Biological therapy has changed the course of inflammatory rheumatic diseases. The safety is well documented in national and international studies. Neurological manifestations are uncommon and it is difficult to establish a clear causal relationship. The neurological signs and symptoms that may appear are multiple and sometimes mimic demyelinating neurological diseases and/or neurodegenerative diseases. Knowledge and disclosure of these cases is essential for a comprehensive management of biological therapy in our patients.
La terapia biológica ha cambiado el curso de las enfermedades reumáticas inflamatorias. La seguridad de la misma está más que documentada en diferentes estudios nacionales e internacionales. La baja frecuencia de las manifestaciones neurológicas dificulta en muchas ocasiones el establecer una relación causal clara entre el tratamiento biológico y la clínica neurológica propiamente dicha. Los síntomas y signos neurológicos que pueden aparecer son múltiples, y en ocasiones simulan enfermedades neurológicas desmielinizantes y/o neurodegenerativas. El conocimiento y el reporte de los mismos es fundamental para realizar un manejo exhaustivo de la terapia biológica en nuestros pacientes.
Since the commencement of the age of biological treatments, the prognosis of most inflammatory rheumatic diseases has improved significantly. Both the drugs that inhibit the proinflammatory cytokine, tumor necrosis factor alpha (TNFα), and those that inhibit other interleukins (IL) and cells that participate in the mechanism of inflammation, have definitively changed the course of many chronic rheumatic diseases.
At present, the safety of these drugs over the intermediate and long term is well-documented in different national and international studies, based mostly on patient registries that are followed prospectively. The outcomes in some of them are divergent, since the sample to be studied, the data collection and even the analysis, are far from being similar. However, in general, all coincide in that infections and hypersensitivity reactions are the most common adverse effects.
The biological drugs currently available in Spain are as follows1,2:
- 1.
Drugs that act as TNFα inhibitors and can block the molecule itself (infliximab, adalimumab, golimumab, certolizumab) or its receptor (etanercept).
- 2.
Drugs that inhibit other IL: tocilizumab, inhibits IL-6, which participates in the inflammatory mechanisms and joint destruction; anakinra, which blocks the activity of IL-1 by competitively inhibiting its binding to the receptor IL-1R1; and ustekinumab (IgG1kappa monoclonal antibody, that inhibits IL-12/IL-23).
- 3.
Drugs that interfere with the activity of certain cell lines: abatacept (which inhibits binding of CD28 and CD80, blocking the costimulatory signal of T cells); rituximab (depletion of CD20-positive B cells).
In this review, we will focus mainly on anti-TNFα drugs since there are nearly no cases of neurological involvement with the remainder, probably, among other things, because they have been available for less time. These neurological manifestations are uncommon during treatment with biological drugs, but this does not mean that they are less important. They can also be irreversible.
Since these agents are being utilized, a number of isolated events and series of cases have been published, although it is still hard to establish a definite cause-and-effect relationship.3–5 The neurological finding most widely described in the literature is the demyelination of the central and/or peripheral nervous system, but others have also been reported: optic neuritis, acute/chronic inflammatory polyneuropathy, mononeuritis multiplex and Guillain-Barré syndrome, among others.6–9 All of the guidelines on the use of biological therapy contraindicate their administration to patients with multiple sclerosis, and precaution when there is a family history of the disease.
However, the debate on whether biological treatment can disguise the development of a preexisting demyelinating disease, such as multiple sclerosis or, on the other hand, is responsible for inducing de novo demyelination in the central and/or peripheral nervous system.9
EpidemiologyIn initial studies, it was reported that the risk of developing a demyelinating disease increased by 30% with the use of biological therapy.10 According to the Spanish registry of adverse events in biological therapies in rheumatic diseases (BIOBADASER), the incidence is low, between 0.3 and 0.6 for each 1,000 person-years of exposure. In BIOBADASER, after a follow-up of 9256 patients (21,425 person-years), 9 cases were reported, meaning that the rate of demyelinating disease in patients treated with anti-TNFα was not greater than that expected in the general population.3 The cases of demyelinating disease were more frequent among patients of more advanced age, men and those diagnosed as having psoriatic arthritis, although in no case were the differences statistically significant.8
PathogenesisThe mechanism that leads a patient to be predisposed to develop a demyelinating disease or experience an exacerbation of that disease once the biological treatment has begun is unknown.
