Inflammatory rheumatic diseases usually affect women of childbearing age treated with biologic drugs. However, there is a lack of literature on the efficacy and toxicity of biologic disease-modifying drugs during pregnancy. The aim of this study was to determine the presence of pregnant patients treated with bDMARDs in a real-world dataset and to examine the impact of pregnancy and lactation on the evolution of rheumatic disease in a registry of Spanish patients.
MethodThis was a multicentre prospective study with a real-world setting. Information was obtained from BIOBADASER registry. Patients included are women who got pregnant until November 2020 from 19 rheumatology units. We conducted proportions, means, and standard deviations (SD) to describe the study population and the use of treatments. T-test and Chi-square test were applied to assess differences between groups.
ResultNinety cases of pregnancy were registered (n=68 full-term pregnancies; n=22 spontaneous miscarriages). Most of the cases discontinued bDMARDs during pregnancy (78.9%) but 13 cases continued treatment during pregnancy, mainly using certolizumab pegol. These cases were obtaining better management of rheumatic disease, although the differences were not statistically significant [DAS28-CRP, 2.9 (SD: 1.6) vs. 2.0 (1.2), p=.255; DAS28-ESR, 2.2 (1.0) vs. 1.7 (.5), p=.266]. No serious adverse events were reported during pregnancy and lactation.
ConclusionBeing pregnant is still an uncommon condition in patients with rheumatic diseases and using bDMARDs. Our results show that rheumatic disease tended to progress better during pregnancy in patients who continued to take bDMARDs.
Las enfermedades reumáticas inflamatorias afectan normalmente a mujeres en edad fértil tratadas con fármacos biológicos. Sin embargo, escasea la literatura sobre la eficacia y la toxicidad de los fármacos modificadores de la enfermedad (FAME) biológicos durante el embarazo. El objetivo de este estudio fue determinar la presencia de pacientes embarazadas tratadas con FAME biológicos en un conjunto de datos del mundo real y examinar el impacto del embarazo y la lactancia en la evolución de la enfermedad reumática en un registro de pacientes españoles.
MétodoEstudio prospectivo multicéntrico en un entorno del mundo real. La información se obtuvo del registro BIOBADASER. Los pacientes fueron mujeres embarazadas hasta el mes de noviembre del 2020, de 19 unidades de Rreumatología. Obtuvimos proporciones, medias y desviaciones estándar (DE) para describir la población de estudio y el uso de tratamientos. Se realizaron las pruebas t y χ2 para evaluar las diferencias entre grupos.
ResultadoSe registraron 90 casos de embarazo (n=68 embarazos a término; n=22 abortos espontáneos). La mayoría de los casos suspendieron el tratamiento con FAME biológicos durante el embarazo (78,9%), pero 13 casos prosiguieron el tratamiento durante el embarazo, utilizando principalmente certolizumab pegol. Dichos casos obtuvieron un mejor manejo de la enfermedad reumática, aunque las diferencias no fueron estadísticamente significativas (DAS28-CRP, 2,9 [DE 1,6] vs. 2 [1,2], p=0,255; DAS28-ESR, 2,2 [1] vs. 1,7 [0,5], p=0,266). No se reportaron episodios adversos graves durante el embarazo y la lactancia.
ConclusiónLa situación de embarazo sigue siendo infrecuente en las pacientes con enfermedades reumáticas que utilizan FAME biológicos. Nuestros resultados reflejan que la enfermedad reumática tendió a progresar mejor durante el embarazo en las mujeres tratadas con FAME biológicos.
Inflammatory rheumatic diseases are one of the major diseases caused worldwide.1 They frequently affect women of childbearing age and therefore interfere with family planning.2
The use of disease-modifying antirheumatic drugs (DMARDs) during pregnancy and breastfeeding by women diagnosed with rheumatic diseases warrants investigation.3 The European League Against Rheumatism (EULAR) made several recommendations on the use of these drugs before and during pregnancy and during breastfeeding in women diagnosed with rheumatic diseases.4 Certain DMARDs, including methotrexate and leflunomide, are contraindicated, and treatment with these agents should be discontinued at least 3 months before conception.4 Similarly, the administration of JAK inhibitors should be accompanied by contraception to prevent possible fetal abnormalities and complications during labor.5
Information on biologic DMARDs (bDMARDs) during pregnancy is limited, with tumor necrosis alpha inhibitors (anti-TNF) being the most studied.6 Anti-TNF agents have proven to be relatively safe and were not teratogenic in animal models. In addition, clinical experience shows that they are safe during the first 2 trimesters and do not entail obstetric complications.7 Certolizumab pegol is noteworthy since its composition limits transmission across the placenta and to breast milk. Consequently, 2 anti-TNF agents have been authorized in pregnant women with rheumatic diseases (certolizumab pegol and adalimumab).8 Nevertheless, current obstetric data on bDMARDs continue to be limited, and the long-term progress of the neonate is unknown.9 Given the lack of information and the fact that anti-TNF data cannot be extrapolated to other bDMARDs, women with rheumatic diseases should interrupt therapy with various drugs (e.g., anakinra, abatacept, tocilizumab, rituximab, sarilumab, ustekinumab, ixekizumab, secukinumab, belimumab, and apremilast) during pregnancy.10
Discontinuation of antirheumatic therapy is harmful both for the mother and for the fetus.11 Poor control of inflammatory activity carries a greater risk of maternal and fetal adverse events, including pre-eclampsia, miscarriage, intrauterine growth restriction, preterm birth, low birth weight, and stillbirth.12
Recently, EULAR published a core data set for inclusion in pregnancy registries in rheumatology.13 Particularly remarkable is the European Network of Pregnancy Registries in Rheumatology (EuNeP), which recruited 3500 patients, with data from 2200 pregnancies from 4 European registries (EGR2 [France], RePreg [Switzerland], RevNatus [Norway] and Rhekiss [Germany]), respectively.14 In addition, other specific international registries such as the European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS) or EUROFEVER of autoinflammatory diseases have also performed pharmacotherapeutic follow-up in pregnant women with rheumatic diseases.15,16 While the design of the registries was homogeneous, the variables collected were different. Furthermore, national models of multidisciplinary care are currently being implemented in the pregnancy of patients with rheumatic pathologies to carry out an exhaustive follow-up, reduce neonatal and pregnancy risks, and evaluate the information obtained.17–19 Real-world data on the use of bDMARDs in pregnant women may be useful for orienting treatment and improving knowledge on the safety and effectiveness of these treatments in affected patients.
