We present the case of a 36-year-old woman with a history of granulomatosis with polyangiitis; chronic kidney disease; systemic arterial hypertension. Debut with dyspnea, weakness, and hemoptysis, she was suspected in atypical pneumonia, discarded, persisting with tachypnea, tachycardia, chest pain. The protocol for pulmonary tuberculosis was started with negative sputum samples, positive blood culture for S. haemolyticus, chest tomography with left pneumothorax and ipsilateral pleural effusion, exudate-type pleural fluid was obtained, acid-fast staining, negative PCR for M. tuberculosis; A follow-up echocardiogram was performed due to a new murmur, reporting valvular vegetation, concluding a diagnosis of pleural tuberculosis and endocarditis as complications of multifactorial origin associated with immunosuppression in granulomatosis with polyangiitis.
Se presenta el caso de femenino de 36 años con antecedentes de granulomatosis con poliangitis; enfermedad renal crónica e hipertensión arterial sistémica. Debutó con disnea, debilidad y hemoptisis, se sospechó en neumonía atípica, descartándose, persistiendo con taquipnea, taquicardia, dolor torácico. Se inició protocolo para tuberculosis pulmonar con muestras de esputo negativas, hemocultivo positivo para S. haemolyticus, tomografía de tórax con neumotórax izquierdo y derrame pleural ipsilateral, se obtuvo liquido pleural tipo exudado, tinción alcohol ácido resistente y PCR para M. tuberculosis negativas; se realizó ecocardiograma de rastreo por soplo de nueva aparición, reportando vegetación valvular, concluyendo diagnóstico de tuberculosis pleural y endocarditis como complicaciones de origen multifactorial asociado a inmunosupresión en granulomatosis con poliangitis.
Granulomatosis accompanied by polyangiitis is a vasculitis of small and medium-sized vessels characterised by periods of remission and relapse, with a typical age of presentation between 35 and 55 years.1 Its aetiology is unknown but it is recognised to be of autoimmune origin and associated with the presence of anti-neutrophil cytoplasmic antibodies (c-ANCA). In histopathological terms, it presents as an inflammatory reaction in blood vessels, affecting the organs supplied by them.2 The symptoms are related to upper respiratory and renal tract involvement, characterised by segmental necrotising glomerulonephritis, making up the classic triad of vasculitis, necrosis, and necrotising inflammation.3 Pulmonary involvement is observed in 50–90% of cases, with alveolar haemorrhage, parenchymal nodules, cavitations, pleurisy and pleural effusion. Cardiovascular signs are less frequent, accounting for 3.3–10% of cases.4,5 The diagnosis of granulomatosis with polyangiitis requires two or more clinical and laboratory criteria, including clear data on inflammation, positive reactants, and compatible images either from radiation or histopathology (Table 1).6 The treatment is based on two phases, an induction phase that aims to quickly minimise inflammation and a maintenance phase to prevent relapses. Both therapies are based on the use of glucocorticoids, the main difference being the dose used and therefore the adverse effects that may occur, especially infectious processes.7 The following article presents a case report on a patient diagnosed with granulomatosis accompanied by polyangiitis who developed pleural tuberculosis and endocarditis as probable complications of multifactorial origin.
Diagnostic criteria for granulomatosis, accompanied by polyangiitis. American College of Rheumatology / European Alliance of Associations for Rheumatology 2022.
| 1. Inflammation or consolidation of the nasal/paranasal sinuses in the images2. Positivity for cytoplasmic neutrophil anticytoplasm or antiproteinase 33. Images of chest with lung nodes, lump, or cavitation4. Granuloma or giant cells in biopsy.A diagnosis is reached on the basis of 2 or more criteria. |
A 36-year-old female with a 12-year history of granulomatosis with polyangiitis treated with prednisone, mycophenolate and rituximab. Chronic kidney disease requiring renal replacement and systemic arterial hypertension. The patient began suffering from high temperature, chest pain, haemoptysis, expectorant cough and wheezing. A detection test for SARS-CoV-2 was negative. Empirical antibiotics were instituted with levofloxacin, and then, in order to continue data collection on the systemic inflammatory response and failure of previous antibiotic treatment, the scheme was scaled to trimethoprim/ sulfamethoxazole and linezolid, assessing allergies referred by the patient, and to vancomycin, cefuroxime and imipenem.