A number of hypotheses that have been proposed suggest that TNFα has a major role in the pathogenesis of multiple sclerosis.11 It is a proinflammatory cytokine that participates in the acute phase of the disease and in the demyelinating process. On the other hand, TNFα also has immunosuppressive properties in the second phase of the disease. These properties are related to the TNFα receptors (TNFR1 and TNFR2), which measure different biological responses to TNFα itself.
In the central nervous system, TNFα is produced by microglia, astrocytes and other cells, such as the monomer precursor transmembrane protein (tmTNF). The TNFα converting enzyme disassociates from the cytoplasmic tail and releases soluble forms of TNFα (sTNF). To carry out its biological functions, the monomeric forms of tmTNF and sTNF should aggregate and form homodimers. Both TNF (tmTNF and sTNF) can bind to both TNFR1 and TNFR2; sTNF has a greater affinity to TNFR1, producing the inflammatory response and apoptosis. tmTNF binds mostly to TNFR2 and promotes cell activation and survival. Transgenic mice have been used to study how the isolated expression of tmTNF can avoid and suppress the progression of experimental autoimmune encephalitis, as it also maintains self-tolerance and resistance to infection. Thus, selective inhibition of the sTNF/TNFR1 signal could be used as a strategy to prevent relapses in multiple sclerosis.11
Taking this theory into account, 4 major hypotheses have been postulated to explain a potential biological relationship between TNF antagonists and demyelinating disease:
- a)
Anti-TNFα do not cross the blood-brain barrier, but they enhance the activity through an increase in the autoreactive peripheral T cells, that can penetrate the central nervous system.12
- b)
The necessarily reduced regulation of TNFR2 for the proliferation of oligodendrocytes and repair of the damage.12,13
- c)
Reduced regulation and production of cytokines like IL-10, and overproduction and regulation of IL-12 and interferon γ (IFN-γ) associated with the demyelinating process.14,15
- d)
Anti-TNFα could camouflage a latent infection that, in turn, could be critical to initiate a demyelinating autoimmune process.16
Kaltsonoudis et al.9 conducted a prospective study in 77 patients with inflammatory rheumatic disease (36 patients with rheumatoid arthritis, 24 patients with psoriatic arthritis and 17 patients with ankylosing spondylitis) who began with anti-TNFα (infliximab, adalimumab, etanercept). All underwent a complete neurological examination, nuclear magnetic resonance of the brain and the entire spine, and a neurophysiology study before and more than 18 months after starting the biological therapy. In the initial scrutiny, they detected lesions compatible with demyelinating disease in magnetic resonance in 2 patients and, thus, decided against the biological treatment. At the end of the study, 4% of the patients (3/75) showed evidence of neurological involvement: peripheral demyelinating neuropathy (2 patients) and optic neuritis. In each case, the biological therapy was interrupted and the neurological disease was treated. The neurological symptoms remitted in all the patients.9 The authors stress the importance of magnetic resonance and the neurophysiological study for the early detection of neurological involvement in these patients; however, to perform these ancillary tests in every patient who begins biological therapy would significantly increase the expense and would probably not be cost-effective.