The objectives of this analysis were to evaluate the presence of pregnant women registered in the BIOBADASER registry, as well as the use of bDMARDs and the impact of pregnancy on the evolution of rheumatic disease in a registry of Spanish patients treated with bDMARDs. We also studied the prevalence of adverse events and miscarriages recorded for the pregnant and breastfeeding patients in the registry.
MethodsStudy designWe performed a real-world multicenter prospective study. Information was obtained from BIOBADASER, a national prospective registry of patients with rheumatic diseases treated with bDMARDs, including biosimilars and targeted synthetic DMARDs, either with approved or off-label indications. BIOBADASER has been collecting patient data continuously since 2000.20
PopulationThe study population included women who became pregnant between 2000 and November 2020 from rheumatology units of Spanish hospitals and remained under active follow-up at the end of the study. Pregnancy was registered in the database as an adverse event.
VariablesWe collected the following data: (1) personal data including sex, date of birth, diagnosis, date of diagnosis, and comorbidities; (2) data on treatment, including types of biologics and dates of initiation and discontinuation, reason for discontinuation, and activity indexes (28-joint Disease Activity Score [DAS28] in rheumatic arthritis [RA] and psoriatic arthritis (PsA) and Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] in spondylarthritis); and (3) data on adverse events, including date of occurrence, type, and classification according to the Medical Dictionary for Regulatory Activities MedDRA, version 19,21 severity, and outcome.
Breastfeeding was also recorded. The delta (Δ) activity index was calculated as remainder of post-pregnancy disease activity minus pre-pregnancy disease activity. Miscarriages and other pregnancy-related problems were recorded as adverse events.
Statistical analysisThe study population was analyzed using descriptive statistics according to the type and distribution of the variables. Proportions means and standard deviations (SD) were applied to describe the study population and the use of treatments. The t-test and chi-squared test was performed to assess differences between groups (discontinuation vs. no discontinuation of bDMARDs after pregnancy). Missing data were reported for each variable when present.
All analyses were performed using Stata version 13.1 (Stata Corp., College Station, TX, USA 2013).
Ethical considerationsEthical approval was granted by the Ethics Committee of the Hospital Clinic of Barcelona (one of the participant centers), which functioned as the reference committee (approval code FER-ADA-2015-01). All patients signed the informed consent document before inclusion.
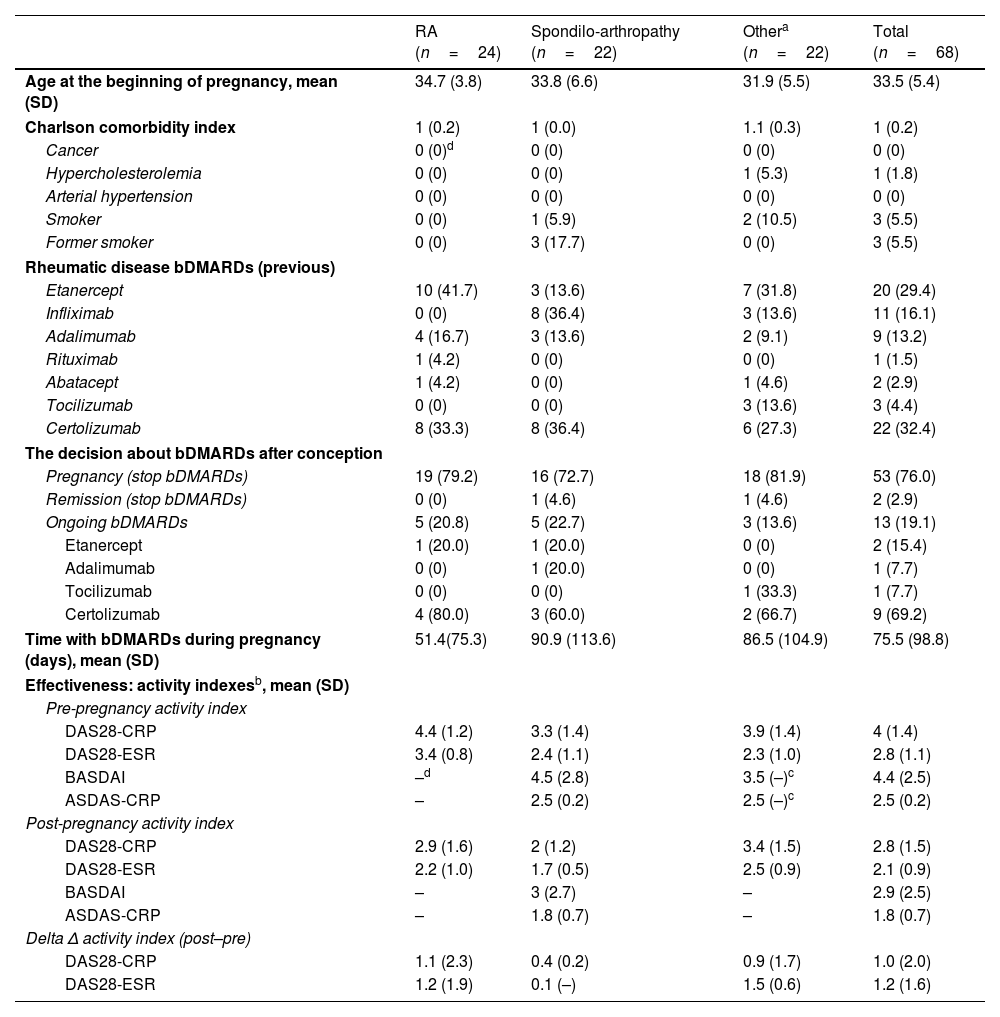
