The aim of this study is to describe the findings of a protocolised odontological evaluation of patients with primary Sjögren’s syndrome (pSS) treated in rheumatology units in the Community of Madrid.
MethodsMulticentric descriptive study in which pSS patients classified according to the American-European consensus of 2002 criteria were included. We collected the demographic, clinical and serological data of each patient. A complete oral examination was performed and salivary flow and the CAOD caries index were collected. The patients completed the visual analogue scale for xerostomia, the Oral Health Impact Profile-14 questionnaire and an oral health questionnaire.
ResultsSixty-one patients were recruited. Ninety-eight percent were women and the mean age of the patients was 57 years. Pathological oral signs (unstimulated salivary flow/salivary glands sialography/scintigraphy) were present in 52.5% of the patients, anti-Ro/anti-La were positive in 90.2%. Ninety-two percent of the patients reported xerostomia and 61% of the patients suffered from hyposialia. Thirty-five patients presented oral mucosa lesions. CAOD index was 16.97 ± 7.93 and visual analogue scale for xerostomia was 46.69 ± 14.43. The results of the OHIP-14 questionnaire were 23.13 ± 14.16. Patients with pathological oral signs obtained a significantly higher Oral Health Impact Profile-14 score (P = .03). We also found that patients with peripheral nervous system involvement obtained a significantly higher Oral Health Impact Profile-14 score (P = .001).
ConclusionsThe presence of xerostomia in this cohort of pSS patients was high and hyposialia was present in 61% of the patients. Oral lesions appeared in more than half of the subjects. Oral health had a negative impact on the quality of life of patients with pSS, being higher in those with pathological objective oral signs and in those with peripheral nervous system involvement.
El objetivo de este estudio es describir los hallazgos de una evaluación odontológica protocolizada en pacientes con síndrome de Sjögren primario (SSp) atendidos en las consultas de reumatología de la Comunidad de Madrid.
MétodosEstudio descriptivo multicéntrico en el que se incluyeron pacientes con SSp clasificados según criterios del consenso Europeo-Americano de 2002. Se recogieron datos demográficos, clínicos y serológicos. Se realizó una exploración oral, se recogió el flujo salival y el índice de caries CAOD. Los pacientes rellenaron la escala visual analógica para xerostomía, el cuestionario Oral Health Impact Profile-14 y un cuestionario de salud oral.
ResultadosSe reclutaron 61 pacientes. El 98% fueron mujeres y la edad media fue de 57 años. El 52,5% presentaban signos orales patológicos (flujo salival no estimulado/sialografía/gammagrafía de glándulas salivales) y el 90.2% antiRo/antiLa+. El 92% de los pacientes referían xerostomía y un 61% hiposialia. Treinta y cinco pacientes presentaron lesiones de la mucosa oral. El índice CAOD fue de 16,97 ± 7,93 y la escala visual analógica para la xerostomía fue de 46,69 ± 14,43. Los resultados del Oral Health Impact Profile-14 fueron de 23,13 ± 14,16. Los pacientes con signos orales patológicos obtuvieron una puntuación en el Oral Health Impact Profile-14 significativamente mayor (p = 0,03), al igual que los pacientes con afectación del sistema nervioso periférico p = 0,001.
ConclusionesLa prevalencia de xerostomía en esta cohorte de pacientes con SSp fue muy elevada y el 61% de los enfermos presentaron hiposialia. Más de la mitad de los pacientes sufrieron lesiones orales. La salud oral presentó un impacto negativo en la calidad de vida de los pacientes con SSp, siendo mayor en aquellos pacientes con signos orales objetivos patológicos y en los que presentaban afectación del sistema nervioso periférico.
Primary Sjögren’s syndrome (pSS) is a chronic and progressive systemic autoimmune disease that typically affects the tear and salivary glands, among other organs. Characterístically it causes lymphoplasmacytic glandular infiltration that leads to the progressive destruction of the same, with the resulting reduction in immunomediated secretion and the appearance of ocular dryness (xerophthalmia) and oral dryness (xerostomia), although it also affects other mucus membranes and internal organs.1
Due to its chronic and progressive nature, this disease has a major impact at a physical level as well as socially and psychologically, increasing patient morbidity. The oral dysfunction leads to an alteration in taste, speech and diet, as well as pain, depression, fatigue and sleep alterations caused by the deterioration in the overall state of health of the patient.2
There is currently no standardised protocol for referral and multidisciplinary collaboration in the treatment and management of dry mouth in l patients with pSS. The aim of the EPOX-pSS study was to obtain a more precise and complete characterisation of the oral state of patients with pSS, and the impact of their oral health on their quality of life. We describe the clinical characteristics of dry mouth, its associated complications, accumulated damage, the severity of dry mouth syndrome and patient quality of life in connection with oral health.
Material and methodsDesignA multicentre descriptive study was undertaken of prevalence, in which 13 hospital rheumatology departments in the Community of Madrid took part, together with the Department of Odontological Clinical Specialities of the Odontology Faculty of the Universidad Complutense, Madrid. The recruitment phase took place during the period from June 2015 to March 2017.
