDAI, respectivamente.
ResultadosLos niveles de fatiga, insomnio moderado y grave presentan una reducción significativa tras el tratamiento de 6 meses con secukinumab. Al mismo tiempo, la calidad de vida reportada por el paciente aumenta notablemente (p = 0,006). Los datos referentes al dolor y a la incomodidad también presentan una notable mejoría tras el tratamiento.
ConclusionesLos pacientes de artritis psoriásica y/o espondilitis anquilosante que inician tratamiento con secukinumab presentan mejoría a los 6 meses en todos los tamaños del efecto del tratamiento, particularmente en el sueño, la fatiga y la calidad de vida. Además, las medidas de desenlace reportadas por los pacientes son un valor clínico adicional y permiten realizar una valoración más exacta y aproximada de su estado real de salud y bienestar.
Psoriatic arthritis is an inflammatory joint disease that occurs in the context of a patient with cutaneous psoriasis or a first-degree family history of the condition.1,2 It has a prevalence of 6–25 cases per 10,000 people and affects 20%–30% of patients with this skin involvement.3,4 Psoriatic arthritis can present as a form of peripheral involvement in the form of asymmetric oligoarthritis or symmetric polyarthritis and/or in the form of axial involvement correlating with inflammatory lumbosacral pain and back stiffness, which can lead to functional impairment and reduced quality of life.5,6
The interleukin IL-23/IL-17A axis is known to be involved in a variety of biological functions such as inflammation, joint damage and injury, and is also present in the pathogenic mechanism of psoriatic arthritis and spondylitis.7 Recent studies have shown that inhibition of the IL-17A receptor with secukinumab, an anti-human IL-17A monoclonal antibody, improves the signs and symptoms of the disease.7–9However, the multifactorial nature of the disease make it difficult to use measures that reflect changes in the course of treatment and its impact on the patient's quality of life.
In recent years, several questionnaires10–12 have been developed to assess the efficacy and effectiveness of health interventions on the premise that the patient's point of view should be included in assessing the relative merits of treatments in clinical trials.13 From this perspective, in our study we analysed the effect of secukinumab on patient-reported variables, namely fatigue, sleep and quality of life in patients with psoriatic arthritis and/or spondyloarthritis.
Material and methodsA multicentre, observational, longitudinal, and prospective 6-month study was conducted in which 39 patients from the Valencia area were selected and consecutive sampling was conducted of all those starting treatment with secukinumab with any rheumatological indication.
The main variables were changes in patient-reported measures, evaluating overall health both at the initial visit and at 6 months using a visual analogue scale (VAS). Fatigue measures were also collected from each patient using the FACIT-fatigue scale. The Insomnia Severity Index was used for sleep assessment and the EuroQol-3L-5D and PsAQoL questionnaires for patient quality of life. The delivery method of secukinumab (as monotherapy or with DMARDs), the administration of previous biologics and the patient's corticosteroid or NSAID medication during the study were used as confounding criteria. In addition, depending on the type of disease (peripheral psoriatic or spondyloarthritis), the DAS28 with velocity or BASDAI, respectively, were recorded.
