To investigate whether there is association between the rs20541 (R130Q) polymorphism in the IL-13 gene with disease susceptibility and clinical subsets in patients with elderly-associated inflammatory chronic diseases.
Material and methodsSeventy-eight patients with giant cell arteritis (GCA), 174 with polymyalgia rheumatica (PMR), 90 elderly-onset rheumatoid arthritis (EORA), and 465 healthy controls from the same geographic area were studied. The rs20541 (R130Q) polymorphism in the IL-13 gene was evaluated by PCR-RFLP. Circulating levels of IL-13 were measured by ELISA.
ResultsA higher frequency of the AA genotype [2.349 (0.994–5.554)], as well as the allele A [1.589 (1.085–2.328] and the A carriers [1.656 (1.021–2.686)] (P<.05) was observed in the GCA patients. No significant differences were observed in the PMR and EORA patients as compared with the healthy controls. Neither difference was observed among the different disease groups studied. In GCA patients, differences in the genotype were associated with a worse prognosis. In PMR patients, the AA genotype was associated with higher levels of serum IL-13 than the GA one. However, such an association was not detected for controls and the other disease groups.
ConclusionsGCA is more frequent in carriers of the rs20541 (R130Q) polymorphism in the IL-13 gene. The utility of this polymorphism to predict the GCA prognosis must be confirmed in studies with a higher number of patients.
Investigar si existe asociación del polimorfismo rs20541 (R130Q) del gen de la IL-13 con la susceptibilidad y la expresión clínica de pacientes con enfermedades inflamatorias crónicas asociadas al envejecimiento.
Material y métodosSe estudiaron 78 pacientes con arteritis de células gigantes (ACG), 174 con polimialgia reumática (PMR), 90 con artritis reumatoide de comienzo en el anciano (EORA), y 465 controles sanos de la misma zona geográfica. El polimorfismo rs20541 (R130Q) para IL-13 se evaluó mediante PCR-RFLP. Los niveles de IL-13 circulante se determinaron por ELISA.
ResultadosEn los pacientes con ACG se observó una mayor frecuencia del genotipo AA [2,349 (0,994-5,554)], así como del alelo A [1,589 (1,085-2,328)] y de portadores de dicho alelo [1,656 (1,021-2,686)] (p<0,05). No encontramos diferencias significativas entre los pacientes con PMR y EORA respecto al grupo control. Cuando comparamos las diferentes patologías entre sí, tampoco encontramos diferencias significativas entre ellas. En los pacientes con ACG las diferencias en el genotipo se asociaron con el pronóstico de la enfermedad. En pacientes con PMR, el genotipo AA se asoció con niveles más elevados de IL-13 circulante comparado con el GA. Sin embargo, esta asociación no se apreció para los controles o las otras enfermedades.
ConclusionesLa ACG es más frecuente en individuos portadores del polimorfismo rs20541 (R130Q) del gen de la IL13. La utilidad de este gen para predecir el pronóstico en ACG debe ser confirmada en estudios con mayor número de pacientes.
The aging process is accompanied by qualitative and quantitative changes in the immune system, which are grouped under the term immunesenesence.1 Consequently, the elderly show increased susceptibility to neoplasms, infections, and autoimmune diseases.2 In this sense, aging is accompanied by the appearance of some age-related diseases, and one of the best examples is giant cell arteritis (GCA), a granulomatous systemic vasculitis with a preference for large and medium caliber arteries.3 The clinical manifestations of cranial GCA symptoms ranges from the classic aortic arch syndrome or less specific manifestations such as fever, weight loss or polymyalgia syndrome.4–7 Polymyalgia rheumatica (PMR) is a syndrome characterized by pain and stiffness of the neck, pelvic girdle and also affects only elderly individuals.8 Although PMR occurs normally in the absence of GCA, some patients may also develop arteritis during the disease.9 Moreover, it has been suggested that PMR and rheumatoid arthritis in the elderly (Eora), especially the seronegative variety, have much in common.10
The T cell-derived cytokine profile of inflammatory diseases associated with aging suggests that it is primarily mediated by a Th1 response. This may be due to an increase in Th1 cytokine expression, such as IFN-γ that plays a crucial role in the development of GCA,11 or in a defect in regulation by activated Th2 cytokines.
