Family planning in women with immune-mediated inflammatory diseases is a challenge for healthcare teams, highlighting the need for standardized available evidence to provide patients with objective and agreed information. This study reflects the work performed by a multidisciplinary team in reviewing available scientific evidence, and the strategy agreed for family planning, pregnancy, postpartum, and breastfeeding in patients with immune-mediated inflammatory diseases.
MethodsA literature search was conducted, information was structured across the different stages (preconception, pregnancy, postpartum and breastfeeding), and an on-site meeting was convened, in which patients and healthcare providers participated.
ResultsSpecific materials, which are included in this work, were developed to guide clinical decisions to be agreed upon by patients and healthcare providers.
ConclusionThese materials meet the need for validated and updated information on the approach and use of indicated drugs for professionals responsible for the management of immune-mediated inflammatory diseases.
El reto terapéutico que supone para los equipos asistenciales la planificación familiar en mujeres con enfermedades inflamatorias inmunomediadas remarca la necesidad de armonizar la evidencia disponible para proporcionar a las pacientes información objetiva y consensuada. Este artículo refleja el trabajo realizado por un equipo multidisciplinar de revisión de la evidencia científica disponible y la estrategia de actuación consensuada en la planificación familiar, embarazo, posparto y lactancia materna de pacientes con enfermedades inflamatorias inmunomediadas.
MétodosSe realizó una búsqueda bibliográfica, se estructuró la información a lo largo de las diferentes etapas (preconcepción, embarazo, posparto y lactancia materna) y se realizó una reunión presencial para consensuar dicha información en la que participaron tanto pacientes como profesionales de la salud.
ResultadosSe desarrollaron materiales específicos incluidos en este trabajo y que pueden servir de guía en la toma de decisiones consensuada entre pacientes y profesionales de la salud.
ConclusiónEstos materiales responden a la necesidad de que los profesionales responsables del manejo de pacientes con enfermedades inflamatorias inmunomediadas posean información validada y actualizada sobre las actuaciones y el uso de fármacos indicados para estas enfermedades.
A large proportion of immune-mediated inflammatory diseases, such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), or axial spondyloarthritis (AxSpA), present in women and may begin in their childbearing years.1 This fact, coupled with the fact that approximately half of all pregnancies are unplanned,2 underscores the importance of family planning in these women. It is vitally important that the therapeutic approach and family planning be provided from a multidisciplinary approach, providing patients with complete and consensual information to aid them in their decision-making.
The main challenge healthcare teams face is that of establishing an appropriate treatment plan to keep the disease under control without endangering the health of the mother or the foetus/ newborn.3,4 Given that not all effective treatments to control disease activity are compatible with the stages of preconception, pregnancy, postpartum, and breastfeeding, the recommendation is that treatment be adjusted before planning any pregnancy, avoiding drugs that present toxicity to the foetus or newborn.5
A study that included 622 women of childbearing age with chronic rheumatological diseases revealed patients’ confusion and lack of information regarding family planning, pregnancy, and breastfeeding, resulting in a high proportion of voluntary treatment discontinuations.6
The few prospective studies addressing the safety of treatments during pregnancy and breastfeeding poses an additional challenge for health professionals.7 Efforts must therefore be coordinated to provide objective, up-to-date, and consensual information to both patients and professionals. This document is the result of the work carried out by a multidisciplinary team with the aim of unifying the criteria to be followed in family planning, pregnancy, postpartum, and breastfeeding in women with RA, PsA, or AxSpA.
MethodsThis document is the result of a multidisciplinary working meeting with the participation of a total of 11 participants, including patients and healthcare professionals. The patient group comprised a total of 5 women of childbearing age with a diagnosis of immune-mediated inflammatory disease (RA, PsA, or AxSpA). Six healthcare professionals also took part: three rheumatologists, a gynaecologist, a hospital pharmacist, and a nurse working in Spanish public healthcare centres in different geographical locations.
The aim of the meeting was to harmonise the strategy for action in female patients of childbearing age with immune-mediated inflammatory diseases during family planning, pregnancy, postpartum, and breastfeeding. This integrated strategy culminated in the creation of briefing documents that were developed in the following phases:
A search of the bibliography was performed covering the following topics: (1) family planning in patients with immune-mediated inflammatory diseases; (2) disease management in both males and females of childbearing age, and (3) pregnancy, postpartum, and breastfeeding in women with these diseases. During the family planning and conception stages, both men and women were considered, while the pregnancy, delivery, postpartum, and breastfeeding phases focused solely on women.