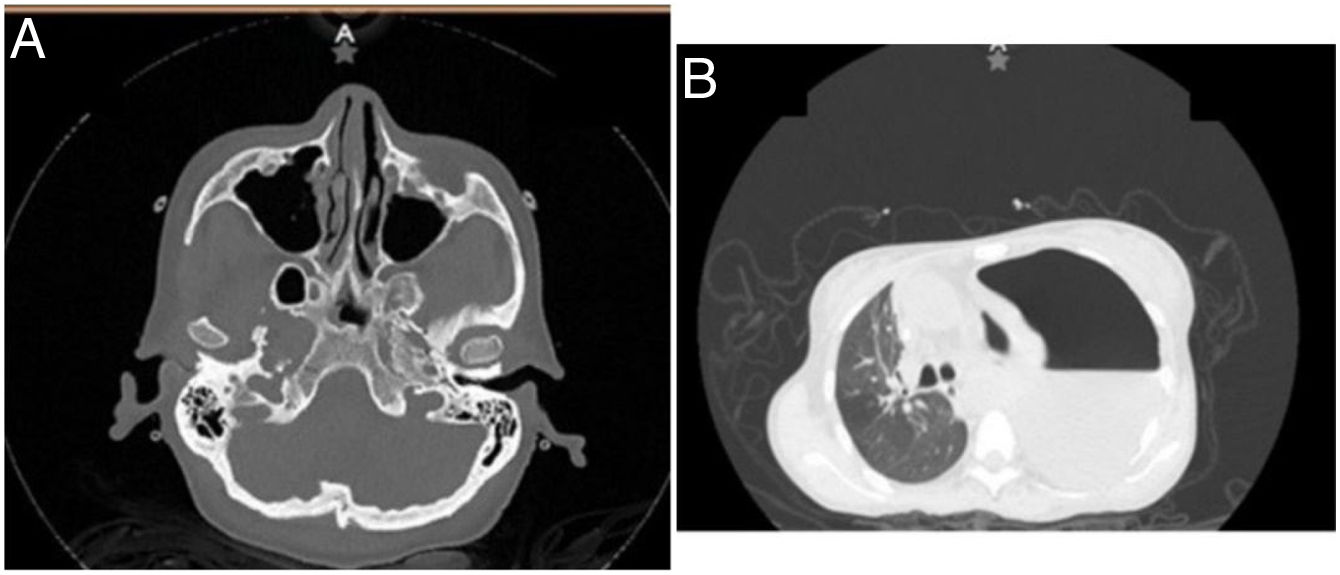
The following haematic biometry paraclinical tests were run: red cells 3,600,000 g/dL; haemoglobin 10 g/dL, haematocrit 30%, mean corpuscular volume (MCV) 82 fL, mean corpuscular haemoglobin (MHC) 27 pg, leukocytes 8.6 k/uL, neutrophils 7.7 k/uL, lymphocytes 0.6 k/uL, creatinine 6.5 mg/dL, blood urea nitrogen (BUN) 40 mg/dL, urea 85.6 mg/dL, serum lactic dehydrogenase (DHL) 188 IU/L, and total serum protein 4.4 g/dL. A simple chest and paranasal sinus tomography scan was run, detecting ethmoidal sinusitis, bilateral maxillary, acute right sphenoidal, septal deviation to the left, frontal sinus hypoplasia, severe left pleural effusion, and tension in the pneumothorax (Fig. 1A and B). For this reason, an endopleural tube was placed to drain off pleural fluid:700 mL with samples for cytochemistry compatible with exudative pleural effusion by DHL 13,933 IU/L. Total proteins were 2.70 g/dL, and glucose 3 mg/dL. The sample was sent to determine adenosine deaminase (ADA) in the face of suspicion of pleural tuberculosis due to high DHL and low glucose. This was reported positive at a rate of 65.05 U/L. Staining of alcohol-acid resistant bacilli (BAAR) was undertaken, plus a PCR for M. tuberculosis in pleural fluid which was negative. Blood culture results were obtained, isolating S. haemolyticus, with reported sensitivity to the empirical approach for up to 28 days. A control chest CT scan was requested, observing an image of cavitation and consolidations compatible with granulomatosis accompanied by polyangiitis. Even with a targeted antibiotic regimen, fever persisted and an aortic murmur was detected. An echocardiogram was requested, finding aortic fungus, which was assessed by the Infectiology Unit, authorising antifungal drugs in the intensive phase and achieving clinical improvement after initiating antituberculosis treatment and targeted antimicrobial therapy.