PatientsPatients with pSS classified according to the criteria of the European-American (EA) Consensus of 20023 were included, who were treated in the private and public healthcare systems of the Community of Madrid in hospital departments specialising in rheumatology.
Selection criteriaThe inclusion criteria were pSS patients who fulfilled the 2002 EA classification criteria, subjects of age and fully capable of taking part in data gathering, and patients who were able to visit the participating odontology department.
Hospital selection and recruitmentAll of the hospitals with a specialised rheumatology department in the Community of Madrid were invited to take part.
Patient recruitmentThe patients who fulfilled the inclusion criteria were invited to take part.
CodificationA code was assigned to each hospital and to each patient (the consecutive number of patients included in each hospital, preceded by the hospital code).
Data gatheringAn individual was assigned to be in charge of the study in each rheumatology department. They were also responsible for recording the minimum demographic data and basic characteristics of the pSS of the patients. Medical data in connection with pSS were obtained from clinical histories and the interview with the patient during visits.
The rheumatologist gave each patient 2 forms. The first form covered demographic data and the characteristics of the pSS of the recruited and coded patient, recorded by the rheumatologist. The second form contained instructions so that the patient could contact the secretary of the participating Odontology department. This document also included the norms and conditions which patients had to comply with to attend their appointment correctly. At the end of the study the patient was given an oral health report with their results, and they were advised about possible interventions. The patient returned the form the filled out by the rheumatologist for the odontologist in question.
Data recordingAll patient data, those supplied by the rheumatologist as well as the study data, were stored in a single database in the Department of Odontological Clinical Specialities in the Odontology Faculty of the Universidad Complutense, Madrid, under the supervision of the head researcher of the study in this department. Statistical analysis of the data was undertaken using version 22.0 of the SPSS program for Windows. Data protection regulations were followed in this process, maintaining confidentiality according to the current law (RD 1720/2007, which develops Organic Law 15/1999, of 13 December, on Personal Data Protection).
Measurements and variablesThe fundamental aim of this study is to obtain a more precise and complete characterisation of oral manifestations, complications and patient quality of life respecting their oral health, so that no specific outcome variable was specified for this study. Sociodemographic data were included to describe the patients. Information was also collected on different aspects that make it possible to characterise the disease, including the presence of each one of the 2002 classification criteria, the presence of systemic manifestations and some of the most typical serological characteristics of pSS (Table 1). The protocol-governed odontological examination of patients is described in Table 2. NSSF (non-stimulated salivary flow) and SSF (stimulated salivary flow) were recorded based on established norms.4
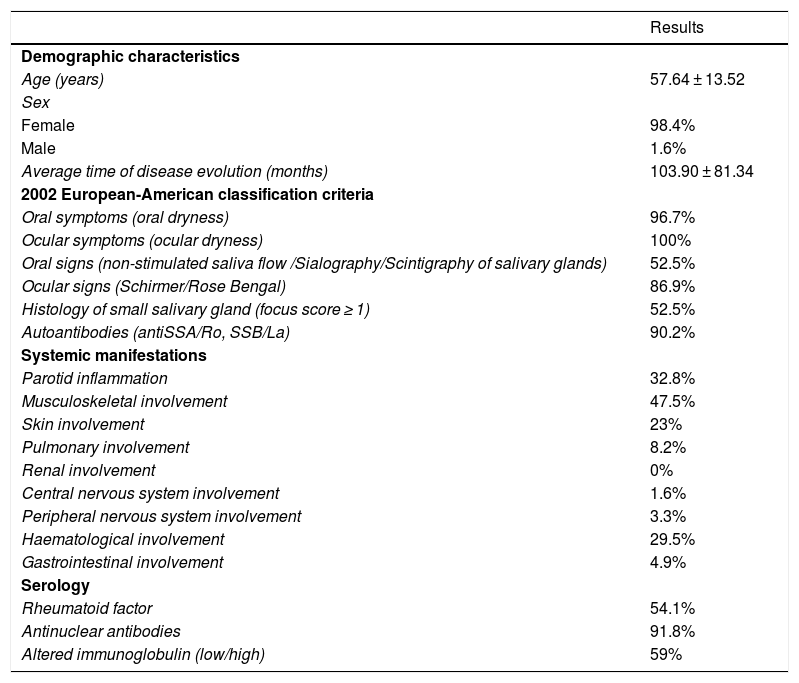
Primary Sjögren syndrome patient characteristics.
