To describe the main demographic and clinical features of patients with spondyloarthropaties in Spain.
Patients and methodsReview of randomized clinical charts of patients with spondyloarthropaties with at least one visit to the rheumatologist in the previous two years. Information was collected on demographic and clinical data (duration of illness, diagnostic category, disease activity, extra-articular manifestations, comorbidity and work disability).
Results1168 patients were included in the study. Their median age was 49.2 years (39.7–60.5), 68.0% were males, and median time of disease was 105.1 months (48.4–192.5). The diagnoses and clinical data such as the BASDAI were reported only in 34.0% of the patients. The most widely used measure of metrology, the Schober test, was missing in 37.7% of the clinical charts. The patients included had the following diagnoses: ankylosing spondylitis (n=629, 55.2%), psoriatic arthritis (n=253, 22.2%), undifferentiated spondyloarthritis (n=184, 16.1%), arthritis associated to inflammatory bowel disease (n=50, 4.4%), and reactive arthritis (n=16, 1.4%). The most common extra-articular manifestations were psoriasis (20.8%), anterior uveitis (19.4%), and enthesitis (16.9%). Some kind of work disability was reported in 8.3% of the patients.
ConclusionsDemographic and clinical characteristics of patients with spondyloarthropaties in Spain do not differ as a whole from other published studies, except for undifferentiated spondyloarthritis, which was more likely in our patients than in other studies. The quality of the records of activity in the clinical charts could be improved.
Describir las características clínicas y demográficas de los pacientes con espondiloartritis en España.
Pacientes y métodosRevisión de historias clínicas aleatorizadas de pacientes con espondiloartritis mayores de 16 años, con al menos una visita al reumatólogo en los 2 años anteriores. Se recogió información sobre datos sociodemográficos y clínicos (tiempo de duración de la enfermedad, categoría diagnóstica, actividad de la enfermedad, manifestaciones extrarticulares, y comorbilidad).
ResultadosSe incluyeron 1.168 pacientes procedentes de 46 hospitales de toda España. El 68% eran varones con valores mediana de edad y tiempo de evolución de la enfermedad de 49,2 años (39,7–60,5) y de 105 meses (48,4–192,5), respectivamente. Los diagnósticos, por orden de frecuencia, fueron: espondilitis anquilosante (n=629, 55,2%), artritis psoriásica (n=253, 22,2%), espondiloartritis indiferenciada (n=184, 16,1%), artritis asociada a enfermedad inflamatoria intestinal (n=50, 4,4%) y artritis reactiva (n=16, 1,4%). Las manifestaciones extrarticulares más comunes fueron: psoriasis (20,8%), uveítis anterior (19,4%) y entesitis (16,9%). Constaba la existencia de incapacidad laboral en el 8,3% de las historias clínicas. Constaban datos clínicos como el BASDAI solo en el 34% y la medida de metrología más utilizada, el test de Schöber, faltaba en el 37,7% de las historias.
ConclusionesLas características sociodemográficas y clínicas de los pacientes con espondiloartritis del estudio emAR II, no difieren de forma global de lo publicado previamente en otros estudios, excepto para el diagnóstico de formas indiferenciadas, que son más frecuentes en nuestros pacientes que en otras publicaciones. La calidad de los registros de actividad en las historias clínicas es mejorable.
Spondyloarthritides (SpA) are a heterogeneous group of diseases characterized by familial aggregation and association with HLA-B27. They include ankylosing spondylitis, psoriatic arthritis, reactive arthritis and arthritis associated with intestinal inflammatory disease,1 which is a substantial overlap between these entities. Their prevalence varies between 0.3% and 1.06% depending on the study.2–4 Ankylosing spondylitis, the prototype of this group of diseases, has a prevalence that is very different between different ethnic groups, in Europe being between 0.08%2 and 0.26%.5
The increasingly frequent use of disease modifying agents and biologics in the management of these patients has led to increased health care needs and demand closer monitoring, which has led to the creation of SpA units, these conditions also lead to demand for care from other medical specialties and put pressure on labor productivity because of their impact on functional ability. Studies in our country have shown that a similar disease, such as rheumatoid arthritis (RA), consumes a significant number of healthcare resources and is accompanied by low work productivity.6 In this sense, it has been seen that patients with RA consume more resources, especially those related to medical care, visits to other specialists or surgery than a population of similar characteristics but without the disease.7
In our country, there is a greater clinical and demographic need and resource consumption, both by patients with SpA,8 as for RA.6 Since the time of these publications, there have been significant changes both in their treatment, with the use of disease modifying drugs and new biological agents, and in the use of new diagnostic criteria,9 so a new approach to the clinical characteristics of these patients may be interesting to have.
