To identify, from the Mexican Public Health System perspective, which would be the most cost-effective treatment for patients with fibromyalgia (FM).
Material and methodsA Markov model including three health states, divided by pain intensity (absence or presence of mild, moderate or severe pain) and considering three-month cycles; costs and effectiveness were estimated for amitriptyline (50mg/day), fluoxetine (80mg/day), duloxetine (120mg/day), gabapentin (900mg/day), pregabalin (450mg/day), tramadol/acetaminophen (150mg/1300mg/dia) and amitriptyline/fluoxetine (50mg/80mg/dia) for the treatment of FM. The clinical outcome considered was the annual rate of pain control. Probabilities assigned to the model were collected from published literature. Direct medical costs for FM treatment were retrieved from the 2006 data of the Mexican Institute of Social Security (IMSS) databases and were expressed in 2010 Mexican Pesos. Probabilistic sensitivity analyses were conducted.
ResultsThe best pain control rate was obtained with pregabalin (44.8%), followed by gabapentin (38.1%) and duloxetine (34.2%). The lowest treatment costs were for amitriptyline ($ 9047.01), followed by fluoxetine ($ 10183.89) and amitriptyline/fluoxetine ($ 10866.01). By comparing pregabalin vs amitriptyline, additional annual cost per patient for pain control would be around $ 50000 and $ 75000 and would result cost-effective in 70% and 80% of all cases.
ConclusionsAmong all treatment options for FM, pregabalin achieved the highest pain control and was cost-effective in 80% of patients of the Mexican Public Health System.
Identificar desde la perspectiva del proveedor de servicios de salud pública en México el tratamiento más coste-efectivo para pacientes con síndrome de fibromialgia (SFM).
Material y métodosMediante un modelo de Markov con 3 estados de salud, definidos por la intensidad del dolor (ausencia o presencia de dolor leve; moderado o severo), en ciclos de 3 meses, se estimaron los costes y las efectividades de amitriptilina (50mg/día), fluoxetina (80mg/día), duloxetina (120mg/día), gabapentina (900mg/día), pregabalina (450mg/día), tramadol/acetaminofén (150mg/1300mg/día) y amitriptilina/fluoxetina (50mg/80mg/día) en el tratamiento del SFM. El resultado clínico de interés fue el porcentaje de control del dolor al año de tratamiento. Las probabilidades asignadas al modelo se obtuvieron de la literatura publicada. Los costes médicos directos del tratamiento SFM se calcularon a través bases de datos del Instituto Mexicano del Seguro Social (IMSS) en 2006 y se expresaron en pesos mexicanos de 2010. El análisis de sensibilidad fue probabilístico.
ResultadosEl mejor control del dolor se obtiene con el uso de pregabalina (44,8%), seguido de gabapentina (38,1%) y duloxetina (34,2%). El tratamiento con menor coste, fue con amitriptilina ($ 9.047,01), seguido de fluoxetina ($ 10.183,89) y amitriptilina/fluoxetina ($ 10.866,01). Al comparar pregabalina vs amitriptilina, el coste anual adicional por paciente con control del dolor se encuentra entre $ 50.000 y $ 75.000 y resulta ser coste-efectivo entre el 70 y el 80% de los casos.
ConclusionesEntre las alternativas de tratamiento para el SFM, pregabalina alcanza el mejor control del dolor y es coste-efectiva hasta en el 80% de los pacientes del sistema de salud público en México.