Taking the scientific evidence and the experts’ experience into account, 2 types of initial documents were developed, targeting either patients or professionals. The documents for patients covered relevant information from the patient’s perspective and included checklists and frequently asked questions, while the documents for professionals were of a scientific nature (checklists, frequently asked questions, and treatment table). Prior to the meeting, patient participants reviewed the patient-specific materials, while healthcare professionals were asked to review both documents. During the face-to-face meeting, attendees were divided into 2 working groups (patients and healthcare professionals) in order to evaluate and adapt the materials separately. In the patient group meeting, the limitations and/or difficulties that these diseases cause in their daily lives and possible measures to address these issues were discussed. The group of healthcare professionals worked on screening the information based on scientific evidence and its relevance for the professionals who care for these patients. The contributions made by each working group were then pooled and incorporated into the original documents for further review. The materials were also reviewed by another 14 healthcare professionals that included rheumatologists, gynaecologists, hospital pharmacists, nurses, and psychologists. All comments and reviews were compiled so as to create the final materials for dissemination and that can be found in summarised form in this paper.
ResultsPreconceptionThe European League Against Rheumatism (EULAR) recommends family planning for all patients of childbearing age and stresses the importance of regularly emphasising the benefits of family planning for individuals with inflammatory diseases.4 Preconception counselling should range from contraceptive measures for individuals who do not wish to become pregnant to treatment options for those who are willing to conceive. In the latter group of patients, the main issues to be addressed will be: potential influence of the disease on fertility, impact of the disease on pregnancy and vice versa, adaptation of treatment, maternal-foetal risk factors, etc.
It is especially advisable that all this information be communicated clearly and consensually, which requires close collaboration between the various care teams, including rheumatologists, primary care physicians, midwives, obstetricians, and hospital pharmacists (especially in patients receiving drugs dispensed in the hospital). Good communication between professionals and patients can significantly reduce the risks and increase chances of success, leading to better compliance and disease control. Table 1 illustrates a list of frequently asked questions surrounding management during the preconception phase.
List of frequently asked questions to be addressed during preconception, pregnancy, postpartum, and breastfeeding in women with immune-mediated inflammatory diseases.
| When will I be able to become pregnant? |
| Before making this decision, it is important that your patients talk to you so that you can plan together to get the disease under control at least 3−6 months before they attempt to become pregnant. |
| Will the medication affect my child? |
| It may be necessary to discontinue some of the medications you are taking before becoming pregnant or during breastfeeding. But if you plan your treatment plan early and correctly, you can receive medication that is safe for both you and your child. |
| If it is the father who takes medication for these conditions, do you have to take any special precautions for your family planning? |
| Some drugs (such as sulphasalazine) can decrease male fertility, so it is sometimes recommended that certain medications be discontinued before planning a pregnancy. In this case, you can advise and inform the patient about which medications he/she can take and which ones should be discontinued. |
| Is it possible that my child will inherit my disease? |
| The chances of the newborn inheriting RA/ PsA are very small. The probability of inheriting AxSpA is estimated to occur in one in 6 couples if one parent carries the HLA-B27 gene. The chances are even lower if they do not carry the HLA-B27 gene. However, the way in which the disease is inherited is complex, so that the patient does not necessarily pass the disease on to her child. |
| What is my risk of miscarriage, foetal malformation, or premature birth? |
| There is a slightly increased risk of miscarriage in women with rheumatic diseases, especially with the use of certain medications and the presence of uncontrolled disease during pregnancy. This risk should be discussed with the patient and they should be reassured by close monitoring. |
| The risk of congenital malformation does not appear to be higher in women with RA than in any other pregnant woman. However, some drugs, such as methotrexate, are known to be teratogenic if taken during pregnancy and should be discontinued early. |
| There is a slightly increased risk of premature births, particularly if the disease is not well controlled, although proper monitoring is associated with a decrease in this risk and this will reassure your patient. |
| Will my disease affect my child's growth? |
| There may be a risk of lower birth weight in women with increased disease activity during pregnancy. |
| Will I be able to have a vaginal delivery? |
| Patients with rheumatic disease can have a vaginal delivery. However, a slight increase in the number of Caesarean sections has been reported in women with uncontrolled rheumatic disease or if they have physical limitations that make vaginal delivery difficult (such as osteoarthritis of the hip). |
| Will I be able to breastfeed my baby and when? |
| Your patient can choose to breastfeed as long as their disease is controlled and the medication they are taking is compatible with it. In each case, how the delivery went and the condition of the newborn must also be factored in. |
| Will I be able to receive epidural anaesthesia? |
| Normally there is no reason why a woman in her condition cannot receive this kind of anaesthesia, always according to the anaesthetist’s criteria. |
| Will my disease activity worsen after delivery? |
| There is a possibility that this may occur, even in those patients who experienced improvement or remission of the disease during pregnancy. However, there are drugs that are compatible with breastfeeding that can help normalise your patient’s daily life after delivery without them having to choose between disease control and breastfeeding. |
| What kind of adapted childcare products will I need to care for my child? |
| Some examples would be articulated beds, height-adjustable tables, prams and buggies, ergonomic backpacks, breastfeeding cushions, etc. They can receive this type of advice in specialised childcare shops and also from your hospital’s nursing team, midwife, and occupational therapists. |
PsA: Psoriatic arthritis; RA: Rheumatoid arthritis; AxSpA: Axial spondyloarthritis.