ResultsBaseline characteristics including exposure to the various bDMARDsOf the 727 women of reproductive age in active follow-up included in the analysis, were recorded a total of 90 pregnancies; 24.4% did not reach full term. The diagnoses made among pregnant patients were RA (37.4%) and spondyloarthropathy (PsA and spondyloarthritis, 31.9%); the remaining pregnancies were in women with other inflammatory rheumatic diseases [juvenile idiopathic arthritis (n=8), enteropathic arthritis (n=1), undifferentiated spondyloarthropathy (n=3), Behcet's disease (n=2), Sapho syndrome (n=1), uveitis without rheumatic disease (n=1), seronegative chronic oligoarthritic (n=1), seronegative chronic polyarthritis (n=2), vasculitis (n=2) and psoriasis (n=1); 26.4%]. Conception occurred at a mean age of 33.5 (SD: 5.4) years, and patients diagnosed with RA were older [34.7(3.8) years]. In addition to rheumatic diseases, various comorbid conditions were reported. Six patients were smokers, one had hypercholesterolemia, and one had a history of cancer. The Charlson comorbidity index was 1.0 (0.2) (Table 1).
Patient and clinical features according to rheumatic disease.
| RA (n=24) | Spondilo-arthropathy (n=22) | Othera (n=22) | Total (n=68) | |
|---|---|---|---|---|
| Age at the beginning of pregnancy, mean (SD) | 34.7 (3.8) | 33.8 (6.6) | 31.9 (5.5) | 33.5 (5.4) |
| Charlson comorbidity index | 1 (0.2) | 1 (0.0) | 1.1 (0.3) | 1 (0.2) |
| Cancer | 0 (0)d | 0 (0) | 0 (0) | 0 (0) |
| Hypercholesterolemia | 0 (0) | 0 (0) | 1 (5.3) | 1 (1.8) |
| Arterial hypertension | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Smoker | 0 (0) | 1 (5.9) | 2 (10.5) | 3 (5.5) |
| Former smoker | 0 (0) | 3 (17.7) | 0 (0) | 3 (5.5) |
| Rheumatic disease bDMARDs (previous) | ||||
| Etanercept | 10 (41.7) | 3 (13.6) | 7 (31.8) | 20 (29.4) |
| Infliximab | 0 (0) | 8 (36.4) | 3 (13.6) | 11 (16.1) |
| Adalimumab | 4 (16.7) | 3 (13.6) | 2 (9.1) | 9 (13.2) |
| Rituximab | 1 (4.2) | 0 (0) | 0 (0) | 1 (1.5) |
| Abatacept | 1 (4.2) | 0 (0) | 1 (4.6) | 2 (2.9) |
| Tocilizumab | 0 (0) | 0 (0) | 3 (13.6) | 3 (4.4) |
| Certolizumab | 8 (33.3) | 8 (36.4) | 6 (27.3) | 22 (32.4) |
| The decision about bDMARDs after conception | ||||
| Pregnancy (stop bDMARDs) | 19 (79.2) | 16 (72.7) | 18 (81.9) | 53 (76.0) |
| Remission (stop bDMARDs) | 0 (0) | 1 (4.6) | 1 (4.6) | 2 (2.9) |
| Ongoing bDMARDs | 5 (20.8) | 5 (22.7) | 3 (13.6) | 13 (19.1) |
| Etanercept | 1 (20.0) | 1 (20.0) | 0 (0) | 2 (15.4) |
| Adalimumab | 0 (0) | 1 (20.0) | 0 (0) | 1 (7.7) |
| Tocilizumab | 0 (0) | 0 (0) | 1 (33.3) | 1 (7.7) |
| Certolizumab | 4 (80.0) | 3 (60.0) | 2 (66.7) | 9 (69.2) |
| Time with bDMARDs during pregnancy (days), mean (SD) | 51.4(75.3) | 90.9 (113.6) | 86.5 (104.9) | 75.5 (98.8) |
| Effectiveness: activity indexesb, mean (SD) | ||||
| Pre-pregnancy activity index | ||||
| DAS28-CRP | 4.4 (1.2) | 3.3 (1.4) | 3.9 (1.4) | 4 (1.4) |
| DAS28-ESR | 3.4 (0.8) | 2.4 (1.1) | 2.3 (1.0) | 2.8 (1.1) |
| BASDAI | –d | 4.5 (2.8) | 3.5 (–)c | 4.4 (2.5) |
| ASDAS-CRP | – | 2.5 (0.2) | 2.5 (–)c | 2.5 (0.2) |
| Post-pregnancy activity index | ||||
| DAS28-CRP | 2.9 (1.6) | 2 (1.2) | 3.4 (1.5) | 2.8 (1.5) |
| DAS28-ESR | 2.2 (1.0) | 1.7 (0.5) | 2.5 (0.9) | 2.1 (0.9) |
| BASDAI | – | 3 (2.7) | – | 2.9 (2.5) |
| ASDAS-CRP | – | 1.8 (0.7) | – | 1.8 (0.7) |
| Delta Δ activity index (post–pre) | ||||
| DAS28-CRP | 1.1 (2.3) | 0.4 (0.2) | 0.9 (1.7) | 1.0 (2.0) |
| DAS28-ESR | 1.2 (1.9) | 0.1 (–) | 1.5 (0.6) | 1.2 (1.6) |
RA: rheumatoid arthritis. SD: standard deviation.