- •
The FACIT Scale is a 13-item questionnaire. Each response is assessed on a 5-point Likert-type scale, with 0 being no problem and 4 being the maximum amount, and includes items such as fatigue, weakness, apathy, lack of energy and the impact these feelings have on the patient. The overall range of the scale is from 0 to 52, with lower values denoting higher levels of fatigue.4,11,14
- •
The Insomnia Severity Index (ISI) is a 7-item questionnaire assessing the nature, severity, and impact of insomnia. The dimensions evaluated are severity of sleep onset and problems with early morning awakening problems, sleep dissatisfaction, sleep difficulties, noticeability of sleep problems by others. Each item is rated on a 5-point Likert scale, with 0 being no problem and 4 very severe problem. Thus, a scale is generated where 0–7 is interpreted as absence of insomnia; 8–14 sub-threshold insomnia; 15–21 moderate insomnia; 22–28 severe insomnia.15
- •
The EuroQol-5D-3L (EQ-5D) is a generic instrument for measuring quality of life by which patients themselves evaluate their state of health by dimensions on mobility, self-care, activities of daily living, pain and discomfort, anxiety, and depression. Each of these is scored as 3 levels of severity and an overall VAS assessment. A third element of the EQ-5D is the social value index, which consists of 5 dimensions (mobility, self-care, activities of daily living, pain/discomfort, and anxiety/depression), each of which is scored from one to three, with 1 being no problems and 3 being extreme problems. The second part of the EQ-5D is a VAS in which the patient assesses his or her general health state on a scale from 0 to 100, where 0 is the best possible health state and 100 the worst, the least significant difference being 10.16