Chronic pain syndromes, such as the fibromyalgia syndrome (FMS), have a significant impact on quality of life and lead to decreased productivity.1–3 The main manifestations of the syndrome are widespread musculoskeletal pain, presence of allodynia and hyperalgesia, often accompanied by sleep disturbance, anxiety, depression, headaches and irritable bowel syndrome, among others.4
The worldwide prevalence reported for FMS is between 3% and 5% of the adult population (20–60 years old), with a significant prevalence in women. In Mexico, the estimated frequency is 0.68% (95% CI, 0.56–0.80).5
The negative impact of FMS is manifested in the daily activities of life and result in lost work and/or school, with an effect on productivity, a reduction that has been estimated up to 65%. Thus, the most important economic impact of FMS occurs in the costs associated with lost productivity, which represent up to 70% of the burden of disease.6 The therapeutic approach for FMS is multidisciplinary and the main objective is to reduce pain and improve functional capacity. Treatment should include: patient education, lifestyle changes, physiotherapy and drug treatment.7
The most frequently studied pharmacological treatment is the use of tricyclic antidepressants. A recent meta-analysis analyzing effect size in reducing pain with amitriptyline in FMS calculated a standardized mean difference (SMD) of −1.64 (95% CI, −2.57 to −0.71).8 Given the high incidence of adverse effects associated with use of tricyclic antidepressants and seeking a better safety profile, selective inhibitors of serotonin reuptake inhibitors (SSRIs) have been an alternative treatment. The same meta-analysis reported a SMD, in the case of pain when using fluoxetine and paroxetine, of −0.39 (95% CI, −0.77 to −0.01) and when treating with selective inhibitors of noradrenaline (SINA) the authors reported an SMD for duloxetine and milnacipran of −0.36 (95% CI, −0.46 to −0.25).9
The combination of at least 2 drugs with different modes of action has also studied. The analgesic effect documented with the combination of tramadol with acetaminophen has identified a reduction of at least 50% of the baseline pain score in 35% of cases and the combination of drugs is well tolerated and showed significant improvement in functionality as measured by the fibromyalgia impact questionnaire (FIQ).10 Furthermore, the combination of amitriptyline and fluoxetine reported larger improvement in FIQ and VAS scores compared to the use of monotherapy.11
Other alternatives are the newly identified auxiliary a2-δ subunit ligands of calcium voltage-dependent channels in the CNS (pregabalin, gabapentin). Several clinical trials have reported a significant improvement of pain in patients with FMS, but also improved functionality, mood and a reduction in sleep disturbances. On the other hand, data from clinical trials have been integrated into a meta-analysis showing a modification of the SMD for pain of −0.28 (95% CI, −0.36 to −0.20), improvement in sleep disturbance of −0.39 (95%, from −0.48 to −0.39) and improved quality of life of −0.30 (95% CI, −0.46 to −0.15).12 It is noteworthy that the Food and Drug Administration has approved at least 3 drugs for the treatment of FMS: pregabalin (in 2007), duloxetine (in 2008) and milnacipran (in 2009).13
One of the problems faced by patients in any health system is access to effective drugs. Many assistance programs are designed with high levels of evidence, but also a policy of cost containment, depending on the availability of often limited resources. Thus, the objective of this study was to identify which of the available drug treatments in the public health system in Mexico for the treatment of FMS is the most cost-effective.
Material and MethodsWe performed an economic analysis of the cost-effectiveness rate, with a Markov model construct used to compare the costs and efficacies of alternative first-line drug available in Mexico for the treatment of FMS.
Alternative Treatment ComparedIn conformity with international guidelines for the treatment of FMS14 and according to the list of drugs approved by the General Health Council for the care of patients in the public health system of Mexico, we identified the following treatment options: amitriptyline (baseline comparator) in an initial dose of 25mg/day, and increased to 50mg/day. The group of SSRIs included fluoxetine, with an initial dose of 20mgdaily and increasing to 80mg/day and duloxetine at a starting dose of 60mg/day and 120mg/day for maintenance. Of the a2-δ ligand drugs, gabapentin was chosen, with an initial dose of 900mg/day and increased, if necessary, to 1200mg/day, as well as pregabalin, with an initial dose of 300mg/day and up to 450mg/day. We included analgesics such as the combination of tramadol (37.5mg) with acetaminophen (325mg) with an initial dose of 150mg/1300mg/day and up to 300mg/2600mg/day. Finally, we added the combination of fluoxetine plus amitriptyline as an alternative, with starting dose of 20mg/12.5mg/day and followed by 80mg/50mg/day.11
Economic ModelThe most important symptom of FMS is chronic pain and its impact is mainly on functionality and quality of life. For the purposes of the model we used the information available through the visual analogue scale (VAS), where 0 represents no pain and 10 the most intense pain imaginable. The functionality in FMS was determined through the FIQ, which measures a multidimensional health status of patients. The FIQ scores are between 0 and 100, where 0 represents the highest functional capacity and 100 the worst state of health.15 The Markov model employed was adapted from a published study by Tarride et al.16 While the model mentioned above was designed for another indication (neuropathic pain), different from the present analysis, the inclusion of VAS as a determining factor in assessing the severity of pain has been widely recommended for the evaluation of patients with fibromyalgia according to current literature.17 On the other hand the assumptions of model were similar, with adult patients ≥18 years of age, diagnosed with fibromyalgia and men and women with chronic musculoskeletal pain. Patients were treated throughout the cycle or the end of the observation period (12 months). According to the natural history of disease we did not consider a risk of death associated with FMS and also not did not consider treatment changes throughout the observation period.