Other included: juvenile idiopathic arthritis, enteropathic arthritis, undifferentiated spondyloarthropathy, Behcet's disease, Sapho syndrome, uveitis without rheumatic disease, seronegative chronic oligoarthritic, seronegative chronic polyarthritis, vasculitis, and psoriasis.
Effectiveness activity indexes DAS28-CRP: Disease Activity Score-28 for Rheumatoid Arthritis with CRP; DAS28-ESR: Disease Activity Score-28 for Rheumatoid Arthritis with ESR; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index and ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score with CRP.
The bDMARDs used before conception were mainly anti-TNF agents, the most common being certolizumab pegol (32.4%). A high percentage of patients discontinued treatment due to their desire to start a family, due to pregnancy (53.76%), or disease remission during pregnancy (2.9%). Only 13 patients (19.1%) continued antirheumatic treatment during pregnancy, mainly with certolizumab (69.2%) (Table 2). However, after pregnancy all patients restarted therapy. In most cases, they continued with the treatment they were taking before pregnancy, although those taking abatacept and rituximab replaced these agents with golimumab and baricitinib, respectively. The concomitant drugs being used by pregnant patients were corticosteroids (33.8%), anti-inflammatory medications (22.1%) methotrexate (29.4%), or other DMARDs (11.7%).
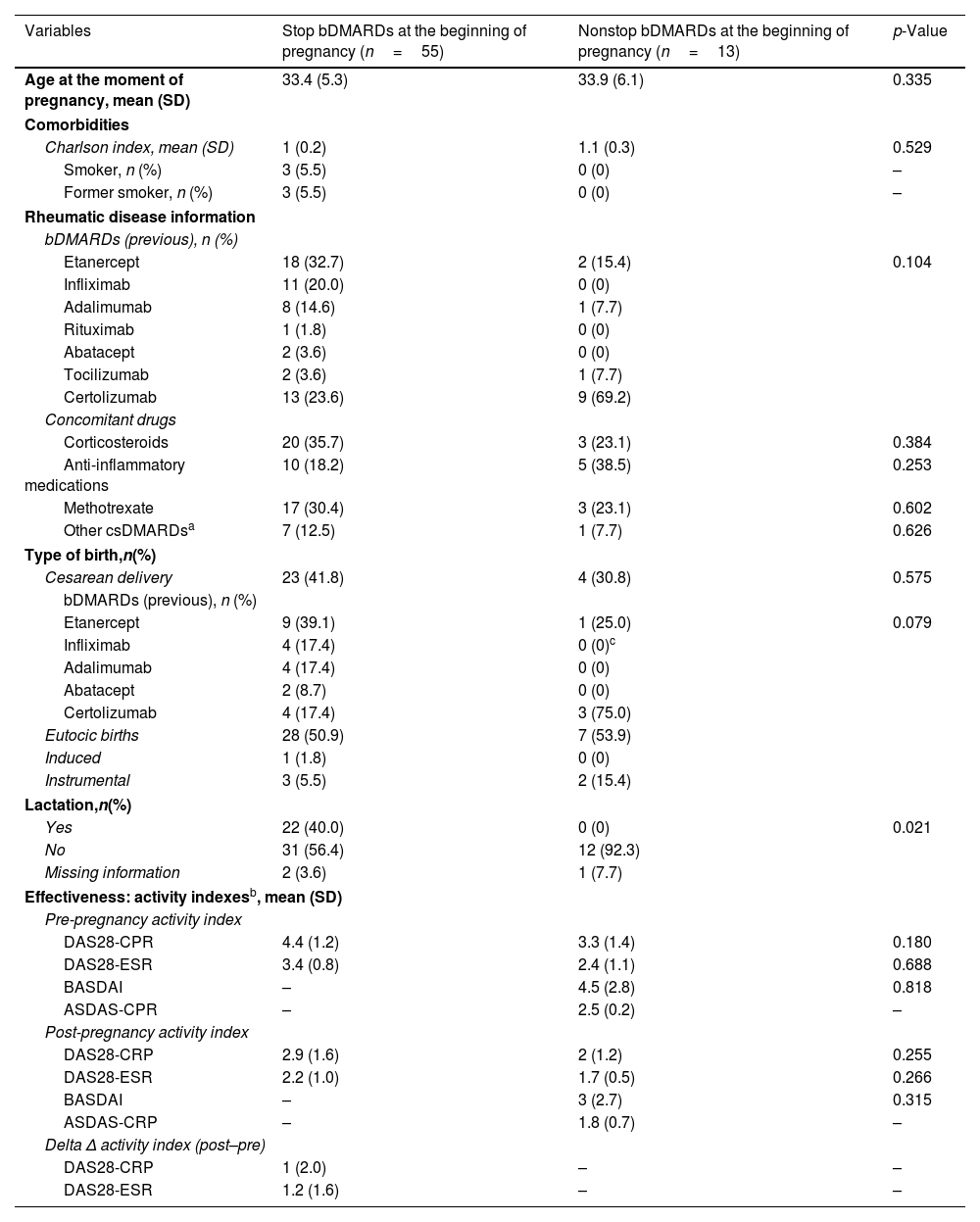
Comparison between cases that continued or interrupted of bDMARDs during pregnancy.