| Results | |
|---|---|
| Demographic characteristics | |
| Age (years) | 57.64 ± 13.52 |
| Sex | |
| Female | 98.4% |
| Male | 1.6% |
| Average time of disease evolution (months) | 103.90 ± 81.34 |
| 2002 European-American classification criteria | |
| Oral symptoms (oral dryness) | 96.7% |
| Ocular symptoms (ocular dryness) | 100% |
| Oral signs (non-stimulated saliva flow /Sialography/Scintigraphy of salivary glands) | 52.5% |
| Ocular signs (Schirmer/Rose Bengal) | 86.9% |
| Histology of small salivary gland (focus score ≥ 1) | 52.5% |
| Autoantibodies (antiSSA/Ro, SSB/La) | 90.2% |
| Systemic manifestations | |
| Parotid inflammation | 32.8% |
| Musculoskeletal involvement | 47.5% |
| Skin involvement | 23% |
| Pulmonary involvement | 8.2% |
| Renal involvement | 0% |
| Central nervous system involvement | 1.6% |
| Peripheral nervous system involvement | 3.3% |
| Haematological involvement | 29.5% |
| Gastrointestinal involvement | 4.9% |
| Serology | |
| Rheumatoid factor | 54.1% |
| Antinuclear antibodies | 91.8% |
| Altered immunoglobulin (low/high) | 59% |
Protocol-governed odontological evaluation.
| Results, % pSS | |
|---|---|
| Directed anamnesis | |
| Ingestion of xerostomising drugs (tricyclic antidepressants, anti-Parkinson’s, phenothiazins, benzodiazepines, anticholinergics, antihypertensives, antihistamines, antipsychotics, tranquillisers, anticonvulsants, antiemetics, anorexics, bronchodilators, decongestants, muscle relaxants, narcotic analgesics, antiarthritics, sedatives and diuretics) | 26.7% |
| Presence of a removable prosthesis | 21.3% |
| Feeling of dry mouth | 91.8% |
| Start of the dry mouth feeling (months) | 98.26 ± 102.01 |
| Presence or not of the following symptoms | |
| Glossodynia | 32.8% |
| Dysphagia | 39.3% |
| Dysgeusia | 34.4% |
| Complementary tests | |
| Patients with reduced non-stimulated salivary flow | 60.7% |
| Patients with reduced non-stimulated salivary flow | 55.7% |
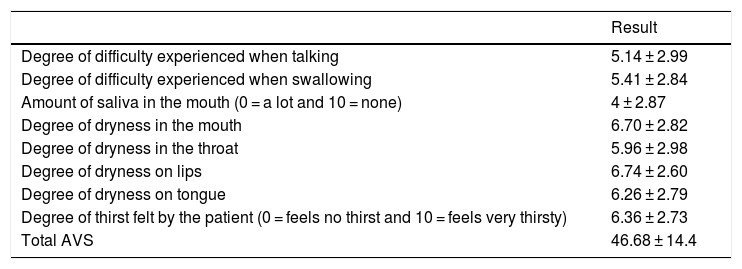
The degree of severity or intensity of the xerostomia perceived by the patient was graded on the analogue visual scale (AVS) for xerostomia, which is composed of 8 items5; patients used this scale to express the discomfort caused by oral dryness: 0 signifies no discomfort and 10 the worst imaginable discomfort. The lines measure 10 cm. The total of the scores of the items may vary from 0 to 80. The scores closest to zero are the ones which are closest to normality, and the closer they are to 10 indicates worse condition of the patient (Table 3).
Analogue visual scale (AVS) for xerostomia.
| Result | |
|---|---|
| Degree of difficulty experienced when talking | 5.14 ± 2.99 |
| Degree of difficulty experienced when swallowing | 5.41 ± 2.84 |
| Amount of saliva in the mouth (0 = a lot and 10 = none) | 4 ± 2.87 |
| Degree of dryness in the mouth | 6.70 ± 2.82 |
| Degree of dryness in the throat | 5.96 ± 2.98 |
| Degree of dryness on lips | 6.74 ± 2.60 |
| Degree of dryness on tongue | 6.26 ± 2.79 |
| Degree of thirst felt by the patient (0 = feels no thirst and 10 = feels very thirsty) | 6.36 ± 2.73 |
| Total AVS | 46.68 ± 14.4 |
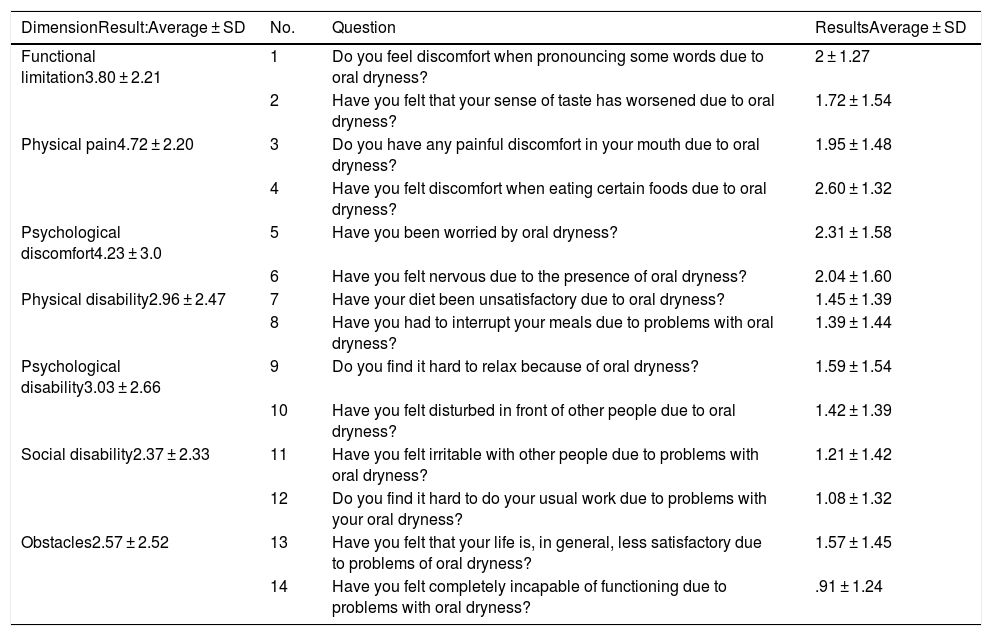