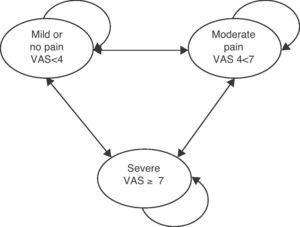
The basic model is shown in Fig. 1. There are 3 health states defined by VAS scores: no pain or mild pain (VAS<4 points), moderate pain (VAS 4–7 points) and severe pain (VAS>7 points). Patients entered into the model had moderate to severe pain. The probability of moving to a lower state of pain was identified by modifying the baseline VAS to a decrease in pain of 50% as a result of treatment and a decrease in the FIQ of 30%. The probability of migrating to a state of worst pain was considered treatment failure, which is a function of loss of effectiveness or treatment discontinuation due to intolerance to adverse events, and these were considered for the cases of antidepressants, anticholinergic, serotonin and gastrointestinal effects as well as dizziness and drowsiness produced by ligands of the a2-δ auxiliary subunit receptors. The cycles of the model were defined by periods of 3 months for a total time horizon of 12 months and 4 cycles were built.
Markov model of the health stages of the fibromyalgia syndrome.
The resulting measures of effectiveness of the model were: (a) the analgesic success rate, measured as the percentage of patients who reduced their VAS by 50% or more compared to the baseline measurement, and b) the rate of overall improvement, calculated by the percentage of patients decreasing the FIQ score by 30% with respect to baseline. Both measures should be achieved and sustained at the end of time horizon (12 months).
Sources of InformationFor probabilities assigned to each of the states of health we conducted a systematic review of the literature, which identified changes in the VAS and FIQ, and the rate of treatment discontinuation secondary to severe adverse events of FMS patients treated with the drug alternatives proposed for the analysis.
The review considered a period of 20 years of publications (1988–2008). We included only randomized clinical trials. Data was obtained from 3 studies using pregabalin,18–20 one using tramadol with acetaminophen,10 2 using duloxetine,21,22 one using gabapentin,23 4 using amytriptillin,11,24–26 3 with fluoxetine23,27,28 and one using a combination of fluoxetine with amytriptillin.11 In these studies we verified the existence of heterogeneity with the Cochrane Q statistic and finally the weighted averages were calculated for the data point; we also calculated 95% confidence intervals for each of the estimates. The data applied to the model is shown in Table 1.
Clinical Efficacy for the Treatment of Fibromyalgia Comparing Different Drugs.