| Variables | Stop bDMARDs at the beginning of pregnancy (n=55) | Nonstop bDMARDs at the beginning of pregnancy (n=13) | p-Value |
|---|---|---|---|
| Age at the moment of pregnancy, mean (SD) | 33.4 (5.3) | 33.9 (6.1) | 0.335 |
| Comorbidities | |||
| Charlson index, mean (SD) | 1 (0.2) | 1.1 (0.3) | 0.529 |
| Smoker, n (%) | 3 (5.5) | 0 (0) | – |
| Former smoker, n (%) | 3 (5.5) | 0 (0) | – |
| Rheumatic disease information | |||
| bDMARDs (previous), n (%) | |||
| Etanercept | 18 (32.7) | 2 (15.4) | 0.104 |
| Infliximab | 11 (20.0) | 0 (0) | |
| Adalimumab | 8 (14.6) | 1 (7.7) | |
| Rituximab | 1 (1.8) | 0 (0) | |
| Abatacept | 2 (3.6) | 0 (0) | |
| Tocilizumab | 2 (3.6) | 1 (7.7) | |
| Certolizumab | 13 (23.6) | 9 (69.2) | |
| Concomitant drugs | |||
| Corticosteroids | 20 (35.7) | 3 (23.1) | 0.384 |
| Anti-inflammatory medications | 10 (18.2) | 5 (38.5) | 0.253 |
| Methotrexate | 17 (30.4) | 3 (23.1) | 0.602 |
| Other csDMARDsa | 7 (12.5) | 1 (7.7) | 0.626 |
| Type of birth,n(%) | |||
| Cesarean delivery | 23 (41.8) | 4 (30.8) | 0.575 |
| bDMARDs (previous), n (%) | |||
| Etanercept | 9 (39.1) | 1 (25.0) | 0.079 |
| Infliximab | 4 (17.4) | 0 (0)c | |
| Adalimumab | 4 (17.4) | 0 (0) | |
| Abatacept | 2 (8.7) | 0 (0) | |
| Certolizumab | 4 (17.4) | 3 (75.0) | |
| Eutocic births | 28 (50.9) | 7 (53.9) | |
| Induced | 1 (1.8) | 0 (0) | |
| Instrumental | 3 (5.5) | 2 (15.4) | |
| Lactation,n(%) | |||
| Yes | 22 (40.0) | 0 (0) | 0.021 |
| No | 31 (56.4) | 12 (92.3) | |
| Missing information | 2 (3.6) | 1 (7.7) | |
| Effectiveness: activity indexesb, mean (SD) | |||
| Pre-pregnancy activity index | |||
| DAS28-CPR | 4.4 (1.2) | 3.3 (1.4) | 0.180 |
| DAS28-ESR | 3.4 (0.8) | 2.4 (1.1) | 0.688 |
| BASDAI | – | 4.5 (2.8) | 0.818 |
| ASDAS-CPR | – | 2.5 (0.2) | – |
| Post-pregnancy activity index | |||
| DAS28-CRP | 2.9 (1.6) | 2 (1.2) | 0.255 |
| DAS28-ESR | 2.2 (1.0) | 1.7 (0.5) | 0.266 |
| BASDAI | – | 3 (2.7) | 0.315 |
| ASDAS-CRP | – | 1.8 (0.7) | – |
| Delta Δ activity index (post–pre) | |||
| DAS28-CRP | 1 (2.0) | – | – |
| DAS28-ESR | 1.2 (1.6) | – | – |
Effectiveness activity indexes DAS28-CRP: Disease Activity Score-28 for Rheumatoid Arthritis with CRP; DAS28-ESR: Disease Activity Score-28 for Rheumatoid Arthritis with ESR; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index and ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score with CRP.