- •
The PsAQoL questionnaire is a specific instrument for assessing quality of life. It consists of 20 yes/no questions derived directly from qualitative patient interviews and is successfully used in diseases such as rheumatoid arthritis, spondylitis, and lupus erythematosus. The total score is calculated as the number of affirmative questions and can range from 0 to 20, with higher values indicating poor quality of life.17
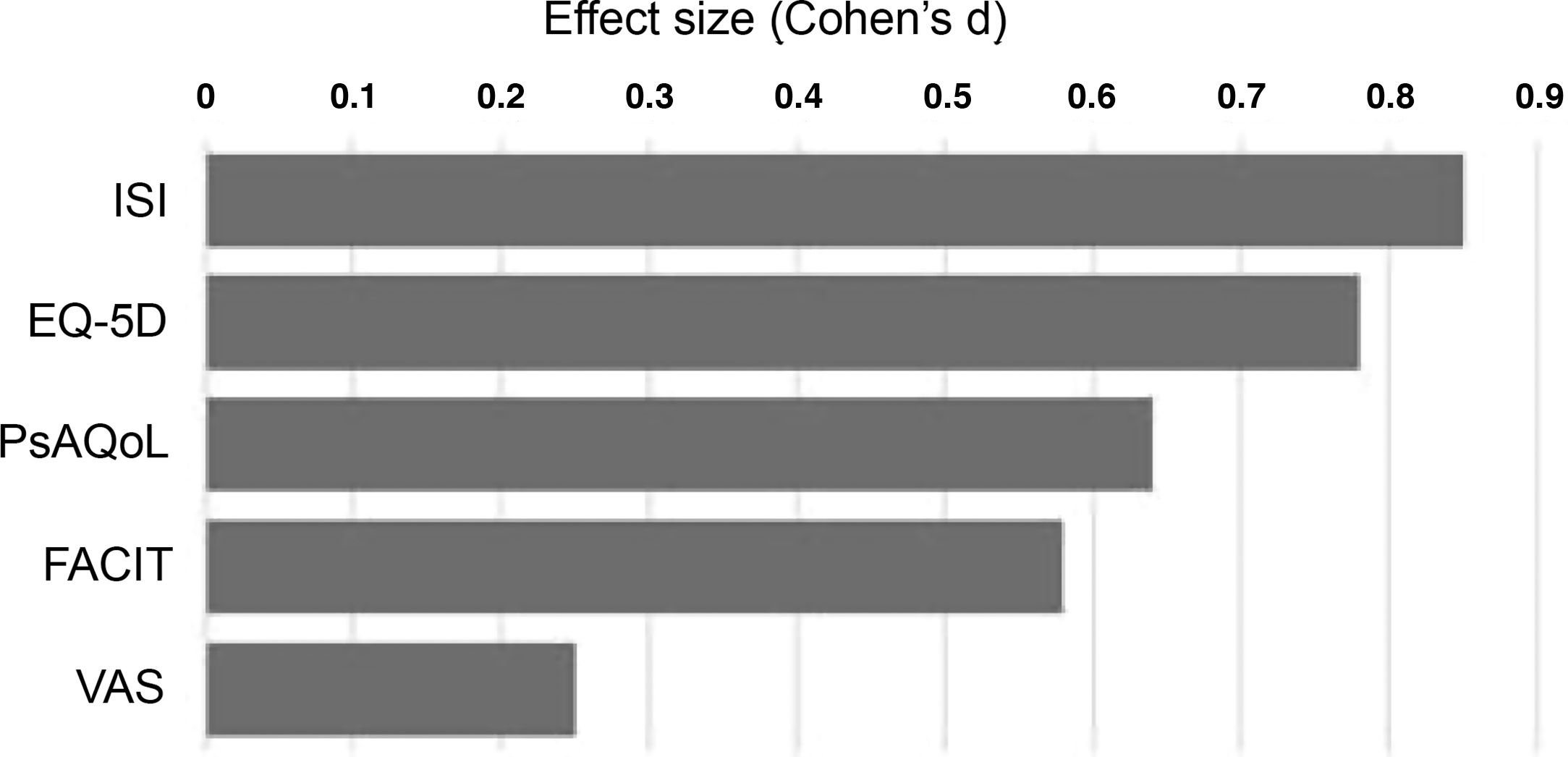
Statistical analysis of the samples was performed using measures of central tendency, changes were analysed after 6 months using the Student's t-test for paired data in the case of the FACIT, VAS, PsAQoL and ISI and the chi-squared test for the dimensions of the EQ-5D questionnaire. Changes in patient-reported measures were analysed in multiple linear regression models with 95% confidence intervals. The effect size of each measure was calculated using Cohen's d (mean difference/aggregate variance). Results were analysed by disease group and overall using Stata v12 (College Station, Tx, USA).
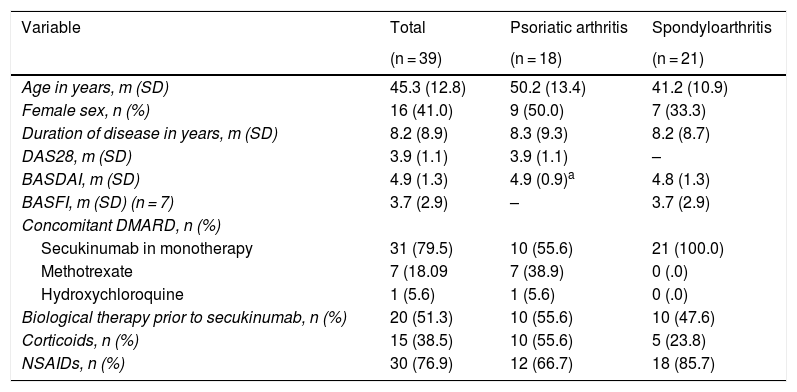
ResultsThe 39 patients under study were classified into two groups according to whether they had psoriatic arthritis or spondyloarthritis, with a mean age of 50.2 ± 13.4 and 41.2 ± 10.9 years, respectively. Of these, 54% had a predominantly axial picture and the remaining 46% had peripheral arthritis. Regarding the drugs used as therapy, treatment either in combination with a disease modifying drug or with corticosteroids was more frequent in the patients with peripheral than in those with axial predominance (Table 1). In addition, approximately half the patients in each group had been treated with a previous biological agent.