| Drug | Reduction in VAS (95% CI) | Reduction in FIQ (95% CI) | Rate of dropout due to adverse events (95% CI) | Source |
| Pregabalin | −0.609 | −0.428 | 0.184 | Crofford,18 Arnold,19 Mease20 |
| (−0.540, −0.631) | (−0.409, −0.437) | (0.145, 0.208) | ||
| Tramadol/acetaminophen | −0.361 | −0.278 | 0.233 | Bennett10 |
| (−0.023, −0.655) | (−0.062, −0.372) | (0.209, 0.256) | ||
| Duloxetine | −0.515 | −0.460 | 0.205 | Arnold21,22 |
| (−0.502, −0.525) | (−0.451, −0.497) | (0.184, 0.222) | ||
| Gabapentin | −0.561 | −0.659 | 0.160 | Arnold23 |
| (−0.505, −0.618) | (−0.593, −0.725) | (0.144, 0.176) | ||
| Amitriptyline | −0.407 | −0.145 | 0.070 | Goldenberg,11 Hannonen,24 Carette25,26 |
| (−0.182, −0.437) | (−0.099, −0.175) | (0.030, 0.121) | ||
| Fluoxetine | −0.249 | −0.219 | 0.192 | Arnold,23,27Wolfe28 |
| (−0.214, −0.338) | (−0.192, −0.260) | (0–159, 0.256) | ||
| Fluoxetine/amitriptyline | −0.373 | −0.337 | 0.161 | Goldenberg11 |
| (−0.196, −0.700) | (−0.210, −0.577) | (0.145, 0.177) |
VAS: Visual analog scale; FIQ: Fibromyalgia Impact Questionnaire; 95% CI: 95% confidence interval.
The information for the calculation of costs was obtained through the identification of resource use and subsequent valuation in 2010.
Mexican pesos. Identification of the type and amount of resources used by patients with FMS was performed using a random sample of 5000 patients served by the Mexican Social Security Institute (IMSS) recorded in the institute's own Medical Operations Information System (SIMO), the Single Information System-Subsystem of hospital discharge (SUI-13)29 and the Single Information System-Subsystem of outpatient care (SUI-27)29 in 2006. The mean±SD age of the patients was 36±14 years, 55% were women.
The unit cost of the drugs was obtained from the website of the IMSS, which reported the purchase prices of medications bought by the institution in 2007.30 The information on the cost of the services offered by the IMSS was identified through the IMSS Timely Information Bulletin.31 All costs were adjusted for cumulative inflation up to December 2010, in accordance with reports by the consumer price index of the Banco de Mexico (4%). The unit cost of each resource and the annual frequency of use are shown in Table 2.
Fibromyalgia Syndrome Attention Costs, IMSS 2010.
| Resources | Price per Unita | Frequency of Use/Annual Occurrence | Annual Costa | Source |
| Services | ||||
| Outpatient family medicine visit | $ 535.00 | 1.75 | $ 936.25 | Boletín de Información Oportuna IMSS31 Subsistema de Información Médico Operativa 13 y 2729 |
| Outpatient specialist visit | $ 850.00 | 0.55 | $ 467.50 | |
| Hospital day costb | $ 4939.00 | 1.10 | $ 5432.90 | |
| Laboratory | $ 84.00 | 2.12 | $ 178.08 | |
| Other diagnostic auxiliaries | $ 3700.16 | 2.22 | $ 8214.36 | |
| Pharmacologic treatmentc | ||||
| Pregabalin (150mg. capsules Box with 28) | $ 324.46 | 1.032 | $ 4229.55 | Portal de transparencia IMSS30 |
| Tramadol/acetaminophen (tablet with tramadol 37,5 mg/acetaminophen 325mg. Box with 20 tablets) | $ 105.95 | 2.190 | $ 1933.60 | |
| Duloxetine (60mg. capsules Box with 14) | $ 325.66 | 730 | $ 8490.33 | |
| Gabapentin (300mg. capsules Box with 15) | $ 105.14 | 1.460 | $ 2558.35 | |
| Amitriptyline (25mg. tablets Box with 20) | $ 40.05 | 688 | $ 730.96 | |
| Fluoxetine (20mg. capsules Box with 14) | $ 6.47 | 1.376 | $ 118.08 | |
| Treatment of drug related adverse eventsd | $ 401.28 | 0.16 | $ 58.40 | |
The study was conducted to support decision makers in the field of social security in Mexico, so the perspective was that of a provider of public health. Initial analysis of cost-effectiveness was performed for a time horizon of one year and later projections were made for 3, 5 and 10 years to estimate the economic impact on each of the alternatives in these cases; we applied a discount rate 5%, both as to efficiency as to the costs.