The validated version in Spanish of the Oral Health Impact Profile 14 (OHIP-14) was used to assess impact on quality of life.6 OHIP-14 is a questionnaire in the form of 14 items or questions. Patients have to answer questions according to the frequency with which they occur; to do this they have to assign a score to the 14 situations which are grouped in 7 dimensions (with 2 questions each) denominated functional limitation, physical pain, psychological discomfort, physical, psychological and social disability, and handicap. The answers are coded on a Likert-type 4-point scale where 0 = never, 1 = almost never, 2 = occasionally/sometimes, 3 = often, 4 = very often/almost always. The score for a dimension varies from 0 to 8 (the sum of both items) and the total test score ranges from 0 to 56 (Table 4). In our case this scale was solely applied to the problem which caused xerostomia or the feeling of a dry mouth in their oral health. Scores closest to 0 indicate a higher quality of life, so that quality of life in connection with oral will be worse the closer a score is to 56.
OHIP-14 xerostomia scale.
| DimensionResult:Average ± SD | No. | Question | ResultsAverage ± SD |
|---|---|---|---|
| Functional limitation3.80 ± 2.21 | 1 | Do you feel discomfort when pronouncing some words due to oral dryness? | 2 ± 1.27 |
| 2 | Have you felt that your sense of taste has worsened due to oral dryness? | 1.72 ± 1.54 | |
| Physical pain4.72 ± 2.20 | 3 | Do you have any painful discomfort in your mouth due to oral dryness? | 1.95 ± 1.48 |
| 4 | Have you felt discomfort when eating certain foods due to oral dryness? | 2.60 ± 1.32 | |
| Psychological discomfort4.23 ± 3.0 | 5 | Have you been worried by oral dryness? | 2.31 ± 1.58 |
| 6 | Have you felt nervous due to the presence of oral dryness? | 2.04 ± 1.60 | |
| Physical disability2.96 ± 2.47 | 7 | Have your diet been unsatisfactory due to oral dryness? | 1.45 ± 1.39 |
| 8 | Have you had to interrupt your meals due to problems with oral dryness? | 1.39 ± 1.44 | |
| Psychological disability3.03 ± 2.66 | 9 | Do you find it hard to relax because of oral dryness? | 1.59 ± 1.54 |
| 10 | Have you felt disturbed in front of other people due to oral dryness? | 1.42 ± 1.39 | |
| Social disability2.37 ± 2.33 | 11 | Have you felt irritable with other people due to problems with oral dryness? | 1.21 ± 1.42 |
| 12 | Do you find it hard to do your usual work due to problems with your oral dryness? | 1.08 ± 1.32 | |
| Obstacles2.57 ± 2.52 | 13 | Have you felt that your life is, in general, less satisfactory due to problems of oral dryness? | 1.57 ± 1.45 |
| 14 | Have you felt completely incapable of functioning due to problems with oral dryness? | .91 ± 1.24 |
The test to evaluate habitual oral hygiene, habits connected with oral health and patient xerostomia contained 16 questions (Table 5). The CAF index was calculated, this being the number of teeth with caries (C), absent (A) or filled (F) per individual in the permanent teeth, and the CAOD index, which was the total of all the CAF scores for each individual, divided by the total number of individuals included in the study.7 Only 28 teeth were considered. This index is a fundamental means of quantifying the prevalence of dental problems in a population.
Managing xerostomia.