Various studies have yielded contradictory results as far as the impact of the disease on fertility is concerned; while some show no correlation between the two whatsoever,8,9 others reveal lower fertility in patients with these diseases.10–12 Likewise, certain drugs have been seen to affect fertility both in women (prednisone [depending on the dose], non-steroidal anti-inflammatory drugs, or prior use of cyclophosphamide) and men (sulphasalazine).10–13 The multidisciplinary team of this study advocated the need to explain that, although these patients may have greater difficulties in conceiving, pregnancy is possible in this population and that there is the possibility of using assisted reproduction techniques in those patients who experience difficulties in conceiving.7
From the point of view of heritability, it is important to explain to the patient that, although the probability of transmitting the disease may be higher than in the general population,14 that does not necessarily mean that they will transmit the disease to their child.
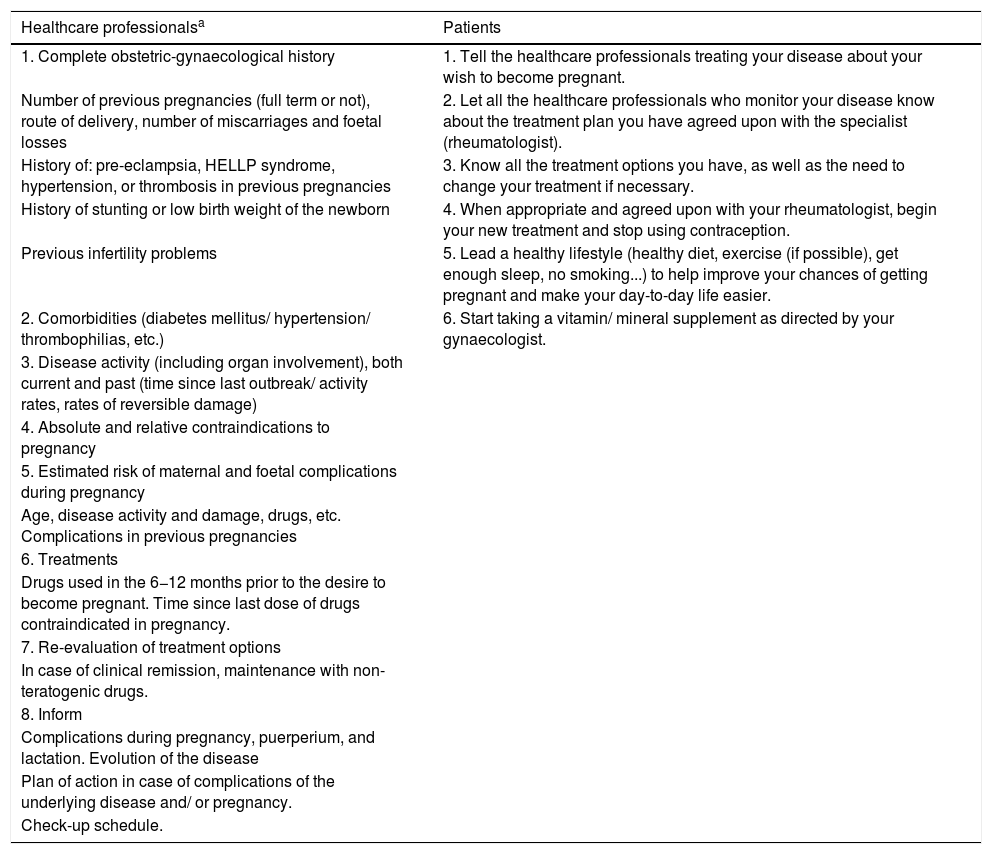
During the preconception phase, a gynaeco-obstetric assessment and rheumatological history-taking is recommended, in addition to the usual prenatal care routinely provided to women (diet, physical activity, risk factors, etc.).15 Likewise, an individual evaluation of the activity of the disease, the treatments used, and the presence of comorbidities or contraindications to pregnancy is recommended, to make it possible to elaborate an effective action and follow-up plan.16Table 2 displays the most relevant considerations, at the discretion of the multidisciplinary team, to be followed during the preconception stage by both patients and healthcare professionals.