Baseline description of the 39 patients in the study.
| Variable | Total | Psoriatic arthritis | Spondyloarthritis |
|---|---|---|---|
| (n = 39) | (n = 18) | (n = 21) | |
| Age in years, m (SD) | 45.3 (12.8) | 50.2 (13.4) | 41.2 (10.9) |
| Female sex, n (%) | 16 (41.0) | 9 (50.0) | 7 (33.3) |
| Duration of disease in years, m (SD) | 8.2 (8.9) | 8.3 (9.3) | 8.2 (8.7) |
| DAS28, m (SD) | 3.9 (1.1) | 3.9 (1.1) | – |
| BASDAI, m (SD) | 4.9 (1.3) | 4.9 (0.9)a | 4.8 (1.3) |
| BASFI, m (SD) (n = 7) | 3.7 (2.9) | – | 3.7 (2.9) |
| Concomitant DMARD, n (%) | |||
| Secukinumab in monotherapy | 31 (79.5) | 10 (55.6) | 21 (100.0) |
| Methotrexate | 7 (18.09 | 7 (38.9) | 0 (.0) |
| Hydroxychloroquine | 1 (5.6) | 1 (5.6) | 0 (.0) |
| Biological therapy prior to secukinumab, n (%) | 20 (51.3) | 10 (55.6) | 10 (47.6) |
| Corticoids, n (%) | 15 (38.5) | 10 (55.6) | 5 (23.8) |
| NSAIDs, n (%) | 30 (76.9) | 12 (66.7) | 18 (85.7) |
BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; DAS28: Disease Activity Score 28-joints; DMARD: disease-modifying antirheumatic drugs; m: mean; NSAIDs: nonsteroidal anti-inflammatory drugs; SD: standard deviation.
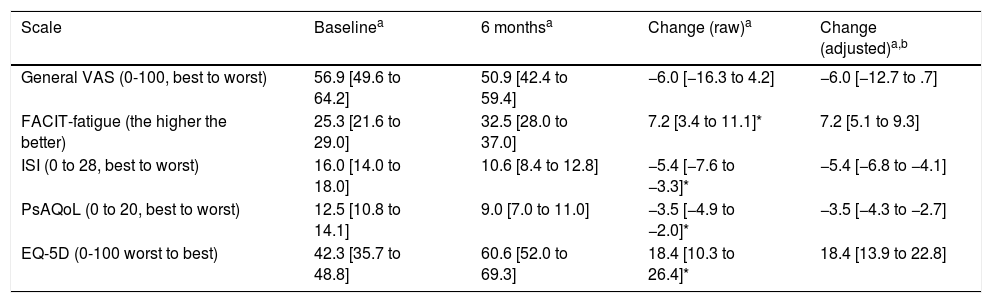
To analyse patient-centred variables and quality of life, questionnaires were conducted at baseline and after 6 months of secukinumab treatment. Except for the Visual Analogue Scale (VAS), all study variables showed significant and relevant changes after treatment with secukinumab. After 6 months of treatment, a significant reduction in fatigue levels was observed (Table 2). Likewise, the quality-of-life questionnaires (PsAQoL and EQ-5D) showed significant improvement after treatment.
Changes in each of the patient centred variables after 6 months of therapy with secukinumab.
| Scale | Baselinea | 6 monthsa | Change (raw)a | Change (adjusted)a,b |
|---|---|---|---|---|
| General VAS (0-100, best to worst) | 56.9 [49.6 to 64.2] | 50.9 [42.4 to 59.4] | −6.0 [−16.3 to 4.2] | −6.0 [−12.7 to .7] |
| FACIT-fatigue (the higher the better) | 25.3 [21.6 to 29.0] | 32.5 [28.0 to 37.0] | 7.2 [3.4 to 11.1]* | 7.2 [5.1 to 9.3] |
| ISI (0 to 28, best to worst) | 16.0 [14.0 to 18.0] | 10.6 [8.4 to 12.8] | −5.4 [−7.6 to −3.3]* | −5.4 [−6.8 to −4.1] |
| PsAQoL (0 to 20, best to worst) | 12.5 [10.8 to 14.1] | 9.0 [7.0 to 11.0] | −3.5 [−4.9 to −2.0]* | −3.5 [−4.3 to −2.7] |
| EQ-5D (0-100 worst to best) | 42.3 [35.7 to 48.8] | 60.6 [52.0 to 69.3] | 18.4 [10.3 to 26.4]* | 18.4 [13.9 to 22.8] |
EQ-5D: thermometer of the EuroQol-5 dimensions; FACIT: Functional Assessment of Chronic Illness Therapy; ISI: Insomnia Severity Index; PsAQoL: Psoriatic Arthritis Quality of Life; VAS: visual analogue scale.