| Question | Yes (%) | No (%) |
|---|---|---|
| Do you smoke? | 13.1 | 86.9 |
| Do you drink alcohol? | 21.3 | 78.7 |
| Do you rinse your mouth with saline solutions or bicarbonate? | 9.8 | 90.2 |
| Do you brush your teeth at least twice a day? | 90.2 | 9.8 |
| Do you know how to brush your teeth correctly? | 96.7 | 3.3 |
| Do you use fluoride toothpaste? | 80.3 | 19.7 |
| Do you use toothpaste that is specifically for xerostomia? | 31.1 | 67.2 |
| Do you use mouthwash? | 50.8 | 49.2 |
| Do you use specific mouthwashes for xerostomia? | 23 | 77 |
| Do you use dental floss or interproximal brushes? | 50 | 50 |
| Do you eat lots of sweets? | 23.3 | 76.7 |
| Do you drink fizzy drinks or sugared drinks: coca-cola, soft drinks, fruit juices…? | 23.3 | 76.7 |
| Do you visit the dentist regularly (at least once a year? | 73.3 | 26.7 |
| Do you drink at least 2 litres of water every day? | 70 | 30 |
| Do you use chewing gum or sweets? | 55 | 45 |
| Do you use any medication for your oral dryness? | 15 | 85 |
Descriptive statistics was used to detail the clinical and analytical characteristics of the patients. Categorical variables are shown as a number and percentage, and quantitative variables are given as an average ± standard deviation. The student t test was used to study the association between the OHIP-14 index score and the characteristics of the patients with pSS. P values ≤ .05 were considered to be statistically significant. The SPSS 22.0 program for Windows was used for statistical calculations.
Ethical aspectsThis study was undertaken according to the Helsinki Declaration and its subsequent revisions. When the patient fulfilled the selection criteria they were asked to take part in the study, which was explained to them, and they were given the corresponding information sheet. After they had read this information and their questions had been answered, they were requested to complete and sign the informed consent document in duplicate. A copy of this was given to the patient, while the other was kept in the researcher’s hospital. All of the patients signed the informed consent document as an indispensible requisite for their inclusion in the study.
Participating rheumatology departments assigned an identification code to each one of their patients to maintain data confidentiality according to current regulations (RD 1720/2007 which develops Organic Law 15/1999, of 13 December, on Personal Data Protection). The file containing the data identifying the patients is kept under the charge of the researcher in each hospital. Those who processed data and the scientific committee have no access to any data that identifies patients.
The project was approved by the Ethics Committee of the main research hospital, in this case, the Ethics Committee of Hospital de La Paz.
ResultsOf a total of 67 patients recruited in 13 hospitals, 61 who fulfilled all of the inclusion criteria were studied. 52.5% of these patients had pathological oral signs (non-stimulated salivary flow/sialography/scintigraphy of the salivary glands), 90.2% of them were antiRo/antiLa+, 91.8% were ANA + and 54% of the subject had systemic involvement. Demographic data, the variables associated with EA 2002 classification criteria, and the clinical and serological manifestations of the disease are shown in Table 1.