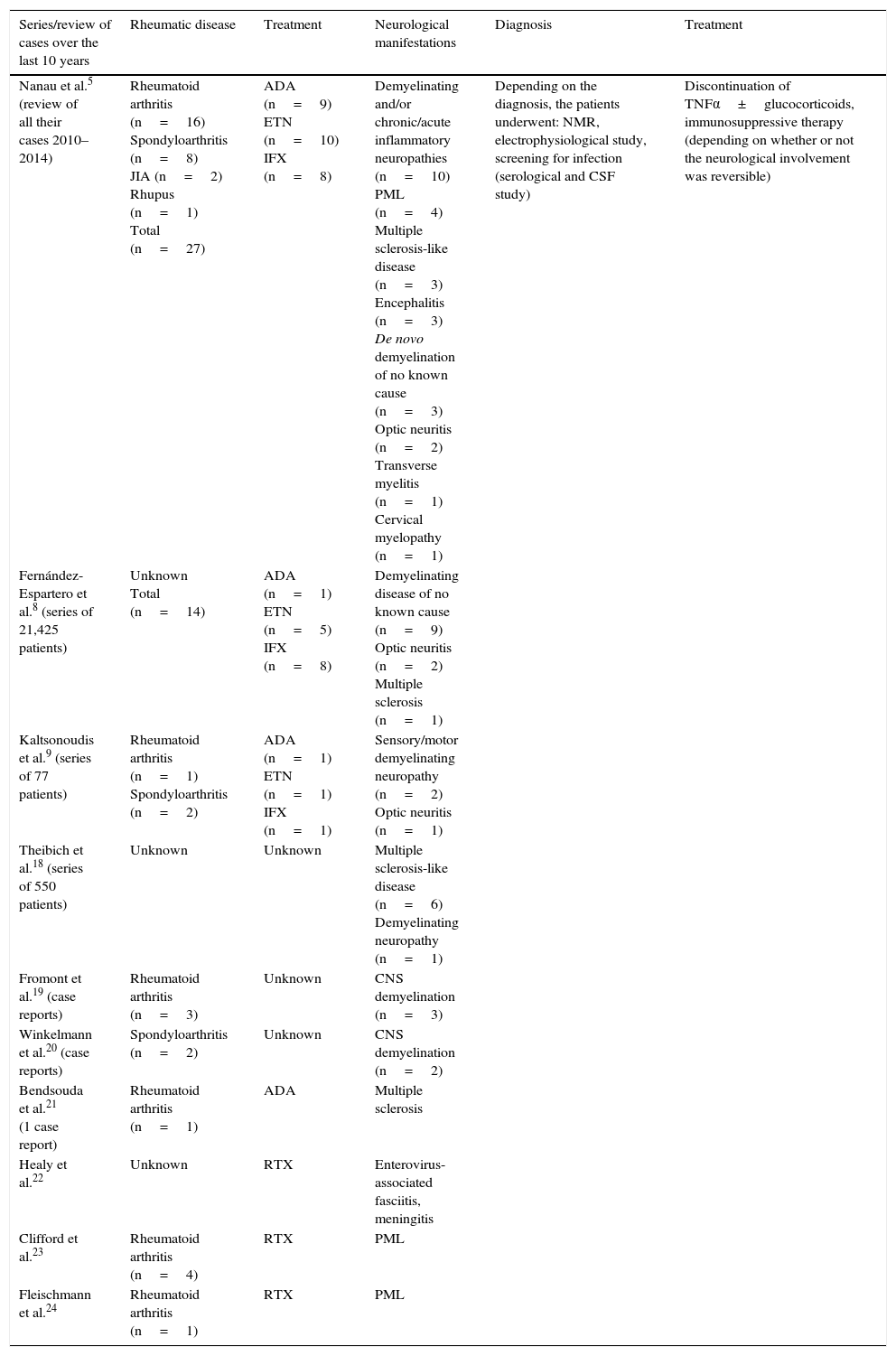
Types of Neurological Involvement and Care ReviewNanau et al.5 carried out a systematic review of the safety of TNFα inhibitors in patients with inflammatory rheumatic diseases.5 They included the publication of clinical trials and case reports published between 2010 and 2014. The majority of the neurological adverse events were observed in patients with rheumatoid arthritis; 50.9% over a 10-year period, according to the authors. The most common finding was the involvement of the central nervous system, the spectrum of demyelinating diseases, optic neuritis and sensory and/or motor demyelinating peripheral neuropathy. The remainder of the neurological events, such as transverse myelitis or progressive multifocal leukoencephalopathy, were less common.5,17
In a French cohort, 33 patients with inflammatory rheumatic disease revealed evidence of demyelinating disease on magnetic resonance. They were treated with anti-TNFα for 3 years, and 2/3 of the patients developed involvement of the central nervous system (encephalitis, transverse myelitis and optic neuritis), and 1/3 showed peripheral involvement (chronic demyelinating polyneuropathy, Guillain-Barré syndrome). The examination of the cerebrospinal fluid showed raised protein levels in 4 cases, oligoclonal bands in 11 and pleocytosis in 4. It was normal in 6 patients, despite central nervous system involvement. In 90% of the cases of peripheral nervous system involvement, the study of the cerebrospinal fluid revealed raised protein levels.5,25
On the other hand, if we take into account the number of times that the images from nuclear magnetic resonance were suggestive of demyelination, the diagnosis of multiple sclerosis itself is not so common.26–28 In many cases, the patient did not meet the diagnostic criteria for multiple sclerosis.29 In a Danish series involving 550 patients receiving biological therapy, 6 developed symptoms suggestive of demyelinating disease and 4 met the diagnostic criteria once the study had been completed.18 In short, not all demyelinating involvement will be a manifestation of multiple sclerosis.