Economic Analysis and Sensitivity AnalysisWe estimated cost-effectiveness ratios model for each alternative pharmacological treatment of FMS and the reasons for cost-effectiveness. We performed a probabilistic sensitivity analysis, with the nonparametric bootstrapping technique and 250 iterations were performed in this estimate. The model was made in Microsoft Excel 2007®, aided by its Simulation tools. Tests for heterogeneity and acceptability curves were performed using the Microsoft Excel 2007® statistical module.
ResultsThe baseline case results are shown in Tables 3 and 4. In relation to the expected costs for the first year of treatment, the lowest cost was obtained for treatment with amitriptyline ($ 9047.01), followed by fluoxetine alone ($ 10,183.89) and fluoxetine in combination with amitriptyline ($ 10,866.08). The duloxetine alternative was more expensive ($ 28,768.49).
Analysis of Cost Effectiveness, Reduction in Visual Analog Pain Scale Score for the Management of Fibromyalgia Syndrome, IMSS 2010.
| Amitriptyline | Pregabalin | Tramadol/acetaminophen | Duloxetine | Gabapentin | Fluoxetine | Fluoxetine+amitriptiline | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Costsa | $ 9047.01 | $ 21129.18 | $ 21942.55 | $ 28768.49 | $ 24495.71 | $ 10183.89 | $ 10866.08 |
| ($ 8626.08, $ 9467.95) | ($ 20694.38, $ 21563.97) | ($ 21536.09, $ 22349.03) | ($ 28374.04, $ 29165.08) | ($ 24108.66, $ 24882.76) | ($ 9779.63, $ 10588.15) | ($ 10349.35, $ 11192.48) | |
| Δ Costs | $ 12082.16 | $ 12895.54 | $ 19721.47 | $ 15448.70 | $ 1136.88 | $ 1819.07 | |
| ($ 11298.43, $ 12865.90) | ($ 11956.65, $ 13834.43) | ($ 16045.51, $ 23397.45) | ($ 13513.98, $ 17383.42) | ($ 924.72, $ 1349.04) | ($ 1592.83, $ 2045.31) | ||
| Reduction in VAS>50% | 22.2% | 44.8% | 17.9% | 34,2% | 38.1% | 6.2% | 13.6% |
| (21%, 24%) | (42%, 48% | (17%, 19%) | (28%, 41%) | (33%, 43%) | (5%, 7%) | (12%, 15%) | |
| Δ reduction in VAS>50% | 22.6% | −4.3% | 12.0% | 15.9% | −16.0% | −8.6% | |
| (21%, 24%) | (−5%, −4%) | (10%, 14%) | (14%, 18%) | (−19%, −13%) | (−10%, −8%) | ||
| CER (VAS) | $ 40752.32 | $ 47132.92 | $ 122816.08 | $ 84067.64 | $ 64234.14 | $ 164465.92 | $ 80173.00 |
| ($ 39972.57, $ 41632.65) | ($ 45172.60, $ 59096.66) | ($ 116601.64, $ 130006.51) | ($ 71831.33, $ 101910.53) | ($ 57987.06, $ 72269.91) | ($ 144102.75, $ 194172.97) | ($ 73446.56, $ 87206.38) | |
| ICER (EVA) | $ 53399.23 | $ −297363.88 | $ 164101.69 | $ 96965.08 | $ −7100.76 | $ 21030.93 | |
| ($ 46893.49, $ 60807.53) | ($ −344064.68, $ −257001.90) | ($ 112537.70, $ 239291.93) | ($ 75381.30, $ 124728.90) | ($−10359.03, −$ 4867.33) | ($ 27005.28, $ 16378.28) |
95% CI: 95% confidence interval; Δ Costs: incremental costs, VAS: Visual analog scale; CER: Cost effectiveness ratio; ICER: Incremental cost effectiveness ratio.