Considerations during the preconception phase for healthcare professionals and patients.
| Healthcare professionalsa | Patients |
|---|---|
| 1. Complete obstetric-gynaecological history | 1. Tell the healthcare professionals treating your disease about your wish to become pregnant. |
| Number of previous pregnancies (full term or not), route of delivery, number of miscarriages and foetal losses | 2. Let all the healthcare professionals who monitor your disease know about the treatment plan you have agreed upon with the specialist (rheumatologist). |
| History of: pre-eclampsia, HELLP syndrome, hypertension, or thrombosis in previous pregnancies | 3. Know all the treatment options you have, as well as the need to change your treatment if necessary. |
| History of stunting or low birth weight of the newborn | 4. When appropriate and agreed upon with your rheumatologist, begin your new treatment and stop using contraception. |
| Previous infertility problems | 5. Lead a healthy lifestyle (healthy diet, exercise (if possible), get enough sleep, no smoking...) to help improve your chances of getting pregnant and make your day-to-day life easier. |
| 2. Comorbidities (diabetes mellitus/ hypertension/ thrombophilias, etc.) | 6. Start taking a vitamin/ mineral supplement as directed by your gynaecologist. |
| 3. Disease activity (including organ involvement), both current and past (time since last outbreak/ activity rates, rates of reversible damage) | |
| 4. Absolute and relative contraindications to pregnancy | |
| 5. Estimated risk of maternal and foetal complications during pregnancy | |
| Age, disease activity and damage, drugs, etc. Complications in previous pregnancies | |
| 6. Treatments | |
| Drugs used in the 6−12 months prior to the desire to become pregnant. Time since last dose of drugs contraindicated in pregnancy. | |