The objective of this paper is to describe the characteristics of patients with SpA in Spain in terms of clinical parameters of activity, occupational disability and comorbidity.
Materials and MethodsDesign, Patient Selection and Data CollectionData were collected from eMAR II, a study of variability in the treatment of RA and SpA made in 2010 to assess the impact of changes in the management of these diseases since the first eMAR study, 10 years earlier. Study characteristics eMAR II are available through the website of the Spanish Society of Rheumatology.10
The sample consisted of medical records of patients over the age of,16 treated in rheumatology departments of Spanish hospitals with at least one visit to a rheumatologist in the 2 years preceding the date of study onset. Stratified sampling was carried out by autonomous communities and second level hospitals (with probability proportional to size) and patients (random equiprobabilistic selection). The criterion for considering if a patient had a diagnosis was the diagnosis of one of these entities by the rheumatologist in charge of the health care of the patient.
The sample size was calculated according to the hypothesis that the proportion of patients requiring surgery increased from 18% to 26% from eMAR I to eMAR II. Under this premise, and assuming an alpha error of 5%, a power of 80%, 15% of incomplete files and a design effect of 2.5, resulted in a sample size of 1410 patients.
Data from the last 2 years were collected from medical records of patients in the standardized data collection. We collected data from these 2 years, given that the uneven assistance record of patients leads to the existence of a variable number of records for each patient and the records of each patient were pooled to prevent this bias. Nonmodifiable data (sex, education, etc.) were collected only once and dynamic data of disease activity (ESR, VAS, number of swollen joints, etc.), which represent the worst and the best situation along the 2-year study, were collected on each visit.
Measures and VariablesInformation was obtained on: (a) demographic data (age, sex, marital status, education level, occupation, and residence), (b) clinical characteristics (date of disease onset, date of first visit and diagnosis, duration, and type of the form of clinical involvement, compliance with modified New York,11 Amor12 diagnostic criteria for ankylosing spondylitis and the European Group for the Study of Spondyloarthropathies13 for intestinal inflammatory disease14 as well as the Rudwaleit criteria for low back pain [later15 modified, select the cutting point for compliance at 2 of the 4 criteria], and the Berlin criteria for axial16 spondylitis, HLA-B27, family history and extra-articular manifestations), (c) activity data and monitoring methods (ESR, CRP, VAS for pain and activity, morning stiffness, joint assessment, painful enthesis [BASDAI]), (d) employment status and functional ability (BASFI), and (e) comorbidity.
The assessment of disease activity by physician and patient was carried out by subjective and VAS scales. In the first case, we asked for best and worst subjective assessment (doctor and patient) on disease activity in the last 2 years. In this case, it called for the collection of compliant classified data printing (doctor and patient) on the best and worst assessment of activity based on explicit annotations or presence of sufficient data on the clinical history. Below are detailed instructions for filling the form:
– Best and worst subjective assessment of the doctor/patient on disease activity in the last 2 years: classify the impression of doctor/patient on best and worst assessment of disease activity in one of the following categories: if printing is explicitly doctor/patient using this data. If not stated explicitly, and data insufficient to classify in a category, fill according to the following:
- 1.
No: VAS activity<10mm or patient in complete remission at the discretion of the physician or by one of the commonly used objective criteria, with or without active treatment of disease/or indication by the patient in absence of pain or morning stiffness and swelling in any joint
- 2.
Mild: VAS activity≥10mm and <40mm or patients with mild activity that does not require treatment modification.
- 3.
Moderate: VAS activity≥40mm and <60mm or patient with moderate activity in which minor amendments to the treatment have been made (e.g. transient increases in doses of NSAIDs or oral corticosteroids or joint infiltration).
- 4.
Severe: VAS activity≥60mm or patient with severe activity who has required major modifications of treatment (e.g. increased dose of DMARD, addition or change of DMARD due to treatment failure for lack of disease control).