In recent years there have been numerous studies of cytokine gene polymorphisms in PMR and GCA.12 However, these studies have focused on pro-inflammatory cytokines rather than Th1 and Th2 cytokines. Th2 immune response is characterized by expression of IL-4, IL-5, IL-9, and IL-13. Theoretically a decrease in the activity of these Th2 cytokines may be accompanied by greater severity of these diseases while Th2 increased activity could be associated with a milder clinical picture. There is only one previous study conducted by Amoli et al.13 in which there was a marginally significant association between GCA and some IL-4 single nucleotide genetic polymorphisms (SNPs).
IL-13 is an immunoregulatory protein produced mainly by activated Th2 cells14 and is involved in the maturation and differentiation of B cells. It promotes expression of CD23 and class histocompatibility molecules, IL15 and isotype switching to IgE in B cells.16 Furthermore, IL-13 decreases the activity of macrophages, thus inhibiting production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, among others. Recently, numerous polymorphisms in the gene for IL-13 have been identified and have been associated with IgE levels and/or allergic diseases.17 One of the SNPs located in exon 4 at position 2044 (switch from G to A, denominated G2044A) causes a change of the amino acid (arginine for glutamine at codon 130, denominated R130Q) and possibly affects ligand-receptor interaction.18,19
The aim of this study was to investigate the association between the R130Q polymorphism of the gene for IL-13 and susceptibility to GCA and 2 other inflammatory diseases associated with aging such as PMR and Eora.
Patients and MethodsPatientsThis study included 78 patients with GCA, 174 patients with PMR and 90 patients with Eora (Table 1). The control group included 465 healthy individuals recruited from the same region who showed no evidence of the diseases listed above. Both patients and controls were Caucasians of Spanish ancestry and residing in the same geographic area in northern Spain, Cantabria. The sampling and studies were conducted after obtaining written informed consent and the study was approved by the regional Ethics Committee.
Demographic, Clinical and Laboratory Characteristics of Patients With Giant Cell Arteritis (GCA), Polymyalgia Rheumatica (PMR) and Elderly Onset Rheumatoid Arthritis (EORA) Included in the Study.
| GCA (n=78) | PMR (n=174) | EORA (n=90) | |
| Women, % | 61.5 | 60.3 | 64.4 |
| Age at diagnosis (mean±SD, years) | 73.7±7.5 | 72.9±7.6 | 70.4±7.5 |
| Positive biopsy/total number of temporal artery biopsies performed, % | 62/75 (82.7%) | 0/64 (0%) | 0/2 (0%) |
| Ischemic manifestations, % | 53.3 | None | |
| VESRSG (mean±SD, mm/min) | 86.4±32.9a | 56.6±30.0 | 66.8±34.1 |
| CRP (mean±SD, mg/dl) | 8.7±5.6b | 4.7±5.1 | 6.3±5.2 |
| Follow up time (mean±SD, months) | 65.6±47.4 | 49.8±45.7 | 72.1±69.3 |
SD: standard deviation; CRP: C reactive protein; ESR: erythrosedimentation rate.
All GCA patients fulfilled the 1990 American College of Rheumatology classification criteria for GCA.20 As shown in Table 1, 82.7% of patients had characteristic histological evidence for GCA in temporal artery biopsies.21 Patients with PMR were diagnosed according to the criteria proposed by Chuang et al.8 We also included in the study patients with PMR with ESR <40mm/1h but who met the other clinical criteria.22 Patients with rheumatoid arthritis (RA) met the 1987 ACR criteria for RA.23 Elderly onset RA was considered if symptoms of the disease began at age 60 or older.24 One third of patients with Eora were rheumatoid factor (RF) positive (280.3±509.7IU/ml) and 25% were positive for anti-cyclic citrullinated peptide (APCC) antibodies (1012±623U/ml).
Data was collected by reviewing medical records of PMR patients and gathered clinical features at diagnosis and during follow-up, CRP and ESR at diagnosis and the initial dose of prednisone. The patients were grouped according to the presence or absence of polymyalgia syndrome and the presence or absence of ischemic events. The ischemic events were defined as the presence of visual loss, jaw claudication, stroke, and/or aortic arc syndromes.4,7 In patients with more than 2 years of follow-up we included analysis of variables such as relapse, duration of treatment with corticosteroids (CS), and cumulative dose of prednisone.5,8,25 In patients with GCA and PMR we assessed the severity of the disease in terms of the presence of relapses and/or recurrences, duration of corticosteroid treatment and its cumulative dose. In patients with Eora we did not systematically assess the severity of the disease.