91.8% of the patients described xerostomia, 60.7% of the patients suffered hyposialia confirmed by NSSF and in 55.7% it was confirmed by SSF. Thirty five patients (57.4%) had lesions of the oral mucosa. The most frequent lesion was oral candidiasis (11.5%), and of the lesions associated with candida, 71.3% were candidiasis subprosthesis. 8.2% of the patients had a fissured tongue, and 13.1% had traumatic lesions. The CAOD index was 16.97 ± 7.93. Patient-perceived xerostomia recorded by the xerostomia AVS was 46.68 ± 14.4; the results corresponding to each one of the questions are described in Table 3. Oral health-related quality of life recorded by the total OHIP-14 index was 23.13 ± 13, where the dimensions with the highest scores were physical pain (4.72 ± 2.2) and psychological discomfort (4.23 ± 3). The scores for each dimension are shown in Table 4. The percentage of patients who use each one of the elements for managing xerostomia is shown in Table 5.
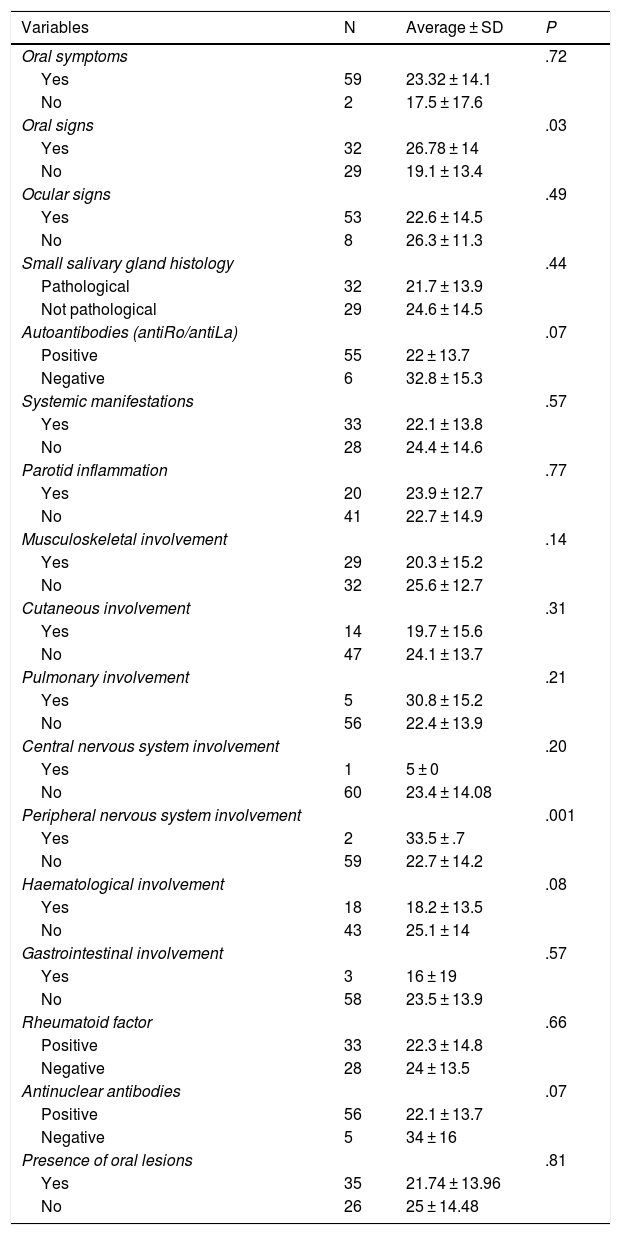
In the association analysis we found that the 32 patients with pathological oral signs obtained an OHIP-14 score significantly higher (26.78 ± 14) than the score for the patients without pathological oral signs (19.1 ± 13.4) (P = .03). This was also the case for both patients with peripheral nervous system involvement vs. those without this alteration (33.5 ± .7 vs. 22.7 ± 14, P = .001). The seronegative patients (6 antiRo/antiLa patients and 5 ANA subjects) tended to have a higher OHIP-14 score than those who were seropositive (antiRo/antiLa 32.8 vs. 22, P = .07; ANA 34 vs. 22.1; P = .07). Although it did not attain statistical significance, the patients with oral lesions obtained an OHIP-14 index score that was lower than that for those without such lesions (21.74 ± 13.96 vs. 25 ± 14.48; P = .81) (Table 6).
OHIP-14 index scores in patients with pSS.
| Variables | N | Average ± SD | P |
|---|---|---|---|
| Oral symptoms | .72 | ||
| Yes | 59 | 23.32 ± 14.1 | |
| No | 2 | 17.5 ± 17.6 | |
| Oral signs | .03 | ||
| Yes | 32 | 26.78 ± 14 | |
| No | 29 | 19.1 ± 13.4 | |
| Ocular signs | .49 | ||
| Yes | 53 | 22.6 ± 14.5 | |
| No | 8 | 26.3 ± 11.3 | |
| Small salivary gland histology | .44 | ||
| Pathological | 32 | 21.7 ± 13.9 | |
| Not pathological | 29 | 24.6 ± 14.5 | |
| Autoantibodies (antiRo/antiLa) | .07 | ||
| Positive | 55 | 22 ± 13.7 | |
| Negative | 6 | 32.8 ± 15.3 | |
| Systemic manifestations | .57 | ||
| Yes | 33 | 22.1 ± 13.8 | |
| No | 28 | 24.4 ± 14.6 | |
| Parotid inflammation | .77 | ||
| Yes | 20 | 23.9 ± 12.7 | |
| No | 41 | 22.7 ± 14.9 | |
| Musculoskeletal involvement | .14 | ||
| Yes | 29 | 20.3 ± 15.2 | |
| No | 32 | 25.6 ± 12.7 | |
| Cutaneous involvement | .31 | ||
| Yes | 14 | 19.7 ± 15.6 | |
| No | 47 | 24.1 ± 13.7 | |
| Pulmonary involvement | .21 | ||
| Yes | 5 | 30.8 ± 15.2 | |
| No | 56 | 22.4 ± 13.9 | |
| Central nervous system involvement | .20 | ||
| Yes | 1 | 5 ± 0 | |
| No | 60 | 23.4 ± 14.08 | |
| Peripheral nervous system involvement | .001 | ||
| Yes | 2 | 33.5 ± .7 | |
| No | 59 | 22.7 ± 14.2 | |
| Haematological involvement | .08 | ||
| Yes | 18 | 18.2 ± 13.5 | |
| No | 43 | 25.1 ± 14 | |
| Gastrointestinal involvement | .57 | ||
| Yes | 3 | 16 ± 19 | |
| No | 58 | 23.5 ± 13.9 | |
| Rheumatoid factor | .66 | ||
| Positive | 33 | 22.3 ± 14.8 | |
| Negative | 28 | 24 ± 13.5 | |
| Antinuclear antibodies | .07 | ||
| Positive | 56 | 22.1 ± 13.7 | |
| Negative | 5 | 34 ± 16 | |
| Presence of oral lesions | .81 | ||
| Yes | 35 | 21.74 ± 13.96 | |
| No | 26 | 25 ± 14.48 |
In this work we present a cohort of pSS patients with xerostomia in a very high percentage of cases (91.8%), hyposialia detected by the NSSF in more than half of the individuals, with a high intensity of oral dryness according to the xerostomia AVS, complications such as glossodynia, dysphagia and dysgeusia in a third of the patients and oral lesions in more than half of the subjects. This group also had a high rate of dental deterioration and a negative impact on quality of life in connection with oral health, as shown in the CAOD and OHIP-14 indices, respectively.