Descriptive analysis was performed using measures of central tendency (mean or median) and dispersion (standard deviation and 25 and 75 quartile) for continuous variables, as adjusted or not normally distributed, and percentages for qualitative variables. The estimates are adjusted to the sampling design using the svy commands in Stata 9.0 (StataCorp, College Station, USA). The median is a statistic that summarizes the central tendency of the variable non-normal distributions, and the use of which avoids the influence of extreme values or outliers.
ResultsOf the estimated sample (No.=1410), we obtained valid information for 1168 patients, representing 82.8% of the theoretical sample.
Sociodemographic68% of patients were male with median age (P25–P75) at the time of review of 49.2 years (39.7–60.5) and 30.4 years (23.2–39.8) at disease onset, and disease duration of 105.1 months (48.4–192.5).
No information on marital status, academic and profession status in 64.2%, 72.5%, and 52.0% of cases were available, although the most common status was: to be married (28.5%), have primary education (11%) and be an unskilled worker (12%). In relation to the place of residence, more than half lived in the same town where the hospital stood (52.1%) and among those residing in a different location, the most common distance to the hospital was between 20 and 50km (41.7%) (Table 1).
Sociodemographic Data.
| Characteristic | Median (P25–P75) |
| Current age (No.=1139) | 49.2 (39.7–60.5) |
| Age at onset of disease (No.=817) | 30.4 (23.2–39.8) |
| Time since onset (months) (No.=1016) | 105.1 (48.4–192.5) |
| Characteristic | No. (%) |
| Gender | Total=1160 |
| Male | 789 (68.0) |
| Female | 371 (32.0 |
| Marital status | Total=1155 |
| Single | 71 (6.1) |
| Married | 329 (28.5) |
| Widowed | 4 (0.3) |
| Separated | 9 (0.8) |
| Not documented | 742 (64.2) |
| Schooling | Total=1149 |
| None | 15 (1.3) |
| Primary | 127 (11.0) |
| Secondary | 89 (7.7) |
| Superior | 85 (7.4) |
| Not stated | 833 (72.5) |
| Profession | Total=1146 |
| Direction business and administration | 16 (1.4) |
| Technical, professional, intellectual | 53 (4.6) |
| Technical and support professionales | 35 (3.0) |
| Service providers | 66 (5.8) |
| Agriculture and fisheries | 65 (5.7) |
| Industry | 19 (1.6) |
| Operators | 50 (4.4) |
| Non-qualified workers | 30 (2.6) |
| Armed forces | 134 (11.7) |
| Housewives | 6 (0.5) |
| Students | 63 (5.5) |
| Not stated | 13 (1.1) |
| 596 (52.0) | |
| Residence | Total=1155 |
| Same area | 602 (52.1) |
| Different area | 489 (42.3) |
| Not stated | 64 (5.5) |
| Distance to hospital | Total=489 |
| less than 20km | 171 (35.0) |
| Between 20 and 50km | 204 (41.7) |
| Over 50km | 102 (20.8) |
| Not known | 12 (2.4) |
The most common diagnosis was that of ankylosing spondylitis (55.2%), followed by psoriatic arthritis (22.2%) and undifferentiated forms (16.1%), 4.4% were related to inflammatory bowel disease and only 1.4% were reactive arthritis. In connection with the classification criteria, more than half of the patients fulfilled ESSG and Amor criteria (61.3% and 55.0%, respectively), 51.2% could be classified as ankylosing spondylitis according to modified New York criteria, and 32.1% and 31.7% as inflammatory back pain or ankylosing according to the Rudwaleit and axial Berlin criteria, respectively. 8.6% did not meet any classification criteria, while 18.2% met all. HLA-B27 was positive in 58.8% of patients. There was a family history of psoriasis, inflammatory bowel disease, oligo- or polyarthritis, uveitis and positive HLA-B27 in 15.3% of cases. The mixed form of clinical involvement was the most frequent (43.0%), followed by the axial (40.1%), and 11.8% with peripheral involvement, with less than 1% entheseal.
Psoriasis was the most common extra-articular manifestation (20.8%), followed by uveitis (19.4%) and enthesitis (16.9%). By contrast, the least common were renal disease (2.1%), neurological (1.3%) and amyloidosis (0.1%); 41.5% of cases did not present any of these manifestations and among those who presented them (34.9%) the usual number was 2 (16.3%) (Table 2).