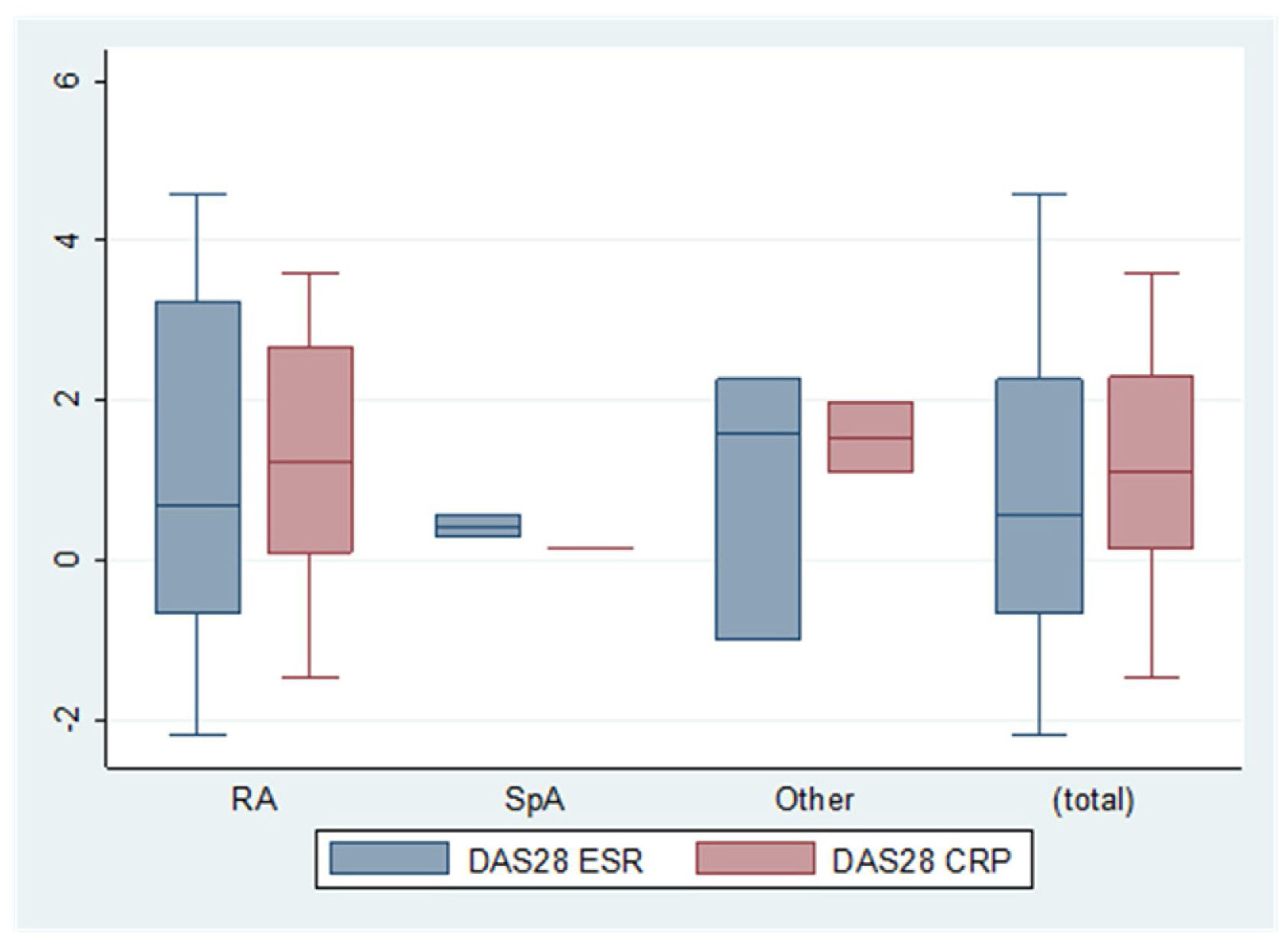
Concerning the difference in efficacy of bDMARDs before and after pregnancy according to the activity indexes, we found a delta activity index for DAS28-CRP (C-reactive protein) of 1.0 (±2.0). Patients who did not interrupt treatment with bDMARDs during pregnancy achieved better results, although the differences were not statistically significant (DAS28-CRP, 2.9 [±1.6] vs. 2.0 [±1.2], p=0.255; DAS28-ESR, 2.2 [±1.0] vs. 1.7 [±0.5], p=0.266) (Fig. 1).
The difference in indexes before and after pregnancy. Delta (Δ) activity indexes for DAS28-ESR, DAS28-CRP. RA: rheumatoid arthritist (n=10; n=6); SpA: spondyloarthritis (n=2; n=1); OTHER: other inflammatory rheumatic diseases [(juvenile idiopathic arthritis, enteropathic arthritis, undifferentiated spondyloarthropathy, Behcet's disease, Sapho syndrome, uveitis without rheumatic disease, seronegative chronic oligoarthritis, seronegative chronic polyarthritis, vasculitis and psoriasis) (n=3; n=2)]. Total (n=15; n=9).