Cost Effectiveness Analysis, Global Improvement (FIQ) of Fibromyalgia, IMSS 2010.
| Amitriptyline | Pregabalin | Tramadol/acetaminophen | Duloxetine | Gabapentin | Fluoxetine | Fluoxetine+amitriptyline | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Costsa | $ 9047.01 | $ 21129.18 | $ 21942.55 | $ 28768.49 | $ 24495.71 | $ 10183.89 | $ 10866.08 |
| ($ 8626.08, $ 9467.95) | ($ 20694.38, $ 21563.97) | ($ 21536.09, $ 22349.03) | ($ 28374.04, $ 29165.08) | ($ 24108.66, $ 24882.76) | ($ 9779.63, $ 10588.15) | ($ 10349.35, $ 11192.48) | |
| Δ Costs | $ 12082.16 | $ 12895.54 | $ 19721.47 | $ 15448.70 | $ 1136.88 | $ 1819.07 | |
| ($ 11298.43, $ 12865.90) | ($ 11956.65, $ 13834.43) | ($ 16045.51, $ 23397.45) | ($ 13513.98, $ 17383.42) | ($ 924.72, $ 1349.04) | ($ 1592.83, $ 2045.31) | ||
| Reduction in FIQ≥30% | 15.2% | 31.6% | 13.7% | 28.5% | 29.1% | 6.6% | 18.8% |
| (14%, 16%) | (295%, 33%) | (13%, 15%) | (23%, 34%) | (25%, 33%) | (5%, 8%) | (16%, 21%) | |
| Δ reduction in FIQ | 16.4% | −1.5% | 13.3% | 13.9% | −8.6% | 3.6% | |
| (15%, 17%) | (−1.5%, −1.3%) | (10%, 16%) | (12%, 16%) | (−10%, −7%) | (3.2%, 4%) | ||
| CER (FIQ) | $ 59715.31 | $ 66968.69 | $ 159660.45 | $ 101030.62 | $ 84175.79 | $ 154820.75 | $ 57706.23 |
| ($ 58580.15, $ 61013.00) | ($ 64183.36, $ 70140.45) | ($ 151582.12, $ 169008.45) | ($ 86325.31, $ 122473.81) | ($ 75989.29, $ 94706.29) | ($ 135651.80, $ 182785.63) | ($ 52864.74, $ 62768.67) | |
| ICER (FIQ) | $ 73669.10 | $ −916515.36 | $ 148006.04 | $ 110739.99 | $ −13262.14 | $ 49434.53 | |
| ($ 64693.85, $ 83889.54) | ($ −1060453.48, $ −792114.35) | ($ 101499.52, $ 215821.37) | ($ 101499.52, $ 215821.37) | ($ −19347.64, $ 9090.74) | ($ 38498.17, $ 63477.62) |
Δ Costs: incremental costs; FIQ: fibromyalgia impact questionnaire, 95% CI: 95% confidence interval; CER: cost effectiveness ratio; ICER: incremental cost effectiveness ratio.
By identifying the health outcomes, the probability of achieving a reduction of VAS over 50% of its baseline score (Table 3) was higher with pregabalin (44.8%), the next best alternative was gabapentin (38.1%) and duloxetine (34.2%). The alternative treatment to lower effectiveness in reducing the VAS score was fluoxetine as monotherapy (6.2%). When analyzing the percentage reduction in the FIQ scale (Table 4), the largest proportion was reached with pregabalin (31.6%), followed by gabapentin (29.1%) and duloxetine (28.5%). The alternative treatment that reached a lower overall improvement measured by the FIQ was fluoxetine as monotherapy.
Treatment with pregabalin, gabapentin or duloxetine have better results in the modification of the VAS and FIQ, but are more costly than its comparator, amitriptyline. Of these 3 comparators, pregabalin provides better clinical outcomes at a lower cost, absolutely dominating gabapentin and duloxetine.
Analysis of net savings per capita, on the horizon of 3, 5 and 10 years, is presented in Table 5; the data show the difference in costs and changes in the FIQ to be gained by treating patients with pregabalin, gabapentin or duloxetine, in relation to expected with regular use of amitriptyline over time. It is seen that in none of these cases there are savings. However, among these 3 alternatives, pregabalin treatment proves to be a saver with greater global improvement at a lower cost at 3, 5 and 10 years of treatment.