Clinical Characteristics.
| Characteristic | No. (%) |
| Diagnosis (No.=1140) | |
| Ankylosing spondylitis | 629 (55.2%) |
| Psoriasis associated spondylitis | 253 (22.2%) |
| Spondyloarthritis associated to inflammatory intestinal disease | 50 (4.4%) |
| Reactive arthritis | 16 (1.4%) |
| Undifferentiated spondyloarthritis | 184 (16.1%) |
| Not stated | 8 (0.7%) |
| Spondyloarthritis criteria compliant | |
| Rudwaleit (inflammatory back pain) | 375 (32.1%) |
| ESSG (spondyloarthritis) | 716 (61.3%) |
| Amor (spondylarthritis) | 643 (55.0%) |
| Berlín (axial spondylitis) | 370 (31.7%) |
| New York (ankylosing spondylitis) | 598 (51.2%) |
| None | 101 (8.6%) |
| Positive HLA-B27 (No.=1147) | |
| No | 252 (22.0%) |
| Yes | 675 (58.8%) |
| Not stated | 220 (19.2%) |
| Family history (No.=1128) | |
| No | 408 (36.2%) |
| Yes | 173 (15.3%) |
| Not stated | 547 (48.5%) |
| Form of clinical affection (1159) | |
| Enthesitic | 9 (0.8%) |
| Axial | 465 (40.1%) |
| Peripheral | 137 (11.8%) |
| Mixed | 499 (43.0%) |
| Not stated | 49 (4.2%) |
| Extra-articular manifestations | |
| Uveitis (No.=1168) | 227 (19.4%) |
| Lung disease (No.=1168) | 31 (2.6%) |
| Cardiovascular (No.=1168) | 45 (3.8%) |
| Neurlogic (No.=1168) | 15 (1.3%) |
| Inflammatory intestinal disease (No.=1168) | 70 (6.0%) |
| Renal (No.=1168) | 25 (2.1%) |
| Osteoporosis (No.=1168) | 58 (5.0%) |
| Amyloidosis (No.=1168) | 1 (0.1%) |
| Psoriasis (No.=1168) | 243 (20.8%) |
| Nail affection (No.=1168) | 50 (4.3%) |
| Enthesitis (No.=1168) | 197 (16.9%) |
| Dactilytis (No.=1168) | 109 (9.3%) |
| None documented (No.=1168) | 485 (41.5) |
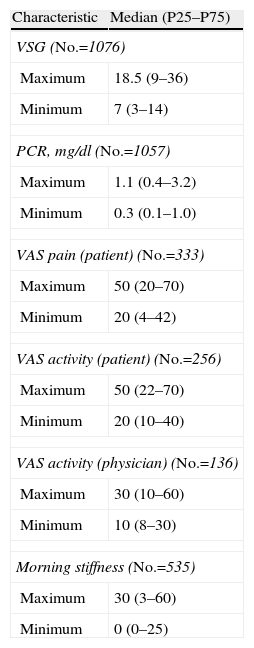
The ESR values ranged from about minimum and maximum median of 7 and 18, with respective figures of 0.3 and 1.1mg/dl for CRP. The assessment, by pain and activity VAS by the patient showed a minimum of 20 and a maximum of 50, with a VAS activity assessed by the physician of 30. The maximum value of morning stiffness was 30min.
Importantly, a considerable number of reviewed records did not contain information on these parameters. The percentages of files without these data were: ESR 8.0%, CRP 9.4%, VAS pain (patient) 71.5%, VAS activity (patient) 78.1%, VAS activity (physician) 88.0% and morning stiffness 54.2%.
The best subjective assessment of disease activity by the physician rated the majority of patients in the mild category (32.6%) or not of that (23.7%). In the worst subjective evaluation, the most frequent were mild or moderate (23.9% and 21.6%) with absence of this information in 35.0% of the stories. The best and worst assessments of the activity of the patient showed distribution patterns that coincide with those made by their physicians (Table 3).