| 7. Re-evaluation of treatment options | |
| In case of clinical remission, maintenance with non-teratogenic drugs. | |
| 8. Inform | |
| Complications during pregnancy, puerperium, and lactation. Evolution of the disease | |
| Plan of action in case of complications of the underlying disease and/ or pregnancy. | |
| Check-up schedule. |
HELLP: haemolytic anaemia, elevated liver enzyme, low platelet count.
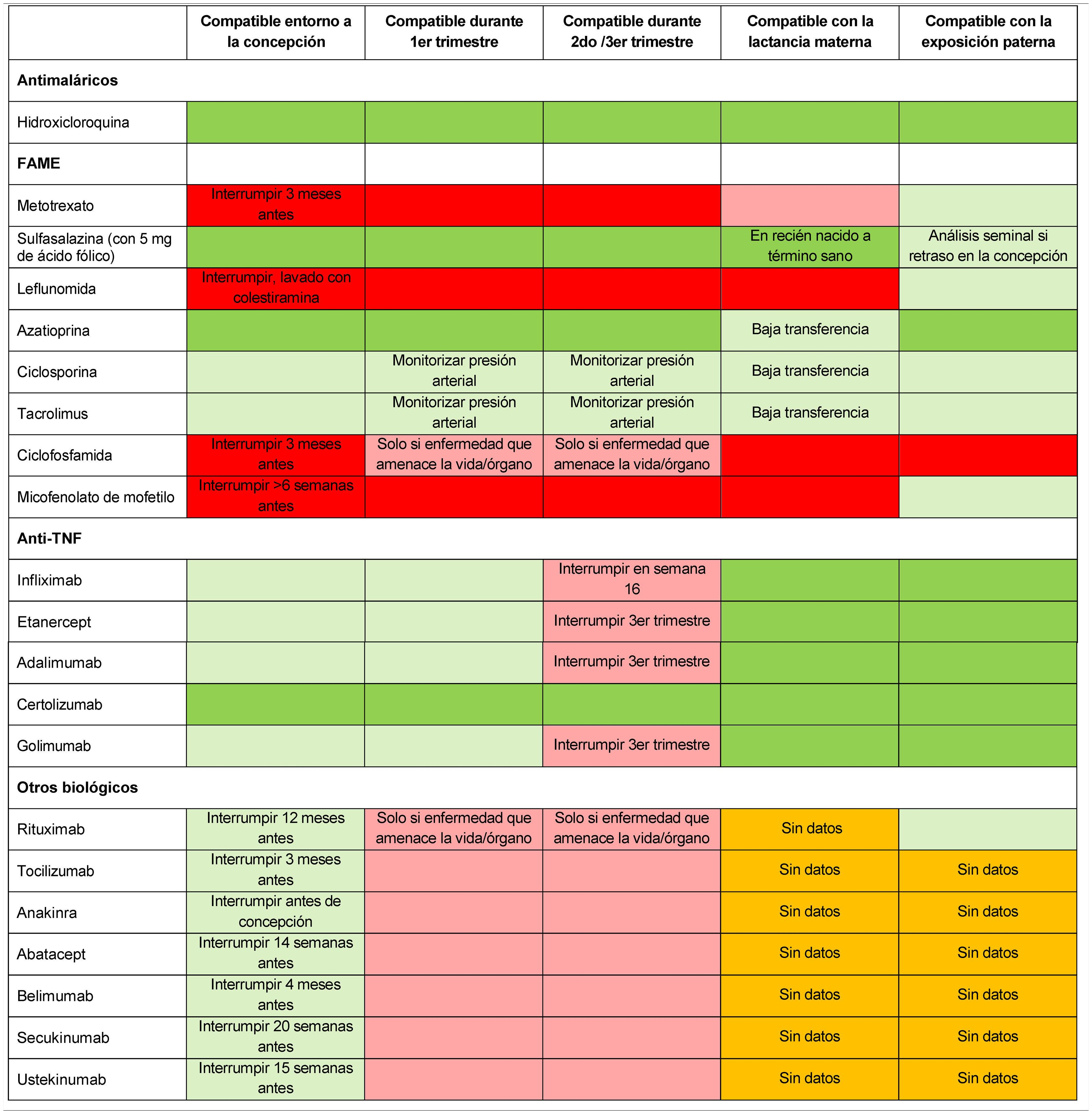
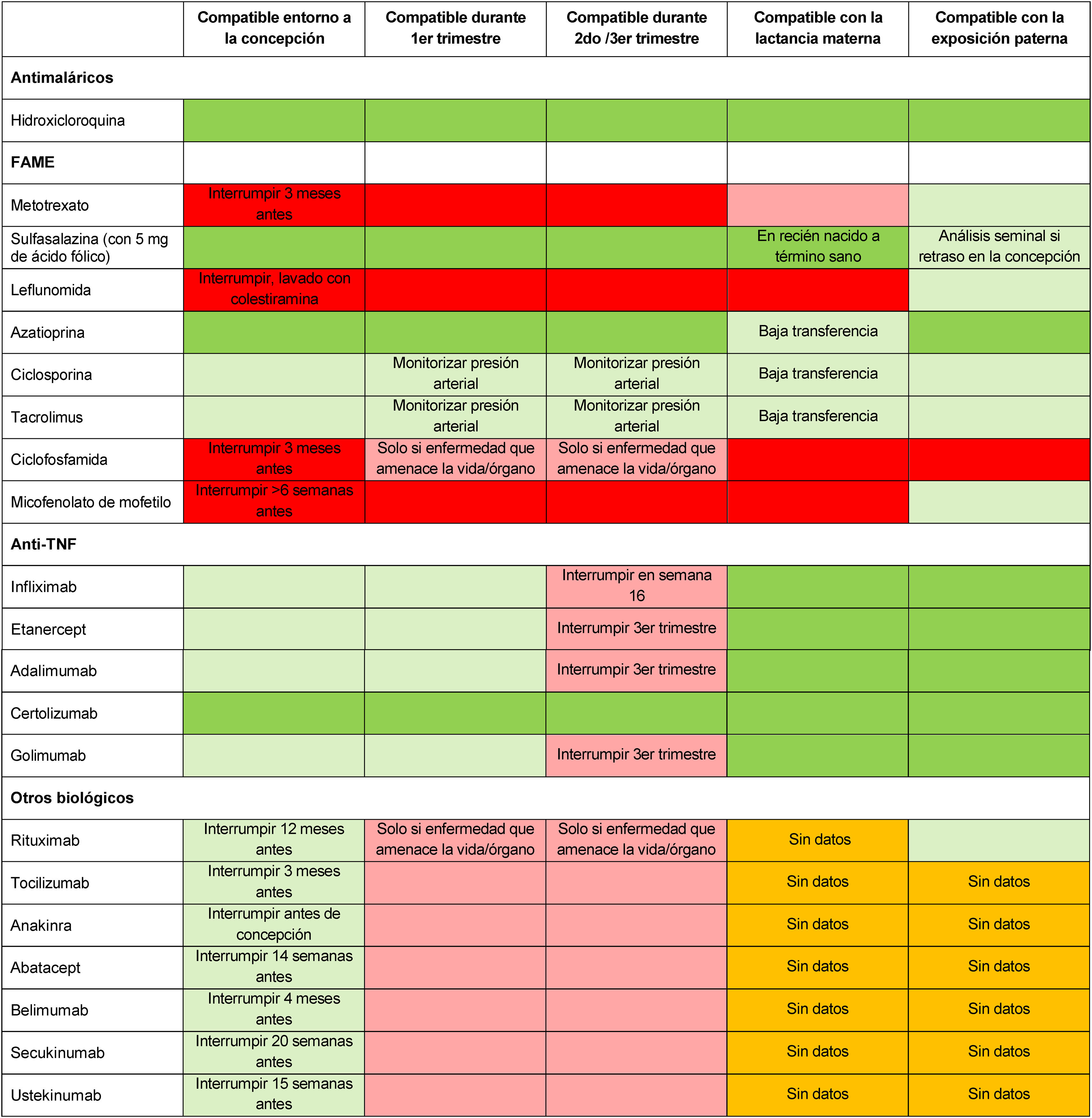
One of the key aspects to be addressed during family planning is whether to continue or adjust treatment. The treatment algorithm should be modified to accommodate the needs of the different stages (preconception, pregnancy, postpartum, and breastfeeding) and generally pursue 2 objectives: (1) efficacy, keeping disease activity under control, and (2) maternal and foetal safety, circumventing the risks to the mother or foetus/ infant posed by the use of drugs that cross the placenta and/ or breast milk.17 The authors highly recommend that low disease activity or remission be achieved for at least 3−6 months prior to pregnancy, which may entail considering postponing pregnancy until the disease is controlled or in remission.17 At this stage, therapeutic options should be appraised on an individual basis and, if necessary, the treatment prescribed by the different healthcare teams should be adjusted and agreed upon with the patient, always taking her preferences into account.5 During pregnancy planning, discontinuation of less safe treatments, such as methotrexate, leflunomide, mycophenolate mofetil, or some biologics is advisable.5,7,18 Treatment with hydroxychloroquine, azathioprine, or sulfasalazine is considered to be compatible with pregnancy.7 Treatment with tumour necrosis factor (TNF) antagonists is deemed safe during a planned pregnancy, inasmuch as it has been demonstrated that