Analysis of Net Savings per Patient in the Management of Fibromyalgia Compared With Amitriptyline, IMSS 2010.
| Pregabalin | ||||
| 1 Year | 3 Years | 5 Years | 10 Years | |
| Cost* | ||||
| Drug treatment | $ −13933.98 | $ −48390.96 | $ −88918.39 | $ 226969.64 |
| Hospital treatment | $ 541.39 | $ 1880.20 | $ 3454.87 | $ 8818.83 |
| Medical visit | $ −214.29 | $ −744.17 | $ −1367.42 | $ −3490.42 |
| Laboratory and diagnostic aids | $ 1530.02 | $ 5313.58 | $ 9763.71 | $ 24922.49 |
| Adverse events | $ −5.32 | $ −18.49 | $ −33.98 | $ 86.74 |
| Total | $ −12082.17 | $ −41959.84 | $ −77101.20 | $ −196805.70 |
| Benefit | ||||
| Reduction in FIQ** | 16.4% | 18.9% | 20.9% | 26.7% |
| Net savings | $ −13953.80 | $ −48459.80 | $ 89044.88 | $ 227292.67 |
| Cost* | Gabapentine | |||
| Drug treatment | $ −16860.32 | $ −58553.78 | $ 107592.56 | $ −274636.81 |
| Hospital treatment | $ 494.36 | $ 1716.85 | $ 3154.73 | $ 8052.62 |
| Medical visit | $ −150.13 | $ −521.41 | $ −958.10 | $ −2445.61 |
| Laboratory and diagnostic aids | $ 1072.04 | $ 3723.03 | $ 6841.07 | $ 17462.26 |
| Adverse events | $ −4.64 | $ −16.09 | $ −29.57 | $ −75.48 |
| Total | $ −15448.70 | $ 53651.40 | $ −98584.44 | $ 251643.02 |
| Benefit | ||||
| Reduction in FIQ** | 13.9% | 16.1% | 17.8% | 22.7% |
| Net savings | $ −51024.69 | $ −177202.38 | $ −325609.37 | $ 831138.47 |
| Cost* | Duloxetine | |||
| Drug treatment | $ −20906.80 | $ −72606.71 | $ −133414.83 | $ 340549.77 |
| Hospital treatment | $ 371.64 | $ 1290.66 | $ 2371.57 | $ 6053.60 |
| Medical visit | $ −133.76 | $ −464.54 | $ −853.58 | $ −2178.81 |
| Laboratory and diagnostic aids | $ 955.09 | $ 3316.88 | $ 6094.78 | $ 15557.30 |
| Adverse events | $ 7.63 | $ −26.51 | $ 48.71 | $ −124.34 |
| Total | $ 19721.47 | $ 68490.21 | $ 125850.76 | $ 321242.03 |
| Benefit | ||||
| Reduction in FIQ** | 13.3% | 16.1% | 17.0% | 21.7% |
| Net savings | $ −88290.73 | $ −306622.69 | $ −563419.18 | $ −1438163.02 |
FIQ: Fibromyalgia impact questionnaire.
aCosts expressed in Mexican pesos 2010.
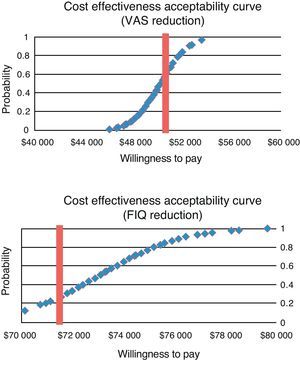
In the probabilistic sensitivity analysis, to construct acceptability curves, from a level of willingness to pay $ 50000 (≈U.S. $ 3850; exchange rate: 13.0 pesos per U.S. dollar) for one additional patient to succeed in changing the VAS, pregabalin is a cost-effective alternative in 80% of cases. The curve is shown in Fig. 2. For measuring the effectiveness of the percentage reduction of FIQ, with a willingness to pay $ 75000 (≈ U.S. $ 5770) for one additional patient, the total improvement cost for pregabalin is effective in 70% of cases (Fig. 2).