The incidence of xerostomia in the general population is estimated to stand at around 20% in individuals over the age of 60 years old, and it is an important indicator of pSS.8 In patients with pSS the prevalence of xerostomia is far higher than it is in the general population, as is shown by the results of our cohort (91.8%). The intensity of the oral symptoms perceived by patients in this study was detected using an AVS for xerostomia, giving a total average score of 46.69. A recent study published by Fernández-Martínez et al.9 and undertaken in patients with pSS found total AVS scores similar to ours, although somewhat lower (41.5), while the scores in their healthy control group amounted to 10. Other works use different scales to express the degree of xerostomia in patients with pSS, such as the study by Tashbayev et al.10 in Oslo, which evaluated the SXI-D (Summated Xerostomia Inventory-Dutch Version). They included 34 patients with pSS and a control group of 32 healthy patients. They found significantly more severe oral dryness in the patients with pSS compared with the healthy control group (average score: 12.1 vs. 5.94). The CODS (Clinical Oral Dryness Score index) ≥ 5 was reached by 62% of the patients with pSS, compared to 0% of those in the healthy control group.
One of the pSS classification criteria is the reduction in the flow of saliva.3 The majority of experts agree that a NSSF < .1 mL/min. and a SSF < .7 mL/min.11 is abnormal, and these values have been taken into account in this work. More than half of the patients in our cohort had hyposalia (60.7% of NSSF and 55.7% of SSF). The percentage of patients with reduced NSSF in our study is lower than the figures published in other works, such as the one by Enger et al.12 in Norway, who reported a reduced NSSF in 81% of 177 patients whose pSS was evaluated, or the study by López-Jornet and Camacho-Alonso13 in Spain, which found a reduced NSSF in 90.91% of the cases in their cohort (33 patients with pSS and 11 with secondary Sjögren syndrome [SS]). These variations may be due to the time of evolution and treatment being received by the patients for their disease. It is also possible that the time of day saliva flow was measured may have an influence, as this is often not specified in studies.
Saliva has important functions, not only as a lubricant, but also as an agent which protects against infections, caries and alterations to the oral mucosa. Although in itself it is not a disease, xerostomia may also affect patients’ quality of life.14,15 It sometimes does not manifest until 50% of the normal volume of saliva has been lost, and it usually appears when multiple glands are affected. Prolonged oral dryness leads to difficulties in speech, mastication and deglutition,16 reducing the retention of removal removable dentures17 and causing oral lesions. In our cohort 32.8% of the patients had glossodynia, 39.3% had dysphagia and 34.4% has dysgeusia. In the above-mentioned study by Enger et al.,12 49% of the patients described a reduction in their sense of taste, 33.8% had repeated fungal infections and 64% were hoarse. Due to their oral dryness, 35% feared going out to eat dinner during celebrations, 62% said they ate slowly, 85% had to get up at night and 72% mentioned speech difficulty.
We used the OHIP-14 index in our study to measure quality of life in connection with oral health. This index is often used in odontology to evaluate oral alterations and how different odontological treatments influence patient quality of life. It was developed by Slade and Spencer in 199718 to discover whether oral condition is an important part of different aspects of people’s lives, such as social functions, psychological and biological factors, discomfort, dysfunction and disability. In our case the scores amounted to 23.13. Previous studies also used this tool, such as the work by Enger et al.,12 who reported an average OHIP-14 index score of 12.7. These authors also found that patients with pSS and high levels of this index scored less in the 5 SF-36 subscales (general health questionnaires). In 2017 Rusthen et al.19 observed that patients with pSS (31 patients) had a significantly lower smell and taste scores than the controls (33 individuals), greater dysgeusia, ageusia and hypogeusia, anosmia or hypoanosmia, burning tongue and halitosis. The OHIP-14 average was significantly higher in patients with pSS (16.2 vs. 2.7) and it is positively associated with dysgeusia, halitosis and burning tongue, which indicates that oral involvement has a marked impact on pSS patients’ general quality of life. These authors did not analyse, like us, variations in the OHIP-14 score depending on different characteristics of patients with pSS. In our cohort we found a significantly greater negative impact on oral health-associated quality of life measured by OHIP-14 in patients with hyposialia revealed by the NSSF test and/or glandular involvement according to 2002 EA classification criteria in sialography or scintigraphy of the salivary glands. We also found a significantly higher OHIP-14 score in patients with peripheral nervous system involvement.
A series of clinical signs are associated with hyposialia, including the presence of dry cracked lips and a cracked, saburral and erythematous tongue. Other frequent findings are stringy saliva, angular cheilitis, traumatic lesions and aphthae.20 A recent systematic review undertaken by our work group on the prevalence of oral lesions in patients with SS21 concluded that patients with SS suffer more oral lesions than patients without this disease. It was also found that the most common types of oral lesion described in the literature in patients with SS were cheilitis angular, atrophic glossitis, recurring oral ulcers and cracks in the back of the tongue. In our cohort 57.4% of the patients had oral lesions, the most frequent of which were candidiasis, a cracked tongue and traumatic lesions, so that our results agree with those of the majority of studies.