Progressive multifocal leukoencephalopathy is a subacute infection of the central nervous system associated with oligodendrocyte destruction by John Cunningham virus (JC virus). Reactivation of JC virus occurs under conditions of immunosuppression. The symptoms include confusion, motor impairment, impaired coordination, speech disorders and visual disturbances. Seizures are not very common. The areas of demyelination can be seen in the occipital and parietal regions.30,31 The confirmation of the diagnosis is obtained when the virus is identified in cerebrospinal fluid culture or in a brain biopsy. As the incidence of JC virus is somewhat greater in patients treated with rituximab, in patients with anti-TNFα it is low, and is comparable to that of the general population.31 A total of 15 cases of JC virus have been reported in the Food and Drug Administration Adverse Event Reporting System database.32 Infliximab and adalimumab have been more closely related to JC virus reactivation, according to the Health Canada Drug Product Database and the World Health Organization (WHO) adverse effects database. However, there are also cases with other anti-TNFα.32 The association between rituximab and progressive multifocal leukoencephalopathy is better known, with the majority of the reported cases occurring in patients with rheumatoid arthritis.23,24 Nevertheless, it should be taken into account that rituximab, in contrast to other biological drugs, is used to treat other nonrheumatic diseases, like hematological and neurological disorders; thus, this other group of diseases would also contribute to increasing the prevalence of progressive multifocal leukoencephalopathy.22,33–36 Other adverse effects related to rituximab are: enterovirus myofasciitis, infectious polyneuropathy (West Nile virus) and encephalitis.34–36
Finally, bradykinetic syndromes are rare. There is only 1 case reported in the literature, in a patient with Parkinson's disease, which was rapidly established 1 year after treatment was begun with anti-TNFα.37 Nevertheless, the database of the Spanish Pharmacovigilance System locates a number of cases relating anti-TNFα with parkinsonian syndromes.2 The Who database provides more than 70 cases that relate anti-TNFα with parkinsonism. However, since the data cannot be accessed, it is impossible to determine whether or not there is a clear causal relationship.38
Paradoxically, there may be a relationship between the family of TNFα receptors and caspases, which participate in the pathogenesis of Parkinson's disease. It has been reported that TNFα could be toxic for midbrain dopaminergic neurons, which are also involved in the development of the disease. If this hypothesis were to be true, TNFα inhibitors would be beneficial, rather than harmful. Belarbi et al.39 carried out an experimental study in rats that demonstrated that inhibiting the synthesis of TNFα reestablished the neuronal function and the cognitive deficits induced by chronic inflammation of the nervous tissue. These authors concluded that the inhibition of TNFα could be a future therapeutic target in the spectrum of neurodegenerative diseases.39 Nonetheless, the results are contradictory and, thus, additional studies will be necessary to shed light on this question. Table 1 is included to summarize the cases reported in the literature over the last 10 years.