DiscussionThe clinical manifestations of granulomatosis with polyangiitis were observed visually in upper and, to a lesser extent, lower airways, as well as cardiovascular and renal involvement, without neglecting the complications associated not only with the natural history of the disease itself but also with the underlying immunosuppressive therapy.8
Among the possible associated complications were opportunistic infections such as tuberculosis, which is the leading cause of infectious death in the world, affecting the economically disadvantaged the most, for example; people in developing countries, as well as those with some degree of immunosuppression. In patients infected with M. tuberculosis, extrapulmonary involvement accounts for 25%, with the pleura being the second main site after lymph nodes, with an incidence of pleural involvement of 3–5% in non-endemic areas and up to 30% in endemic areas. The diagnosis of pleural effusion was reached using imaging studies, and the aetiology through direct analysis of the pleural fluid. Chest X-ray and CT scan are the preferred imaging studies for the diagnosis of pleural effusion, with findings detected in about 20–50% of patients and 86%, respectively. Effusions caused by tuberculosis tend to be straw-coloured, with elevated DHL in 75% of cases, high protein concentrations in 55–75% of cases, in addition to low glucose levels and a pH around 7.3. Cytological analysis revealed a predominance of neutrophils as the effusion progressed at an estimated ratio of 3:1 with respect to neutrophils.9
The identification of mycobacteria in pleural fluid is the conventional way to reach the diagnosis; the initial form is alcohol-resistant acid staining such as Ziehl Nielsen, however, its yield is low with 10% of cases detected, increasing to 20% in patients with HIV or empyema, since those patients have a higher bacilli load.9
The gold standard of diagnosis is based on pleural fluid culture (although some literatures indicate that pleural biopsy can also be considered) in the search for M. tuberculosis with a diagnostic specificity of 100% and a low positive rate of 25–37%. This was using the Lowenstein-Jensen medium, while liquid culture mediums such as the BACTEC system presents positive results in 70% of cases. Regarding the analysis of sputum or bronchoalveolar lavage, since this is a bacterium located in the pleura and not in the lung parenchyma, the yield from the culture is minimal, ranging from 0 to 30%.9
Genetic tests are rapid tests (compared to cultures) to detect small amounts of genetic material of M. tuberculosis. The most common method is PCR, which has a general sensitivity that varies between 28 and 81%, with specificity from 91 to 100%. This low sensitivity, compared to other diagnostic tests, is associated with the low number of bacilli in pleural fluid, therefore negative PCR results would not rule out the diagnosis and the measurement of breakdown products associated with their presence. This represents a more reliable technique, at least in the case of this type of tuberculosis presentation.9
Pleural biopsy is an invasive procedure with various complications inherent in its undertaking, such as subcutaneous emphysema, fever or bleeding. The main findings are caseating granulomas and even the same bacteria with an approximate sensitivity of 69–97%, it being important to note that sensitivity increases if this is combined with genetic tests.10,11
A diagnostic method increasingly in use is the ADA, given its high sensitivity of 92%, specificity of 90%, positive predictive value (PPV) of 98% and negative predictive value (NPV) of 98.9%. Cut-off points can vary from 40 to 45 U/L, even 60 U/L, taking into account that the results may be affected by the patient profile and the local prevalence of tuberculosis. In endemic regions, values of 40 U/L or more confer a high PPV, while in areas of low prevalence such as developed countries, values equal to or less than 30 U/L confer a high NPV. Another tool to consider in places of high prevalence is the DHL/ADA ratio, with values less than 15, which points to tuberculous effusion and decreases the probability of a bacterial origin, with sensitivity and specificity of 89% and 84%, respectively, although this does not provide a diagnostic value higher than ADA.7,10,11
The quantification of interferon-gamma (IFN-γ) in pleural fluid provides a sensitivity of 89% and a specificity of 97% in the diagnosis of tuberculous effusions, with values greater than 140 pg/mL and, if combined with high ADA results, the sensitivity approaches 100%.9