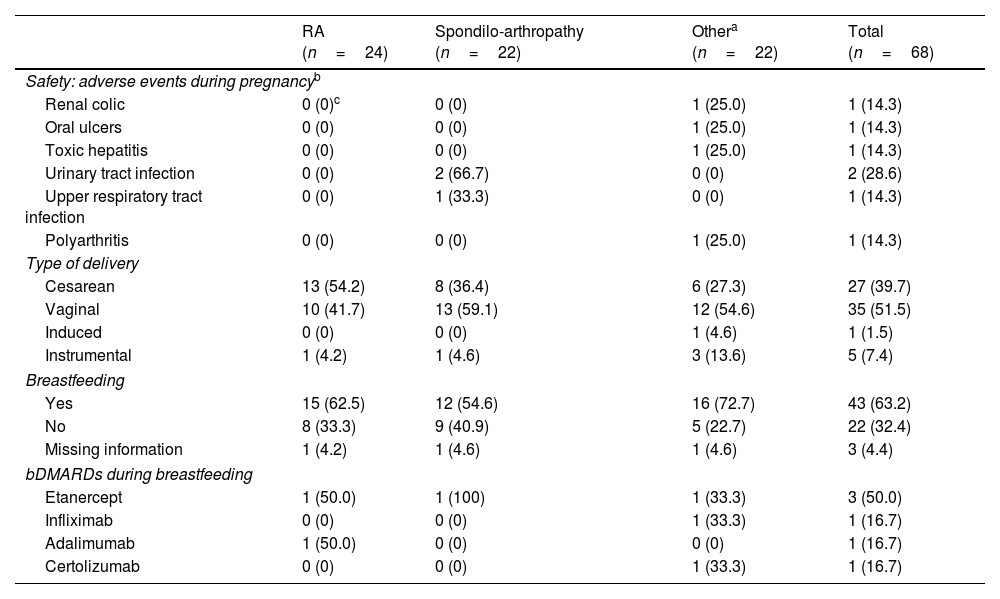
Regarding the safety of bDMARDs during pregnancy, mild adverse events have been observed in patients who did discontinue treatment: urinary tract infections, oral ulcers, upper respiratory tract infection, renal colic, hepatic toxicity and flare of polyarthritis. There were no adverse effects during pregnancy such as preeclampsia, diabetes mellitus, preterm delivery, fetal complications and altered APGAR score (Appearance, Pulse, Grimace, Activity). No serious adverse effects were recorded during pregnancy (Table 3).
Pregnancy outcomes. Safety of bDMARDs and breastfeeding.
| RA (n=24) | Spondilo-arthropathy (n=22) | Othera (n=22) | Total (n=68) | |
|---|---|---|---|---|
| Safety: adverse events during pregnancyb | ||||
| Renal colic | 0 (0)c | 0 (0) | 1 (25.0) | 1 (14.3) |
| Oral ulcers | 0 (0) | 0 (0) | 1 (25.0) | 1 (14.3) |
| Toxic hepatitis | 0 (0) | 0 (0) | 1 (25.0) | 1 (14.3) |
| Urinary tract infection | 0 (0) | 2 (66.7) | 0 (0) | 2 (28.6) |
| Upper respiratory tract infection | 0 (0) | 1 (33.3) | 0 (0) | 1 (14.3) |
| Polyarthritis | 0 (0) | 0 (0) | 1 (25.0) | 1 (14.3) |
| Type of delivery | ||||
| Cesarean | 13 (54.2) | 8 (36.4) | 6 (27.3) | 27 (39.7) |
| Vaginal | 10 (41.7) | 13 (59.1) | 12 (54.6) | 35 (51.5) |
| Induced | 0 (0) | 0 (0) | 1 (4.6) | 1 (1.5) |
| Instrumental | 1 (4.2) | 1 (4.6) | 3 (13.6) | 5 (7.4) |
| Breastfeeding | ||||
| Yes | 15 (62.5) | 12 (54.6) | 16 (72.7) | 43 (63.2) |
| No | 8 (33.3) | 9 (40.9) | 5 (22.7) | 22 (32.4) |
| Missing information | 1 (4.2) | 1 (4.6) | 1 (4.6) | 3 (4.4) |
| bDMARDs during breastfeeding | ||||
| Etanercept | 1 (50.0) | 1 (100) | 1 (33.3) | 3 (50.0) |
| Infliximab | 0 (0) | 0 (0) | 1 (33.3) | 1 (16.7) |
| Adalimumab | 1 (50.0) | 0 (0) | 0 (0) | 1 (16.7) |
| Certolizumab | 0 (0) | 0 (0) | 1 (33.3) | 1 (16.7) |
Despite the safety of bDMARDs mentioned above, a high number of spontaneous abortions (22/90: 24.44%) were recorded in women with rheumatic diseases treated with bDMARDs in the BIOBADASER registry. The diagnoses presented were rheumatoid arthritis (n=10), psoriatic arthritis (n=6), juvenile idiopathic disease (n=2), undifferentiated spondyloarthropathies (n=2), 1 ankylosing spondylitis and another patient had a combination of several diagnoses. Regarding the bDMARDs used were mainly anti-TNF drugs [certolizumab pegol (n=5), etanercept (n=4), adalimumab (n=2) and infliximab (n=1)], and other molecules [golimumab (n=4), tocilizumab (n=2), baricitinib (n=1), ustekinumab (n=1) and rituximab (n=1)]. Six of the patients were naive, the remaining patients had been treated with other bDMARDs previously [second therapeutic line (n=9) or third (n=6) or fourth (n=1)]. Finally, it should be noted that six patients were receiving concomitant methotrexate and 10 were taking corticosteroids.
At full term, most of the 68 patients had a normal delivery, with spontaneous initiation and completion and no complications (i.e., vaginal; 51.5%). Cesarean delivery was necessary in 27 cases and was more common in women diagnosed with rheumatoid arthritis (n=13/27) who had discontinued treatment with bDMARDs (n=23/27). Due to the high rate of cesarean deliveries observed, the type of bDMARDs drug has been specified in Table 2. Table 3 shows the comparison between patients who discontinued bDMARDs and those who continued treatment during pregnancy. Furthermore, 7.4% of deliveries were instrumental, and 1.5% had to be induced.