Summary of the Series of Patients With Rheumatic Diseases Reported Over the Past 10 Years.
| Series/review of cases over the last 10 years | Rheumatic disease | Treatment | Neurological manifestations | Diagnosis | Treatment |
|---|---|---|---|---|---|
| Nanau et al.5 (review of all their cases 2010–2014) | Rheumatoid arthritis (n=16) Spondyloarthritis (n=8) JIA (n=2) Rhupus (n=1) Total (n=27) | ADA (n=9) ETN (n=10) IFX (n=8) | Demyelinating and/or chronic/acute inflammatory neuropathies (n=10) PML (n=4) Multiple sclerosis-like disease (n=3) Encephalitis (n=3) De novo demyelination of no known cause (n=3) Optic neuritis (n=2) Transverse myelitis (n=1) Cervical myelopathy (n=1) | Depending on the diagnosis, the patients underwent: NMR, electrophysiological study, screening for infection (serological and CSF study) | Discontinuation of TNFα±glucocorticoids, immunosuppressive therapy (depending on whether or not the neurological involvement was reversible) |
| Fernández-Espartero et al.8 (series of 21,425 patients) | Unknown Total (n=14) | ADA (n=1) ETN (n=5) IFX (n=8) | Demyelinating disease of no known cause (n=9) Optic neuritis (n=2) Multiple sclerosis (n=1) | ||
| Kaltsonoudis et al.9 (series of 77 patients) | Rheumatoid arthritis (n=1) Spondyloarthritis (n=2) | ADA (n=1) ETN (n=1) IFX (n=1) | Sensory/motor demyelinating neuropathy (n=2) Optic neuritis (n=1) | ||
| Theibich et al.18 (series of 550 patients) | Unknown | Unknown | Multiple sclerosis-like disease (n=6) Demyelinating neuropathy (n=1) | ||
| Fromont et al.19 (case reports) | Rheumatoid arthritis (n=3) | Unknown | CNS demyelination (n=3) | ||
| Winkelmann et al.20 (case reports) | Spondyloarthritis (n=2) | Unknown | CNS demyelination (n=2) | ||
| Bendsouda et al.21 (1 case report) | Rheumatoid arthritis (n=1) | ADA | Multiple sclerosis | ||
| Healy et al.22 | Unknown | RTX | Enterovirus-associated fasciitis, meningitis | ||
| Clifford et al.23 | Rheumatoid arthritis (n=4) | RTX | PML | ||
| Fleischmann et al.24 | Rheumatoid arthritis (n=1) | RTX | PML |
ADA, adalimumab; CNS, central nervous system; CSF, cerebrospinal fluid; ETN, etanercept; IFX, infliximab; JIA, juvenile idiopathic arthritis; NMR, nuclear magnetic resonance; PML, progressive multifocal leukoencephalopathy; RTX, rituximab; TNF, tumor necrosis factor.
We should not forget the secondary manifestations of the underlying rheumatic disease. Taking into account the fact that most rheumatic diseases have a more or less important systemic component, the differential diagnosis is essential and indispensable. At times, it may be difficult to distinguish whether the neurological involvement is produced by the treatment or is due to the disease itself, even after doing the appropriate ancillary tests. Moreover, the heterogeneity of the series of cases and of the isolated cases reported in the literature makes it difficult to establish a causal relationship between the anti-TNFα drug and the neurological involvement.
Recommendations and ConclusionsThe interruption of the biological treatment should be considered if there is any reservation about the diagnosis. This decision must be made by the clinician, taking into account the risk-benefit of discontinuing biological therapy in each patient on an individualized basis. When the neurological involvement is serious, the standard treatment is the administration of glucocorticoids. Immunoglobulins have not often been necessary.5,25 The remission of the neurological signs is not always complete in all the patients, even when the biological therapy has been interrupted. The decision to restore it depends on neurological concerns and on whether a cause–effect relationship has been established. In clear terms, “not everything that happens to the patient will be due to the biological therapy”. Nevertheless, if the doubt exists, the best approach is to discontinue the drug and, if the patient's disease is still active, the therapeutic target should be changed.
In conclusion, as rheumatologists, we must be aware of and be familiarized with the adverse effects of the therapies we administer in our routine clinical practice, although some may be infrequent. It is of the utmost importance that they be reported, even if we cannot be sure of a cause-and-effect relationship. Spanish rheumatologists are fortunate to count on the BIOBADASER registry, which facilitates our work when we need to review the effects that we must bear in mind and their distribution among the Spanish population.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Tejera-Segura B, Ferraz-Amaro I. Terapias biológicas y manifestaciones neurológicas. ¿Qué sabemos? 2017;13:102–106.