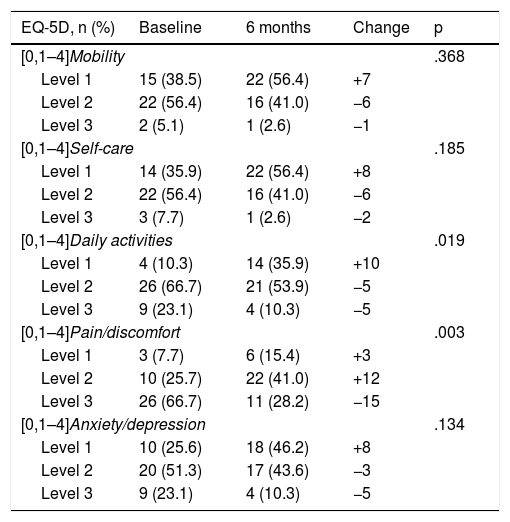
The subitems where the greatest differences were obtained after 6 months of treatment with secukinumab were the pain and discomfort variables (p = .003). In turn, the indicators referring to daily activities as a whole also showed significant improvement (p = .019) (Table 3). In contrast, we found no significant differences in the variables referring to mobility, self-care, and anxiety (p > .05) (Table 3).
Changes in the EuroQuoL-5D after six months’ treatment with secukinumab.
| EQ-5D, n (%) | Baseline | 6 months | Change | p |
|---|---|---|---|---|
| [0,1–4]Mobility | .368 | |||
| Level 1 | 15 (38.5) | 22 (56.4) | +7 | |
| Level 2 | 22 (56.4) | 16 (41.0) | −6 | |
| Level 3 | 2 (5.1) | 1 (2.6) | −1 | |
| [0,1–4]Self-care | .185 | |||
| Level 1 | 14 (35.9) | 22 (56.4) | +8 | |
| Level 2 | 22 (56.4) | 16 (41.0) | −6 | |
| Level 3 | 3 (7.7) | 1 (2.6) | −2 | |
| [0,1–4]Daily activities | .019 | |||
| Level 1 | 4 (10.3) | 14 (35.9) | +10 | |
| Level 2 | 26 (66.7) | 21 (53.9) | −5 | |
| Level 3 | 9 (23.1) | 4 (10.3) | −5 | |
| [0,1–4]Pain/discomfort | .003 | |||
| Level 1 | 3 (7.7) | 6 (15.4) | +3 | |
| Level 2 | 10 (25.7) | 22 (41.0) | +12 | |
| Level 3 | 26 (66.7) | 11 (28.2) | −15 | |
| [0,1–4]Anxiety/depression | .134 | |||
| Level 1 | 10 (25.6) | 18 (46.2) | +8 | |
| Level 2 | 20 (51.3) | 17 (43.6) | −3 | |
| Level 3 | 9 (23.1) | 4 (10.3) | −5 | |
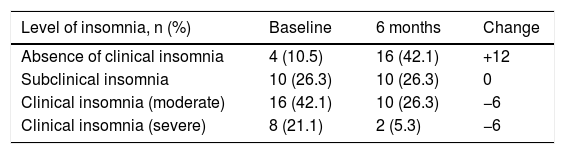
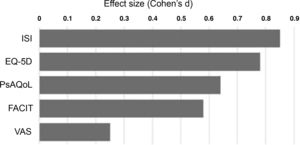
Finally, after 6 months of therapy with secukinumab, a significant change was observed in the variables referring to moderate and severe insomnia, with a notable reduction in both (p = .006) (Table 4). This is the variable where the greatest differences were observed between baseline and after treatment (Fig. 1), followed by self-reported quality of life.