Breastfeeding and bDMARDsAfter delivery, 63.2% of the patients breastfed their infants during the first's months; 32.4% resorted to artificial lactation with formula. Six patients received anti-TNF agents while breastfeeding, mainly etanercept (50.0%) (Table 3). No mild or severe adverse events were reported during breastfeeding.
DiscussionThe main findings of the present study were as follows: few adverse events were reported for the 90 patients included in the analysis. No serious adverse events were reported; Rheumatic disease tended to progress better during pregnancy in patients who continued to take bDMARDs; the pre-pregnancy treatment used was mostly certolizumab, and patients who discontinued treatment during pregnancy restarted mainly with the same bDMARDs after pregnancy.
Our results are consistent with previous studies. Certolizumab is the reference drug and, consequently, the most frequently used in this type of patient. Previous studies have evaluated the safety of bDMARDs (specifically ustekinumab and vedolizumab), finding that no serious adverse events were reported, as in this study.22 Similarly, several previous studies that have evaluated the activity of rheumatic disease (specifically, juvenile idiopathic arthritis and PsA) during and after pregnancy, found that disease activity was generally low, with no major alterations during pregnancy and diminished activity in patients treated with anti-TNF agents during pregnancy.23,24 However, several studies of patients with rheumatic disease found an association between an increased risk of active disease and pregnancy.25,26 An increase in the use of artificial breastfeeding with formula has been observed concerning other data from the Spanish population.27
Bröms et al.21 also found an increased frequency of cesarean deliveries in women diagnosed with autoimmune diseases such as psoriasis and PsA. Therefore, consistent with other studies, the authors recommend active follow-up of pregnant women diagnosed with rheumatic diseases owing to the greater probability of problems during labor.28–30
Our study has both strengths and limitations. Given the absence of pregnant patients in clinical trials and the limited availability of safety and efficacy data for bDMARDs during pregnancy, our study provides information that could prove useful for daily clinical practice. However, our findings are limited by the number of pregnancies included. Similarly, BIOBADASER is a register of safety in which adverse event reporting is voluntary, with the result that both pregnancies, adverse events, and disease activity data could be under-reported. As part of quality control, the participating investigators were contacted by e-mail and by telephone before this analysis to reinforce the need to report all pregnancies in the patients included in the registry.
ConclusionIn conclusion, our analysis provides new data on the safety of bDMARDs before and during pregnancy in women diagnosed with rheumatic diseases. Our results indicate that the presence of pregnant women with rheumatic diseases and in treatment with bDMARDs is infrequent. Although the findings of this study are limited by the number of pregnancies included in the analysis, our results did not detect serious adverse events in pregnant patients. Continuing biological treatment during pregnancy could have positive effects on the evolution of the rheumatic disease. A greater number of patients and longer follow-up are necessary for more accurate evaluation of the safety of bDMARDs in pregnant women with rheumatic diseases.
Data availabilityThe data that support the findings of this study are available from the Spanish Society of Rheumatology, although availability is subject to restrictions. The data were used under license for the current study and are therefore not publicly available. However, the authors can make data available upon reasonable request and with the permission of the Spanish Society of Rheumatology.
AuthorshipAll named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author contributionsRCC, CMJ, and CS-P designed the study. CS-P and FS-A contributed to data management and statistical analysis. CS-P and CMJ drafted the publication. All the authors performed a critical review of the article.
All the authors contributed to the discussion and interpretation of the results. The final version of the manuscript was read and approved by all the authors.
FundingThis research is supported by the Research Unit of the Spanish Society of Rheumatology. BIOBADASER is supported by the Spanish Agency of Medicines and Medical Devices (AEMPS), Biogen, Bristol-Myers Squibb (BMS), Celltrion, Janssen, Lilly, Merck Sharp and Dohme (MSD), Novartis, Pfizer, Regeneron, Roche, and Samsung Bioepis.
Conflict of interestsThe authors declare that they have no conflict of interest.
We are grateful to all the researchers of the BIOBADASER group for their collaboration. We thank the CRAs Jesus T. Sanchez-Costa and Nuria Montero for their help in BIOBADASER.




![The difference in indexes before and after pregnancy. Delta (Δ) activity indexes for DAS28-ESR, DAS28-CRP. RA: rheumatoid arthritist (n=10; n=6); SpA: spondyloarthritis (n=2; n=1); OTHER: other inflammatory rheumatic diseases [(juvenile idiopathic arthritis, enteropathic arthritis, undifferentiated spondyloarthropathy, Behcet's disease, Sapho syndrome, uveitis without rheumatic disease, seronegative chronic oligoarthritis, seronegative chronic polyarthritis, vasculitis and psoriasis) (n=3; n=2)]. Total (n=15; n=9). The difference in indexes before and after pregnancy. Delta (Δ) activity indexes for DAS28-ESR, DAS28-CRP. RA: rheumatoid arthritist (n=10; n=6); SpA: spondyloarthritis (n=2; n=1); OTHER: other inflammatory rheumatic diseases [(juvenile idiopathic arthritis, enteropathic arthritis, undifferentiated spondyloarthropathy, Behcet's disease, Sapho syndrome, uveitis without rheumatic disease, seronegative chronic oligoarthritis, seronegative chronic polyarthritis, vasculitis and psoriasis) (n=3; n=2)]. Total (n=15; n=9).](https://static.elsevier.es/multimedia/21735743/0000001900000009/v1_202311081440/S2173574323000771/v1_202311081440/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w937trqSwLGgTrQM2QjUSRyU=)