Activity of Disease (I): General Parameters.
| Characteristic | Median (P25–P75) |
| VSG (No.=1076) | |
| Maximum | 18.5 (9–36) |
| Minimum | 7 (3–14) |
| PCR, mg/dl (No.=1057) | |
| Maximum | 1.1 (0.4–3.2) |
| Minimum | 0.3 (0.1–1.0) |
| VAS pain (patient) (No.=333) | |
| Maximum | 50 (20–70) |
| Minimum | 20 (4–42) |
| VAS activity (patient) (No.=256) | |
| Maximum | 50 (22–70) |
| Minimum | 20 (10–40) |
| VAS activity (physician) (No.=136) | |
| Maximum | 30 (10–60) |
| Minimum | 10 (8–30) |
| Morning stiffness (No.=535) | |
| Maximum | 30 (3–60) |
| Minimum | 0 (0–25) |
| No. (%) | |
| Activity: best subjective evaluation (physician) | No.=1155 |
| None | 274 (23.7%) |
| Mild | 377 (32.6%) |
| Moderate | 82 (7.1%) |
| Severe | 21 (1.8%) |
| Not stated | 401 (34.7%) |
| Activity: worst subjective evaluation (physician) | No.=1157 |
| None | 78 (6.7%) |
| Mild | 277 (23.9%) |
| Moderate | 250 (21.6%) |
| Severe | 147 (12.7%) |
| Not stated | 405 (35.0%) |
| Activity: best subjective evaluation (patient) | No.=1161 |
| None | 287 (24.7%) |
| Mild | 383 (33.0%) |
| Moderate | 113 (9.7%) |
| Severe | 37 (3.2%) |
| Not stated | 341 (29.4%) |
| Activity: worst subjective evaluation (patient) | No.=1159 |
| None | 71 (6.1%) |
| Mild | 274 (23.6%) |
| Moderate | 264 (22.8%) |
| Severe | 197 (17.0%) |
| Not evaluated | 353 (30.4%) |
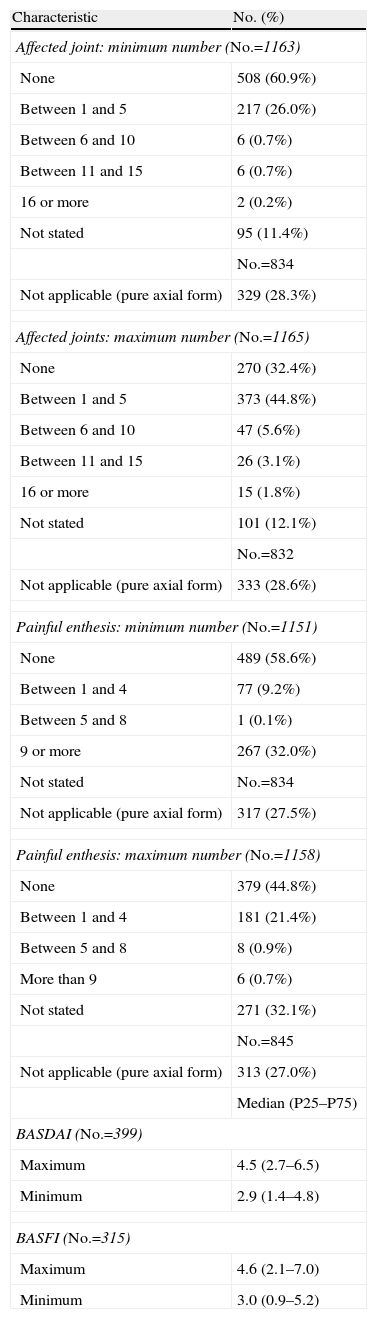
Most forms were not pure axial joint counts with a minimum of 0 (60.9%) or between 1 and 5 (26.0%) and the maximum was between 1 and 5 joints (44.8%) or not (32.4%). In enthesitic forms, the most common did not affect entheses in the minimum count (58.6%) and 0 (44.8%) or between 1 and 4 (21.4%) in the maximum. SpA activity and functional capacity median values showed a maximum and minimum of 2.9 and 4.5 for the BASDAI and 0.4 and 1.0 for the BASFI (Table 4).
Disease Activity (II): Specific SpA Characteristics.