immunoglobulin G does not cross the placenta during the first trimester19,20 (Table 3). If considering anti-TNF treatment, it is important to consider the potential placental transfer throughout the pregnancy and the possibility of being able to use the drug throughout the pregnancy, in order to avoid a change of treatment during gestation. Within the group of biologics, certolizumab pegol (CZP) is the only one with prospective studies of placental transfer and levels in breast milk that support the update of its label to include its potential use during pregnancy and breastfeeding in women with immune-mediated inflammatory diseases.2,21,22 There are limited data regarding the compatibility of other biologics during pregnancy.7
Compatibility of major drugs in conception, pregnancy, breastfeeding, and paternal exposure.
DMARDs: disease-modifying antirheumatic drugs; TNF: tumour necrosis factor.
The colour of the cells indicates, from darkest (black) to lightest grey, the following categories: not recommended for use based on scientific evidence; conditionally not recommended for use based on limited scientific evidence; conditionally recommended for use based on limited scientific evidence, or recommended for use based on scientific evidence. White cells indicate that there are no data available. Adapted by Götestam et al.,5 Sammaritano et al.,7 Flint et al.18
In the case of unplanned pregnancies, it is important to assess whether there has been exposure to non-recommended drugs. In this case, the exact date of exposure should be confirmed and an ultrasound examination of the foetus should be performed.5
PregnancyIt is important to explain the most common symptoms associated with pregnancy, noting that these can vary from one patient to another, and that attention must be paid to any changes in symptomatology that can be attributed to the disease.