DiscussionChronic pain syndromes, especially those of musculoskeletal origin, have been addressed in health services, as they have increased as a complaint, affecting the economically active population and represent a high impact in terms of direct, indirect and even non-tangible costs.
Our results show that the best clinical effect of treatment of FMS is expected with the use of duloxetine, gabapentin and pregabalin. Of the 3 alternatives, pregabalin achieves the highest proportion of patients with clinical success for pain control (44%) and the greatest impact on the overall improvement measured by FIQ functionality (31%). Also among the three, pregabalin leads to lower costs.
Thus, in the comparison between these 3 treatment options for FMS, pregabalin dominates absolutely, providing better clinical outcomes at lower costs. By comparing pregabalin with standard treatment, which in this case corresponds to amitriptyline, the former leads to better clinical outcomes but at a higher cost. The additional cost, the system will have to assume for one additional patient in order to reach better pain control and improved functionality, is between $ 50000 and $ 75000 dollars annually. In a hypothetical level of willingness to pay $ 50000 for one additional patient for adequate pain control and $ 75000 for overall improvement, pregabalin is a cost-effective alternative in 60% and 80% of cases, respectively, for the treatment FMS in the public health system in Mexico. It is correct to say that Mexico has not yet accepted cost-effectiveness thresholds as measures of effectiveness as used in this investigation, nor have these been seen by other authors. However, it is worth mentioning that levels of $ 50000 or $ 75000 are below the GDP per capita in the country (≈ $ 130000/U.S. $ 13900),32 so that even under these parameters, the results of the research indicate that the alternatives would be highly cost-effective, as suggested by the World Health Organization.33
The data obtained in this study are similar to those reported by Choy et al.34 who performed a model for the health system in the UK, where the comparators used were amitriptyline, duloxetine, tramadol and gabapentin. In the latter model it was estimated that with a willingness to pay 30000 pounds per year of life adjusted for quality of life (QALY), pregabalin is a cost-effective alternative in 60% of cases. On the other hand, a recent study in the United States of America,35 which analyzed the use of duloxetine versus routine treatment, showed an incremental cost-effectiveness per QALY gained of about U.S. $ 47500 as a first-line treatment and U.S. $ 16565 as a second-line treatment in a 2 year time horizon. In this regard it is consistent with our analysis and duloxetine could be an second line option, since our study, the ICER of this alternative was high in comparison to pregabalin.
The medical literature shows little in relation to economic analyses for FMS, and there are other models made for non-drug alternatives that are shown to be cost-effective compared with placebo and require additional infrastructure.36,37 In the data shown in both the UK and Mexico, there is consistency with this pharmacoeconomic behavior, so it can be taken into account as a viable alternative for the treatment of FMS. It is important to consider that even if the perspective with which we worked in the study considered only direct costs, social responsibility with health institutions to reinstate the individual to his or her workplace and restore the quality of life of patients may justify the additional costs necessary to use new treatment alternatives.
The limitations of this study are related to the construction of a model which attempts to represent the reality of the clinical course of FMS and the potential changes associated with different treatments compared. The model is fed information from external sources, so there is no joint distribution of costs and clinical outcomes, and also there is no “face to face” comparison between the various treatment alternatives. However, tests were conducted to ensure that the information contained in the model had sufficient internal validity to be taken into account and in this way, the biases found in the results of this research are those associated with the publications themselves, without a differential bias for any of the treatment alternatives compared.
The information obtained from this economic evaluation between drug alternatives for the treatment of FMS may be useful to support decision makers in health systems similar to institutions offering public health services as in Mexico.
Funding SourcesPfizer S.A. de C.V financed the present study.
Conflict of InterestPfizer S.A. de C.V financed the present study without this generating any legal or result compromise. Upon reviewing the manuscript Dr. Joaquín F Mould Quevedo was an employee of Pfizer S.A de C.V.
Please, cite this article as: Arreola Ornelas H, et al. Análisis de coste-efectividad en el tratamiento farmacológico del síndrome de fibromialgia en México. Reumatol Clin. 2012;8:120–7.