| Characteristic | No. (%) |
| Affected joint: minimum number (No.=1163) | |
| None | 508 (60.9%) |
| Between 1 and 5 | 217 (26.0%) |
| Between 6 and 10 | 6 (0.7%) |
| Between 11 and 15 | 6 (0.7%) |
| 16 or more | 2 (0.2%) |
| Not stated | 95 (11.4%) |
| No.=834 | |
| Not applicable (pure axial form) | 329 (28.3%) |
| Affected joints: maximum number (No.=1165) | |
| None | 270 (32.4%) |
| Between 1 and 5 | 373 (44.8%) |
| Between 6 and 10 | 47 (5.6%) |
| Between 11 and 15 | 26 (3.1%) |
| 16 or more | 15 (1.8%) |
| Not stated | 101 (12.1%) |
| No.=832 | |
| Not applicable (pure axial form) | 333 (28.6%) |
| Painful enthesis: minimum number (No.=1151) | |
| None | 489 (58.6%) |
| Between 1 and 4 | 77 (9.2%) |
| Between 5 and 8 | 1 (0.1%) |
| 9 or more | 267 (32.0%) |
| Not stated | No.=834 |
| Not applicable (pure axial form) | 317 (27.5%) |
| Painful enthesis: maximum number (No.=1158) | |
| None | 379 (44.8%) |
| Between 1 and 4 | 181 (21.4%) |
| Between 5 and 8 | 8 (0.9%) |
| More than 9 | 6 (0.7%) |
| Not stated | 271 (32.1%) |
| No.=845 | |
| Not applicable (pure axial form) | 313 (27.0%) |
| Median (P25–P75) | |
| BASDAI (No.=399) | |
| Maximum | 4.5 (2.7–6.5) |
| Minimum | 2.9 (1.4–4.8) |
| BASFI (No.=315) | |
| Maximum | 4.6 (2.1–7.0) |
| Minimum | 3.0 (0.9–5.2) |
Joint involvement in patients with SpA was not assessed by 68 joints counts or other methods in most of the files (52.6 and 58.9%). Something similar happened with entheseal involvement, as a 13 enthesis count was neither used (58.3%) nor was other equivalent method (74.3%). Similarly, no usual assessment of the overall situation of the patient by VAS (65.6%) or other procedures (65.6%) or nocturnal spinal pain (60.2% and 62.2% for VAS for other procedures) was performed. The duration of morning stiffness was the evaluation method used always in 9.7% of patients, usually in 12.4% and occasionally in 22.2%.
The acute phase reactants were used systematically, both ESR (67.3%) and PCR (66.4%). On the contrary, in general, activity indices such as BASDAI (58.2%), or functional capacity scores such as BASFI (66.7%) were not never calculated. With regard to spinal mobility parameters, the most common situation was the absence of these measures on all visits of the study period, with percentages of the “never” response of 37.7% for Schober, 44.6% for chest expansion, 46.1% for occiput-wall distance and 54.4% for lateral flexion of the trunk. It is worth noting that the data record of clinical activity was very uneven, ranging from the presence of data for ESR in 1076 files and BASFI in 315.
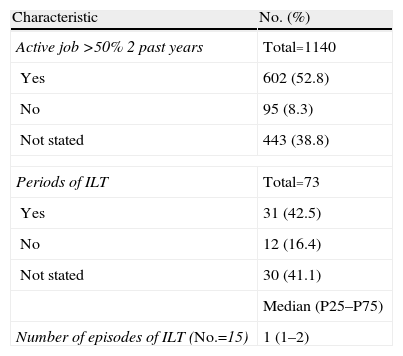
Employment StatusAs for work activity, 52.8% of cases (602 patients) had an active job for more than 50% of the study period of 2 years, while some had episodes in which it did not work (ILT) in 8.3% with a median value of ILT episodes (Table 5). These results should be interpreted with caution, since the efficiency in collecting these data was not entirely correct due to various reasons. On the one hand, it is quite common in the medical records to not record this type of information and, secondly, the collection of work disability was not limited exclusively to the disease under study, but might have included other more or less disabling processes (Table 5).
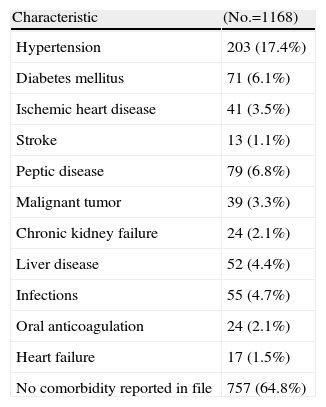
ComorbidityInformation was collected on the history of other chronic diseases. The most common conditions were hypertension (17.4%), peptic disease (6.8%) and diabetes mellitus (6.1%). There was no evidence in the medical history of these comorbidities in 65% of patients, which does not mean that they were not present (Table 6).