At diagnosis, patients with isolated PMR received an initial dose of prednisone of 10mg/day (5mg/12h). GCA patients received an initial dose of prednisone between 40 and 60mg/day. Those with ischemic complications also received 31g IV pulses of methylprednisolone/day. The reduction in steroid treatment was individualized according to medical judgment.
Genotyping of the R130Q Polymorphism of the IL-13 GeneThe genomic DNA of the individuals studied was extracted from blood using a DNA purification kit (Gentra, GENERATION® DNA Purification kits, MN, USA). The IL13 rs20541 (R130Q) polymorphism18 was determined by gene amplification using 2 primers: sense 5′-CTTCCGTGAGGACTGAATGAGACGGTC-3′ and antisense 5′-GCAAATAATGATGCTTTCGAAGTTTCAGTGGA-3′. The amplification conditions were 4min at 94°C, 1min at 69°C, and 2min at 72°C, and then subjected to 35 cycles of 30s at 94°C, 45s at 67°C, and 30s at 72°C, 5min for extension at 72° C. The PCR products were digested by addition of 0.25U NlaIV and incubation at 37°C for 3.30h. The expected size fragments after digestion in the normal genotype was 210 base pairs (bp) and 26bp, whereas the mutant allele was determined by the presence of 3 bands of 178, 32, and 26bp.
Quantification of Serum IL-13Determining the concentration of IL-13 in serum was performed using ELISA. The serum was collected and stored at −80°C until analysis. Serum levels of IL-13 were determined by an ELISA kit (Pelikine Compact Human IL-13, Sanquin, Amsterdam, The Netherlands). The detection limit was 3pg/ml.
Statistical AnalysisStatistical analysis of data was performed using SPSS 15.0 (Chicago, IL, USA). The association between PMR and GCA or Eora and the alleles or genotypes of the IL-13 gene was estimated using odds ratios (OR) and confidence intervals at 95% (CI). The frequencies of alleles and genotypes were compared using the chi-square test. To assess differences between groups in the case of numerical variables, we used the Mann–Whitney and Kruskal–Wallis tests as indicated. Differences were considered significant when the P value was <.05.
ResultsDemographic and Clinical CharacteristicsThe main demographic, clinical, and laboratory characteristics of different populations are shown in Table 1. Most patients with GCA had a positive temporal artery biopsy. Half of patients had ischemic GCA complications. In addition, patients with GCA had higher levels of ESR and CRP compared with patients with PMR and Eora. We found no statistically significant differences between ESR and CRP between PMR and Eora.
The R130Q Polymorphism of the Gene for IL-13 Is Associated With Susceptibility to Giant Cell ArteritisThe allelic distributions of the study populations were in Hardy–Weinberg equilibrium, except for patients with PMR (P=.025). Allelic and genotypic distributions of the R130Q IL-13 gene polymorphism in different groups of patients compared with controls are shown in Tables 2–4. Patients with GCA showed a higher frequency of AA genotype and allele A and a carrier of this allele compared with the control group (Table 2). There were no significant differences in the distribution of the R130Q polymorphism of the IL-13 gene among patients with PMR or Eora and healthy controls (Tables 3 and 4). When comparing the different diseases with each other, we find no significant difference between them (Supplementary Tables 1, 2, and 3). When analyzing the 3 age-related pathologies together (Supplementary Table 4) we observed a similar trend to that observed in patients with GCA.
Allelic and Genotype Frequencies of the rs20541 (R130Q) Polymorphism of the Il-13 Gene in Patients With Giant Cell Arteritis (GCA) Compared to Healthy Controls.
| Genotype | Control | ACG | P | OR (95% CI) |
| n=465 | n=78 | |||
| IL-13 [db SNP ID rs20541 (R130Q)] | ||||
| Genotype frequency | ||||
| GG | 301/465 (64.7) | 41/78 (52.6) | Reference | |
| GA | 139/465 (29.9) | 29/78 (37.2) | .104 | 1.532 (0.914–2.567) |
| AA | 25/465 (5.4) | 08/78 (10.3) | .046 | 2.349 (0.994–5.554) |
| Allelic frequency | ||||
| G | 741/930 (79.7) | 111/156 (71.2) | Reference | |
| A | 189/930 (20.3) | 45/156 (28.8) | .017 | 1.589 (1.085–2.328) |
| Frequency of allelic carriers | ||||
| Allele G | 440/465 (94.6) | 70/78 (89.7) | .095 | 0.497 (0.216–1.146) |
| Allele A | 164/465 (35.3) | 37/78 (47.4) | .039 | 1.656 (1.021–2.686) |
Allelic and Genotypical Frequencies of the rs20541 (R130Q) Polymorphism of the IL-13 Gene in Patients With Polymyalgia Rheumatica (PMR) Compared to Healthy Controls.