Changes in levels of insomnia after 6 months’ treatment.
| Level of insomnia, n (%) | Baseline | 6 months | Change |
|---|---|---|---|
| Absence of clinical insomnia | 4 (10.5) | 16 (42.1) | +12 |
| Subclinical insomnia | 10 (26.3) | 10 (26.3) | 0 |
| Clinical insomnia (moderate) | 16 (42.1) | 10 (26.3) | −6 |
| Clinical insomnia (severe) | 8 (21.1) | 2 (5.3) | −6 |
Level of significance: p = .006.
Treatment with secukinumab during the 6-month study period in patients with psoriatic arthritis and spondyloarthritis produced a marked improvement in all the treatment effect variables on the different scales studied, with the most notable changes being in sleep, quality of life and fatigue (Fig. 1).18 This generalised improvement was reported in both the patients undergoing treatment with secukinumab in monotherapy and those under combination therapy and in both the patients naïve to biological treatment and refractory to previous biologic therapies. Each of the patient-reported variables was analysed using validated questionnaires with high reliability and internal consistency.2,10 These data are highly relevant, as the patient’s perception of a change in their own health is an important indicator of the success of the treatment.13,16
Current treatment for psoriatic arthritis includes classical antirheumatic drugs such as methotrexate19 as well as a variety of biological drugs such as the anti-TNF agents. However, many patients do not respond to either the classical DMARDs or TNF antagonists, and most of those who initially respond do not achieve complete remission of disease.20–22 Therefore, there remains an unmet need for the treatment of spondyloarthritis and a need for research and development of alternatives to existing therapies.23 The results obtained in our study are of great clinical relevance, therefore, as they seem to suggest that 6 months’ therapy with secukinumab (both in patients who had received previous anti-TNF therapy and those who had not), in addition to improving the objective clinical response data that we use in our routine clinical practice, significantly improves patient’s perceived quality of life, as well as self-reported symptoms of fatigue, insomnia, pain and discomfort.6,24–26
Our study showed a clear improvement in patient-reported quality of life, as well as a reduction in insomnia, pain, and fatigue levels after 6 months of treatment with secukinumab. Because psoriatic arthritis and spondyloarthritis can be debilitating in terms of their impact on patient quality of life and generate psychosocial problems, biological agents such as secukinumab have the potential to replace current therapies due to marked improvements in self-reported quality of life.27
These results are consistent with those reported in pivotal studies of secukinumab. Specifically, in the Future 1 study, secukinumab showed clinically meaningful and sustained improvement over time in the various patient-reported outcome measures (PROMs) studied, including global disease parameters, pain, physical function, and fatigue in subjects with active psoriatic arthritis.18,28,29
Limitations of the study, which would require further testing and population expansion, include the fact that although we initially differentiated patients according to axial or peripheral form (Table 1), we could not take these considerations into account when analysing the study results due to the small sample size. The same considerations apply to the monotherapy and combination therapy criteria. In any case, the study does show that secukinumab showed efficacy after 6 months’ treatment in the key domains of the study, with patients experiencing less fatigue and insomnia and an overall improvement in quality of life.30 In addition, and considering the chronic nature of the disease and the prescribed treatment, longer-term studies of this type could be considered to re-evaluate in routine clinical practice the persistence of the short-term effect shown in relation to the different outcome measures mentioned and reported by the patient.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Garrido JJL, Pérez AG, Torres AP, Cid AR, Almela CM, Cubillo MDP, et al. Estudio prospectivo multicéntrico de experiencia en práctica clínica real en el control de medidas de desenlace reportadas por el paciente (PRO) diagnosticado de artritis psoriásica y/o espondiloartritis y que inicia tratamiento con secukinumab. Reumatol Clin. 2022;18:25–29.