Consideration should be given to the possibility of testing for latent tuberculosis infection, the purpose of which is to identify patients who are candidates for pharmacological treatment. The tuberculin test is run intradermally and evaluated at 48–72 hours, with positive results ranging from 5 to 15 mm in diameter in the skin reaction (wheal) produced. Depending on the characteristics of the patient, such as immunosuppression, being an HIV carrier, radiographical evidence, belonging to an endemic area or occupational exposure, sensitivity ranges from 71 to 82%. False negatives may occur in immunosuppressed patients (due to HIV infection or drug therapy) or recent bacterial infection, while false positives may be seen in areas with a high presence of mycobacteria and a history of use of bacillus Calmette and Guérin (BCG). Over the years and due to the increasing limitations of the tuberculin test, the interferon-gamma release assay (IGRA) has been increasingly used. This represents a reflection of the cellular immune response and may present greater sensitivity at the time of reaching the diagnosis, especially with the Quantiferon (81–86%) and T-SPOT (90–95%) methods. This is based on the fact that they are not associated with cross-reaction with the presence of BCG or other mycobacteria, as in the case of tuberculin.12
On the other hand, endocarditis represents an infectious condition at the valvular level in the heart: known risk factors are the use of intravenous drugs, structural congenital alterations and immunodeficiency status. The clinical data that supports the diagnosis are the appearance of further regurgitation, fever, vascular, immunological or rheumatological phenomena, as well as in-house data considered to be major criteria (Duke's) such as positive blood cultures (present in 90% of cases) and echocardiography (sensitivity of 50–90% and specificity of 90%) compatible with typical findings such as fungus, lumps, or regurgitation. The American Heart Association (AHA) points out that the gold standard for diagnosis is the identification of fungus. This is most commonly detected by echocardiography, since it can assess the valvular anatomy as well as the structure and function of the heart. Worthy of special mention is the transoesophageal modality with a sensitivity of 90–100% compared to the transthoracic approach with a sensitivity of 36–69%.13
Various risk factors for the development of multiple infectious processes were found in the patient, such as the underlying diseases which she was a carrier of, such as granulomatosis accompanied by polyangiitis and chronic kidney disease. Hospital stay and therapies due to the comorbidities described above also led to the development of immunosuppression (prolonged use of glucocorticoids and being a carrier of a catheter for haemodialysis). In addition, important to consider are the clinical symptoms mentioned during the description of the case. In this table, the result of ADA was 65.05 U/L, greatly reinforcing the diagnosis of pleural tuberculosis, since this met the cut-off point set in this type of area. Also, to be considered is the fact that currently, in this region of Mexico, the prevalence of tuberculosis is higher than the national average. This is despite a negative PCR result whose sensitivity is not higher than ADA. Other negative results were Ziehl Nielsen staining, both sputum and pleural fluid. With the persistence of fever and the appearance of further heart murmur, this justified the suspicion of endocarditis, evidenced by the well-known gold standard: echocardiography. After initiating targeted antifungal and antibiotic therapy, the patient presented clinical improvement, Monitoring was continued externally by rheumatology, due to granulomatosis accompanied by polyangiitis.
ConclusionsThis case represented a diagnostic challenge as this was a patient with granulomatosis coupled with polyangiitis, revealing pleural tuberculosis and endocarditis jointly. This is an unusual concomitant presentation, since although renal and intrapulmonary complications (especially of the upper airways) were expected. Extrapulmonary complications such as pleural effusion and, even more so, cardiac complications are rare but not non-existent, as this clinical case showed. At the time of reaching a diagnose with the tools available in the region, it was, however, possible to obtain a clinical improvement and the patient was successfully discharged from the hospital.
The nature of these pathologies makes it essential for the clinician to adopt a multifactorial approach, evaluating the symptoms, comorbidities and their natural history, as well as the treatment and the potential effects associated with this, always underlining the fact that it is not diseases that are treated but rather patients.
FundingThe authors declare that they did not receive any funding from public or private institutions to run this study.
Conflict of interestThe authors declare no conflict of interest.