| Genotype | Controls | PMR | P | OR (95% CI) |
| n=465 | n=174 | |||
| IL-13 [db SNP ID rs20541 (R130Q)] | ||||
| Genotype frequency | ||||
| GG | 301/465 (64.7) | 107/174 (61.5) | Reference | |
| GA | 139/465 (29.9) | 52/174 (29.9) | .796 | 1.052 (0.714–1.550) |
| AA | 25/465 (5.4) | 15/174 (8.6) | .126 | 1.688 (0.858–3.322) |
| Allelic frequency | ||||
| G | 741/930 (79.7) | 266/348 (76.4) | Reference | |
| A | 189/930 (20.3) | 82/348 (23.6) | .207 | 1.209 (0.900–1.623) |
| Frequency of allelic carriers | ||||
| Allele G | 440/465 (94.6) | 159/174 (91.4) | .132 | 0.602 (0.310–1.171) |
| Allele A | 164/465 (35.3) | 67/174 (38.5) | .448 | 1.149 (0.802–1.647) |
Allelic and Genotypical Frequencies of the rs20541 (R130Q) Polymorphism of the Il-13 Gene in Patients With Elderly Onset Rheumatoid Arthritis (EORA) Compared to Healthy Controls.
| Genotype | Controls | EORA | P | OR (95% CI) |
| n=465 | n=90 | |||
| IL-13 [db SNP ID rs20541 (R130Q)] | ||||
| Genotype frequency | ||||
| GG | 301/465 (64.7) | 50/90 (55.6) | Reference | |
| GA | 139/465 (29.9) | 36/90 (40.0) | .065 | 1.559 (0.971–2.503) |
| AA | 25/465 (5.4) | 04/90 (4.4) | .947 | 0.963 (0.322–2.885) |
| Allelic frequency | ||||
| G | 741/930 (79.7) | 136/180 (75.6) | Reference | |
| A | 189/930 (20.3) | 44/180 (24.4) | .214 | 1.268 (0.871–1.847) |
| Frequency of allelic carriers | ||||
| Allele G | 440/465 (94.6) | 86/90 (95.6) | .716 | 1.222 (0.415–3.599) |
| Allele A | 164/465 (35.3) | 40/90 (44.4) | .098 | 1.468 (0.929–2.319) |
Supplementary material associated with this article can be found in the online version available at http://dx.doi.org/10.1016/j.reumae.2012.07.003.
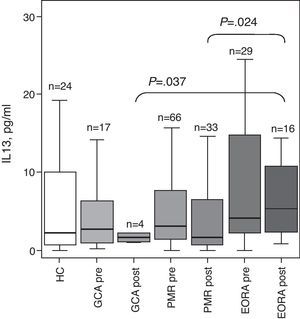
Since the study focuses on the analysis of a polymorphism in the gene for IL-13 we analyzed the differences in the levels of IL-13 in the serum of patients included in the study (Fig. 1). The data show a nonsignificant increase in the concentration of IL-13 concentration in patients with active Eora with respect to healthy controls and a borderline statistical significance (P=.066) in patients with GCA and PMR. After steroid treatment, the levels decreased in all 3 groups of patients although the Eora group's posttreatment levels remained significantly higher than that of GCA and PMR (GCA post vs Eora post P=.037 and PMR post vs Eora post P=.024).
Serum concentration of IL-13 in patients with giant cell arteritis (GCA), polymyalgia rheumatic (PRM) and elderly onset rheumatoid arthritis (EORA) in comparison to healthy controls (HC) paired for age and gender. Results are expressed as boxplots with a median and interquartile range for each group. Data was obtained in the active phase of the disease (pre) and in remission after steroid treatment (post). The number of subjects in each group and the statistical significance when P was under .05 is shown.