One of the main complications of hyposialia is an increase in the caries index. This is due to the dissolving of hard dental tissue by dental plaque acids. The higher the caries index is shows greater loss of teeth and a larger number of replaced teeth.22,23 The average CAOD of the patients with pSS in our study was 16.97, so that more than half of their teeth were missing, with cavities or replaced, showing a higher degree of dental deterioration. Le Gall et al.24 found a somewhat greater number than ours in 31 patients with pSS. The CAOD in their cohort amounted to 20.3, which was significantly higher than in the control group. The study by Pedersen et al.25 contains a dental evaluation and caries index, with the DMFS index (Decayed, Missing, and Filled Surfaces); the patients with pSS (20 patients) had a significantly higher DMFS score than the healthy controls (20 individuals). Other authors, Olate et al.,26 use the COPD index (the total of permanent teeth with caries, missing or filled, including the said extractions, in the total number of individuals examined). They obtain a score of 7 (which is considered to be high) in their 35 patients with SS. These variations in the CAOD index may be due to time over which the disease has evolved and patient hygiene. If we make comparisons with a healthy adult population, some studies in India and Nepal show how the CAOD varies from 4.49 to 5.1 in populations aged from 20 to 56 years old.27,28
One of the limitations of this work is the size of its sample. Given that it did not reach the expected number, all of the researchers taking part were asked to prolong the period for patient inclusion that had been set. The expected number of patients was not attained after prolonging the said period, either. We believe that the problem with recruitment has been due to the complication it involved for patients, as they had to travel to a hospital other than their own, after having made an appointment themselves on a day other than the date when they visited their rheumatologist. Another limitation of this study is the lack of a record of other comorbidities, such as arterial hypertension or diabetes mellitus, as these may have influenced the appearance of oral alterations such as xerostomia and/or hyposialia and the presence of oral lesions.
ConclusionsIn our cohort of patients with pSS in habitual clinical practice a high proportion of patients were found to have xerostomia (91.8%) and hyposalia was detected in 61% of individuals. Half of the subjects had oral lesions, the most frequent of which were candida infections, alterations to the tongue and traumatic lesions. Normal everyday life was also deteriorated by the appearance of difficulty in speech and in the diet of the patients. The appearance of caries and the loss of teeth gave rise to a high CAOD index. Alteration of oral health in these patients with pSS had a negative impact on their social, psychological and biological functions, as well as on their quality of life, as shown by the OHIP-14. The impact was greater when patients had hyposialia and/or glandular involvement detected by sialography and/or scintigraphy of the salivary glands, and in patients with peripheral nervous system involvement. We therefore believe that the availability of this homogeneous group of patients may facilitate the standardised use of different tools to diagnose and evaluate xerostomia in patients with pSS, as this would lead to greater uniformity in the clinical and therapeutic multidisciplinary management of pSS in odontology and rheumatology departments.
FinancingThis projects was financed by the SORCOM (Sociedad Madrileña de Reumatología)-MSD grant, 2015.
Key points- •
The patients with pSS developed oral lesions and dental deterioration.
- •
Oral health has a negative impact on the quality of life for patients with pSS.
The authors have no conflict of interests to declare.
We would like to thank the Sociedad de Reumatología de la Comunidad de Madrid (SORCOM).
Gonzalo Hernández Vallejo (Departamento de Especialidades Clínicas Odontológicas, Facultad de Odontología, Universidad Complutense de Madrid), M. Ángeles Blázquez (Hospital Severo Ochoa, Madrid), Cristina Bohórquez (Hospital Príncipe de Asturias, Madrid), Gema Bonilla (Hospital La Paz, Madrid), Tatiana Cobo (Hospital Infanta Sofía, Madrid), Jesús García Vadillo (Hospital La Princesa, Madrid), Jorge Juan González Martín (Hospital San Chinarro, Madrid), Oscar Illera (Hospital Infanta Sofía, Madrid), Leticia Lojo (Hospital Infanta Leonor), Francisco Javier López Longo (Hospital Gregorio Marañón), Sheila Melchor (Hospital Doce de Octubre), María Teresa Navío (Hospital Infanta Leonor), Laura Nuño (Hospital La Paz), María Carmen Ortega (Hospital Infanta Elena), Diana Peiteado (Hospital La Paz), Sheila Recuerdo (Hospital Fundación Jiménez Díaz), Patricia Richi (Hospital Infanta Sofía, Madrid), Ana Rodríguez (Hospital Ramón y Cajal, Madrid), Martina Steiner (Hospital Infanta Sofía, Madrid), Marta Valero (Hospital San Chinarro, Madrid).
The researchers of the EPOX-pSS group are listed in Appendix 1.
Please cite this article as: Fernández Castro M, López-Pintor RM, Serrano J, Ramírez L, Sanz M, Andreu JL, et al. Evaluación protocolizada odontológica en el paciente con síndrome de Sjögren primario. Proyecto EPOX-SSp: metodología y objetivos. Reumatol Clin. 2021;17:25–31.