Comorbidity.
| Characteristic | (No.=1168) |
| Hypertension | 203 (17.4%) |
| Diabetes mellitus | 71 (6.1%) |
| Ischemic heart disease | 41 (3.5%) |
| Stroke | 13 (1.1%) |
| Peptic disease | 79 (6.8%) |
| Malignant tumor | 39 (3.3%) |
| Chronic kidney failure | 24 (2.1%) |
| Liver disease | 52 (4.4%) |
| Infections | 55 (4.7%) |
| Oral anticoagulation | 24 (2.1%) |
| Heart failure | 17 (1.5%) |
| No comorbidity reported in file | 757 (64.8%) |
The eMAR II is a study of variability of clinical practice in RA and SpA, which provides a wealth of information on the characteristics of patients seen in rheumatology units in our country and the conditions under which health care is provided for these patients. This is a retrospective study, conducted on the medical records of patients, which may influence some of the variability observed, because not all data were collected in a clinical interview, although it is a good way to assess the clinical practice.
The results of this study allow for a comprehensive characterization of patients of our country, which have an age and sex distribution similar to that of other series.8,17,18
By comparing the data in this study with REGISPONSER,8 striking differences in the classification of patients with the diagnostic categories of “psoriatic arthritis” and “undifferentiated spondyloarthritis” were seen going from 17.5% to 14.4% in REGISPONSER to 22.2% and 16.1% in our study, as well as a decrease of the category “ankylosing spondylitis” which dropped from 61.5% in REGISPONSER to 55.2% in the eMAR II study. The reasons for these differences may be methodological (equal probability random sampling in Emar II and selection of patients by physicians in REGISPONSER) but may also reflect changes in diagnostic criteria for these diseases. In this regard, it has been observed that the results of different studies show a lower proportion of undifferentiated SpA.17,18
Similarly, the increased frequency of peripheral joint involvement is striking, reduced from 17.4% in REGISPONSER19 to 54.8% in Emar II. The high percentage of peripheral arthritis in this series is remarkable, which can be explained by the presence of a large group of patients with psoriatic arthritis or undifferentiated SpA, although given the nature of the work it is difficult to draw definitive conclusions. On the other hand, the differences between the two studies regarding other features are of lesser magnitude. Thus, the percentage of patients with axial and mixed forms is 77.8% versus 83%, the presence of enthesitis and extra-articular manifestation is 22.6% versus 16.9% and the incidence of uveitis is similar in both studies (15.8% versus 19.4%).
As in other series8,17,20 ankylosing spondylitis was the single most frequently diagnosed entity within this group of diseases and psoriasis, uveitis and enthesitis the most frequent8 extra-articular manifestations.20
With regard to disease activity, it is noteworthy that monitoring was conducted primarily through laboratory tests, and much less frequently by clinical assessment of patients. Although, as noted at the beginning of the discussion, these data may be missing from the medical record but were taken into account at the time of the interview; however, another possibility is that clinical activity of SpA in practice is not routinely measured.
52.8% of patients in the eMAR II study had an active working life, a percentage similar to that found in another study,21 although higher than that observed in other works.22,23 Although the comparison is of interest, we must take into account that local differences in the job market may influence this outcome, so patients on the SpA British biologic21 registry worked full time in 43.5% and part time in 11.4%. 47% of patient files without an active working life did not specify how many cases were unfit for work due to SpA, nor the number of temporary periods of disability motivated by the rheumatic disease in question. In the REGISPONSER data, from 2004, 25.6% of patients with ankylosing spondylitis showed permanent disability,24 defined as a situation that legally allowed them to collect a pension, but patients may not have a job because of their disease, even without a legally recognized disability. As mentioned, when speaking of the disease activity, it is conspicuously absent in the files regarding work disability of patients and we cannot ensure that they were stated during the interview, but in any case, had not been registered, which means that there is little attention paid to these aspects, although it may have a high impact on the quality of life of these patients.
We found in the literature that most patients have at least one documented associated comorbidity22; it is difficult to make comparisons with the eMAR II study patients, since in most clinical files information regarding this aspect was not available. The prevalence of hypertension, diabetes mellitus and other cardiovascular risk factors did not differ significantly from those found in the general population.25–27 The prevalence of malignant tumors (3.3%) is slightly higher than that seen in the general population (between 2% and 3%).28
In this paper there is great variability in the management of these patients, at least on the recorded history, verifying that in most cases there are not the minimum data needed for evaluation of patients, rather emphasizing laboratory, ESR or CRP at the expense of the clinical data assessment, such as duration of morning stiffness, physician or patient VAS, or BASDAI or BASFI indices, as recommended. Despite the limitations of this study, it is difficult to think that indices like BASDAI or entheses counts, or other metrology measures, are carried out but are not reflected in the medical record, so we may assume that the clinical management of these patients may be improved in many of our rheumatology units.