Several studies have shown that disease symptoms may improve during pregnancy, especially in patients with RA.17,23,24 However, symptoms may remain unchanged or even worsen in some cases and approximately 50% of patients have been seen to require treatment to control disease activity during pregnancy.3 This will depend to a large extent on the disease activity prior to conception and during pregnancy, so it is recommended that the disease be as controlled as possible throughout pregnancy. Likewise, treatment discontinuation due to pregnancy is associated with an increased risk of pre-term delivery25 and it is vital that patients not make any changes to their medication without first consulting with their treating physician. Table 1 displays the response to pregnancy-related questions at the discretion of the multidisciplinary team.
Regardless of whether the pregnancy is planned or unplanned, the appropriateness of treatment in the different trimesters of pregnancy must be assessed on a case-by-case basis.26 Some medications, such as methotrexate and leflunomide, should be withdrawn due to their contraindication during pregnancy.5,27 Other drugs can be continued, such as sulphasalazine supplemented with folic acid5 or hydroxychloroquine.28 A recent study has demonstrated that the use of antimalarials during pregnancy is associated with a lower risk of pre-eclampsia.29 As for TNF antagonists, CZP is the only one compatible with all 3 trimesters of gestation with minimal placental transfer as the absence of Fc in its structure prevents its transfer mediated by neonatal FcRn receptors.18,30 In the prospective CRIB study, minimal levels of CZP were detected in the plasma of newborns.22 An earlier study revealed that placental transfer was lower with CZP than with adalimumab and infliximab.31,32 Etanercept exhibits less placental exchange than adalimumab and infliximab and will therefore be an alternative in cases in which CZP cannot be used. Treatment with other anti-TNF agents (infliximab, etanercept, adalimumab, or golimumab) should be interrupted in the third trimester if the disease is controlled, to avoid the risk of placental transfer.7 There are not sufficient safety data with respect to other biologics do and, thus, should be discontinued during pregnancy.4,33Table 3 describes the compatibility of the leading drugs for the treatment of RA, PsA, and AxSpA during conception, pregnancy, breastfeeding, and paternal exposure.
During this stage, it is fundamental that the different care teams that will follow the pregnancy (gynaecologists, obstetricians, midwives, primary care physicians and nurses), treat the immune-mediated inflammatory disease (rheumatologists), and validate and dispense the treatments in most cases (hospital pharmacists) be coordinated and brought together.
Childbirth and postpartumIn the opinion of the multidisciplinary team, it is important that during this period, the pregnant woman be informed of the different methods of delivery and the implications they have for her health. RA, in particular, has been associated with increased Caesarean births and pre-eclampsia, as well as with an increased risk of low birth weight.34 This again underlines the importance of maintaining adequate disease control throughout pregnancy. Despite this, it is important to note that vaginal delivery is possible in most of these patients. Table 1 illustrates the answers to some of these questions in order to help professionals provide as clear and complete information as possible.
It has been reported that disease activity sometimes worsens after delivery, which can complicate the care of the newborn.17,24 Studies have proven a 50%–90% incidence of reactivation of inflammation in patients during the first 4 weeks following delivery.10 Consequently, monitoring the status of the disease throughout this stage is essential.10 The specialist must assess and adjust treatment based on the patient’s condition, always in line with the therapeutic objectives, i.e., to control disease activity without compromising the health of the newborn.2,4 The multidisciplinary team also considered it relevant that patients be informed that they can ask for help from different professionals (paediatrician, nurses, physiotherapists, occupational therapists) to adapt the various parenting tasks to their functional possibilities.
BreastfeedingScientific evidence supports the benefits of breastfeeding for the health of newborns.35 It is important to note that breastfeeding is possible for patients with immune-mediated inflammatory diseases. It is advisable that the decision to breastfeed be make jointly by the patient and the specialists, bearing in mind the mother’s wishes, the medication she is using, the level of control of the disease, and the needs of the newborn.26 During this stage, the disease must also be controlled as much as possible to reduce the intrinsic difficulties that this period entails and that could be aggravated by the disease itself. The option of not breastfeeding or resorting to mixed breastfeeding can also be considered if the mother so wishes or the circumstances so require.