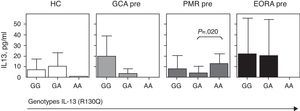
Since the R130Q polymorphism induces a functional amino acid change, we wanted to analyze the impact of this polymorphism in the serum concentration of IL-13 in different patient groups. Since the R130Q polymorphism was very rare, data was not significant, although there was a trend toward decreased levels of serum IL-13 in healthy subjects compared to AA and GG GA. The same trend was also observed in patients with GCA and Eora. In contrast, patients with PMR and the AA genotype showed higher levels of circulating IL-13 (Fig. 2). When we analyzed the 3 groups of patients together there were no significant differences in levels of circulating IL-13 for the different genotypes (Figs. 3 and 4).
Serum concentration of IL-13 in relation to SNP R130Q of the IL-13 gene. Data is presented separately for patients with active giant cell arteritis (GCA; No.=15), polymyalgia rheumatica (PMR; No.=71) and elderly onset rheumatoid arthritis (EORA; No.=28) and healthy controls paired by age and gender (HC; No.=21).
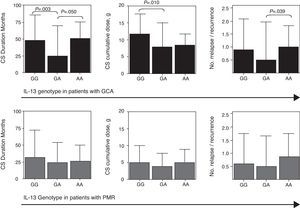
Duration in months of steroid treatment (CS, left), cumulative steroid dose (in middle) and number of relapses (right) in relation to the SNP R130Q of the IL-13 gene. Data is presented separately for active giant cell arteritis (GCA, n=55; superior panel) and polymyalgia rheumatica (PMR, n=120; inferior panel).
Serum IL-13 concentrations in relation to the SNP R130Q of the IL-13 gene. Data is presented jointly for patients with active disease in any of the 3 cases (No. total=114: giant cell arteritis [GCA; No.=15], polymyalgia rheumatica [PMR; No.=71] and elderly onset rheumatoid arthritis [EORA; No.=28]).
To evaluate the influence of both the genotype of IL-13 as studied and circulating levels of IL-13 in different clinical subgroups, we analyzed patients with GCA according to presence or absence of ischemic signs or polymyalgia syndrome and the PMR patients according to ESR levels (“low” or less than 40mm/1sth and “classic” or greater than 40mm/1sth), and patients were subdivided by the polymyalgic Eora onset or classic form. Although we did not have detailed information on the activity and prognosis of patients with Eora, we also analyzed the possible influence of genotype on the presence of typical autoantibodies (RF and ACCP) and their titer. We found no significant association between genotype and circulating levels of IL-13 with any of the above variables.
We evaluated the prognosis in patients with GCA and PMR by analyzing more than 2 years of follow up for the presence of relapses and/or recurrences, duration of corticosteroid treatment and its cumulative dose. As shown in Fig. 3 (top panel), patients with GCA, the GA genotype was associated with a shorter duration of treatment with corticosteroids and a lower cumulative dose of the same, probably due to a lower rate of relapse/recurrence. We found no relationship of these prognostic factors with circulating levels of IL-13.
DiscussionIL-13 is an immunoregulatory protein produced mainly by activated Th2 cells14 and is involved in the maturation and differentiation of B cells.15 This cytokine is considered part of the Th2 immune response and a reasonable hypothesis is that the imbalance in the Th1/Th2 balance could be involved in the pathogenesis of chronic diseases associated with age and are the result of a dysfunction in immune system regulation. According to this hypothesis, a genetic predisposition in the expression of genes coding for Th2 cytokines could influence the severity and/or susceptibility of these 3 diseases. There is only one previous study conducted by Amoli et al.13 in which there was a marginally significant association between GCA and some SNPs for IL-4. However, these results were more significant when considering only those patients HLA-DRB1*04, suggesting an interaction between HLA-DRB1 and IL-4 contributing to increased susceptibility for the disease.13
Therefore, taking into consideration the failure of previous studies in determining the influence of Th2 cytokine genes, we analyzed for the first time the influence of the rs20541 (R130Q) polymorphism in the gene encoding the IL-13 in a large series of patients with inflammatory diseases associated with aging.