In summary, the results of this study show a broad characterization of different aspects of patients in our country. These data allow a better understanding of the disease and therefore may be useful for planning care and service demands.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of InterestThe emAR II study has been financed by Abbot.
emAR II study group: C. Escudero. Hospital Virgen de Valme (Sevilla); N. Chozas, I. Maries, A. Fernandez, F. Medina. Hospital Puerta del Mar; M. Guzmán. Complejo Hospitalario Virgen de las Nieves; I. Ureña, V. Irigoyen, M. Lopez, P. Espiño, S. Manrique. Hospital Carlos Haya; E. Collantes, P. Font, D. Ruiz, M. Granados, M.J. Pozuelo. Hospital Reina Sofía; I. Moreno, A. Garcia López. Hospital Virgen del Rocío; J.M. Pina. Hospital de Barbastro; R. Roselló, C. Vázquez. Hospital General San Jorge; J. Beltrán, A. Pecondón, E. Giménez, F. Jimenez, J. Marzo, M. Medrano. Hospital Universitario Miguel Servet; J. Babío, T. Tinturé, S. González, C. Ordás, M.E. García. Hospital de Cabueñes; J. Ballina, S. Alonso. Hospital Universitario Central de Asturias; L. Espadaler, J. Fernandez, J. Fiter. Hospital Son Dureta; S. Bustabad. Hospital Universitario de Canarias; C. Rodríguez, A. Naranjo, S. Ojeda. Hospital Universitario Dr. Negrín; J. Tornero, J.A. Piqueras. Hospital General Universitario de Guadalajara; E. Júdez, G. Sánchez. Hospital Universitario de Albacete; L. Pantoja, C. López. Hospital El Bierzo; J. Medina, G. Iglesias, M. Alvarez. Complejo Asistencial de Palencia; J. Alegre, M.R. Colazo, J.L. Alonso, B Álvarez. Complejo Asistencial de Burgos; T: Pérez Sandoval. Complejo Asistencial de León; J. Del Pino, C. Montilla, S. Gómez, R. López, M. Sánchez. Complejo Asistencial de Salamanca; F.X. Arasa. Hospital Tortosa Verge de la Cinta; S. Castro. Hospital Universitario de Tarragona Joan XXIII; S. Ordóñez, D. Boquet. Hospital Universitario Arnau de Vilanova; J. Calvet. Corporación Sanitaria Parc Taulí; D. de la Fuente, V. Rios, M. Nolla. Hospital Universitario de Bellvitge; A. Martínez. Hospital Universitario de la Ribera; M.A. Belmonte Serrano. Hospital General de Castellón; R. Negueroles. Hospital Universitario La Fe; J. García, F. Gamero, E. del Rincón. Complejo Hospital de Cáceres; R. Veroz. Complejo Hospitalario Llerena-Zafra; E. Pérez-Pampín. Hospital Universitario de Santiago; L. Fernandez. Complejo Hospital de Orense; R. Miguélez, J. Godó. Hospital de Móstoles; A.M. Ortíz, E. Vicente, E. Tomero, A. Casado, M.J. Aria. Hospital Universitario de La Princesa; E. Cuende, C. Bohorquez. Hospital Universitario Príncipe de Asturias; J.M. Rodríguez, A. Aragón, J. García, J. Zubieta. Hospital Universitario de Getafe; C. Martínez. Hospital Clínico San Carlos; I. Mateo, A. de Juanes, E. Enríquez. Hospital 12 de Octubre; F.J. López-Longo. Hospital Universitario Gregorio Marañón; J. Maese. Hospital La Paz; E. Pagán, M.J. Rubira, P. Mesa. Hospital Los Arcos; N. Rivera. Hospital de Basurto; C. Rodríguez. Hospital Comarcal de Melilla; B. González Álvarez. Complejo Hospital Universitario Virgen Candelaria; A. Zea, C. Diaz-Miguel, A. Cifuentes. Hospital Ramón y Cajal.
Please cite this article as: Casals-Sánchez JL, et al. Características de los pacientes con espondiloartritis seguidos en unidades de reumatología en España. Estudio emAR II. Reumatol Clin. 2012;8:107–13.