The data shows an association of this polymorphism with susceptibility to GCA. Although the differences found in this study are significant only for patients with GCA, PMR patients show a similar trend. However, the study's statistical power for comparisons in allele frequencies and genotype of patients with PMR is low, ranging between 12% and 34%, so the results should be taken with caution and confirmed in studies containing a higher number of patients. Although this study shows a significant association between the analyzed polymorphism and GCA we cannot exclude that other polymorphisms within this gene or its receptor may be associated with susceptibility and/or severity of these diseases. In fact, the polymorphism studied in this work is more clearly associated with asthma and atopy, especially in children. In contrast, the association of other polymorphisms in the gene for IL-13, such as rs20541 (R130Q), rs1800925 (IL13-1055), and rs2243204 have been described in other inflammatory rheumatic disease, which we did not evaluate.26–28
Moreover, SNPs may have different biological effects depending on the cell type upon which the cytokine acts or the inflammatory environment in which the effect develops.29 In this regard, functional studies conducted in the rs20541 (R130Q) SNP have demonstrated functional consequences on transcriptional activity, increased activity or signaling through its protein, or changes in serum protein levels in different cell types.30,31 Therefore, in this study we not only investigated the possible genetic association between the R130Q polymorphism and genetic susceptibility to disease but also its correlation with circulating levels of IL-13. It has been suggested that patients homozygous for the mutation of this IL-13 gene exhibit increased levels of circulating IL-1332. It appears that the presence of the AA genotype is associated with decreased levels of serum IL-13 in both healthy controls and in patients with GCA and Eora. In contrast, in PMR this relationship is the opposite, with a higher level of circulating IL-13 in heterozygous subjects, which corresponds to what is published.32 In any case, this data should be taken with caution because of the low frequency of this genotype and the absence of similar studies.
Serum and synovial fluid IL-13 have been found increased in patients with various types of arthropathies, including RA.32,33 In addition, serum levels of IL-13 have been correlated with the presence of autoantibodies, such as RF.33 In our study, although no significant differences with healthy controls were seen, patients with Eora had a tendency to have higher circulating levels of IL-13, which remained despite treatment. Although we found a significant correlation between levels of circulating IL-13 and autoantibody characteristic of RA, our study clearly differs from the above33 in regards to the study population, Eora only, and therefore on the frequency of these autoantibodies.
The association of the GA genotype with a lower rate of relapse/recurrence, and therefore with a shorter duration and cumulative dose of corticosteroid treatment in patients with GCA is of interest given the current lack of prognostic markers for this disease, but should be confirmed in a larger series of patients.
In summary, the rs20541 (R130Q) polymorphism of the IL13 gene is associated with susceptibility to GCA. The utility of this gene to predict prognosis in GCA should be confirmed in studies with more patients. Further studies are needed regarding Th2 cytokine genes that allow us to clarify this issue.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
FinancingThis study has been financed with grants from the Fundación Marqués de Valdecilla-IFIMAV and the Fondo de Investigación Sanitaria (PI050475 and PI080098). Lorena Álvarez-Rodríguez was financed with a research grant from the Fundación Marqués de Valdecilla-IFIMAV. Iñaki Beares and Marta González (financed by the Fundación Marqués de Valdecilla-IFIMAV) and Carolina Santa Cruz (financed through a research grant from Schering-Ploug, Spain).
Conflict of InterestNone.
We are especially grateful to Iñaki Beares and Marta González (financed by the Fundación Marqués de Valdecilla-IFIMAV) and Carolina Santa Cruz (financed through a research grant from Schering-Ploug, Spain) for their technical advice. We would also like to thank all of the physicians from the Rheumatology Department who have monitored patients and thank both patients and controls for their participation in the study.
Please cite this article as: Álvarez-Rodríguez L, et al. Análisis del polimorfismo rs20541 (R130Q) del gen de la IL-13 en pacientes con enfermedades inflamatorias crónicas asociadas al envejecimiento. Reumatol Clin. 2012;8:321–7.














![Serum IL-13 concentrations in relation to the SNP R130Q of the IL-13 gene. Data is presented jointly for patients with active disease in any of the 3 cases (No. total=114: giant cell arteritis [GCA; No.=15], polymyalgia rheumatica [PMR; No.=71] and elderly onset rheumatoid arthritis [EORA; No.=28]). Serum IL-13 concentrations in relation to the SNP R130Q of the IL-13 gene. Data is presented jointly for patients with active disease in any of the 3 cases (No. total=114: giant cell arteritis [GCA; No.=15], polymyalgia rheumatica [PMR; No.=71] and elderly onset rheumatoid arthritis [EORA; No.=28]).](https://static.elsevier.es/multimedia/21735743/0000000800000006/v1_201305061648/S2173574312000962/v1_201305061648/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w937trqSwLGgTrQM2QjUSRyU=)