When breastfeeding is chosen, guarantees must be made to ensure that the treatment administered is compatible with breastfeeding, avoiding drugs that pass into breast milk. Certain drugs, like sulfasalazine or hydroxychloroquine, are not contraindicated during breastfeeding, while methotrexate or leflunomide are. With respect to anti-TNF agents, continuing with these medications is not contraindicated, given that very little of them actually transfers to breast mild.5 Based on the publication of the prospective CRADLE study, CZP is compatible with breastfeeding, as it has minimal transfer into breast milk (<0.2%).36 Other biologics, such as anakinra, ustekinumab, abatacept, or tocilizumab, among others, have not presented data about their use in nursing mothers.5
DiscussionTwo of the most innovative features of this study are its multidisciplinary and integrated approach, in which each member of the team contributed their point of view and experience in managing patients with immune-mediated inflammatory diseases, and updating their treatment plan, taking into account recent changes in the recommendations of the various drugs indicated for these diseases.
The approach to pregnancy in women with immune-mediated inflammatory diseases is a major challenge for healthcare workers. This fact, together with the limited number of studies available that examine the safety of treatments during pregnancy and breastfeeding, often leads to a cautious approach and discourages these women from becoming pregnant or breastfeeding.37 However, in the opinion of the multidisciplinary team, preconception counselling should be based on the scientific evidence currently available and tailored to patients’ willingness.
It has been reported that patient involvement in treatment decision-making contributes to better adherence to treatment38 and that concerns about family planning and pregnancy in patients with chronic inflammatory diseases are often unsatisfactorily resolved.39 Therefore, five patients with immune-mediated inflammatory diseases joined the multidisciplinary working group and contributed their own experience and highlighted the information most relevant to them. However, the multidisciplinary team felt that the responsibility for correct information should not only depend on patient involvement, but also on the different health professionals involved providing clear, objective, and consensual information.
During preconception counselling, it is very important to stress the importance of keeping the disease under control and to plan treatment in advance, so as to ensure the compatibility of the drugs with preconception, pregnancy, and breastfeeding.16
In order to adapt treatment to the different stages, which drugs have a possible deleterious effect on pregnancy and breastfeeding must be evaluated, as well as the benefits and risks associated with discontinuing them at each stage of the process.26 In the case of breastfeeding, the lack of up-to-date information on the benefits versus the risks associated with the use of drugs during this stage has to often lead professionals to advise against this option.40 For this reason, the aim is to provide information so that the patient can make an informed choice regarding the method of feeding her child and, if breastfeeding is her decision, so that this can take place as normally as possible.41 In this context, it is important to promote measures that encourage the dissemination of rigorous information during congresses, conferences, scientific articles, and educational materials.42 This information should be accessible to all professionals who treat these patients, including paediatricians, midwives, obstetricians, rheumatologists, nurses, primary care physicians, and pharmacists.
In short, new treatments and advances in the management of diseases such as RA, PsA, and AxSpA have helped to “normalise” the lives of patients who now face new challenges in their lives. In some cases, they have made it possible to control their disease better, enabling them to avoid having to give up motherhood and/ or breastfeeding. This paper highlights the importance of multidisciplinary work in the treatment of women of childbearing age with immune-mediated inflammatory diseases who wish to become pregnant and/ or breastfeed.
FundingThis work was funded by UCB to conduct the multidisciplinary meeting and disseminate materials derived from the meeting. The authors of the manuscript were responsible for analysing and interpretating the data, as well as drafting and revising the manuscript.
Conflict of interestJMB has received funding for courses, conferences, and lectures from Novartis, Pfizer, UCB, Lilly, GSK, and Abbvie. JAML has received funding for conferences, courses, and lectures from Novartis, Pfizer, Lilly, Abbvie, and UCB. All other authors declare no conflicts of interest.
The authors would like to thank the following researchers for their help in drafting the papers resulting from the multidisciplinary meeting: Paloma Vela (Alicante), Victor Martinez Taboada (Santander), Elisa Trujillo (Canary Islands), Montserrat Romera (Barcelona), Andrea Pluma (Barcelona), Maria Victoria Hernandez (Barcelona), Maria Luz Garcia Vivar (Bilbao), Rafael Caliz (Granada), Esteban Rubio (Sevilla), Cristina Sobrino (Madrid), Jose Maria Pego (Vigo), Encarna Perez (Alicante), Paloma Vallejo (Madrid), Leticia Leon Mateos (Madrid). They also thank the patients who participated in the multidisciplinary meeting. The documents were written with the help of Trialance in the scientific writing and sponsored by UCB Pharma.
Please cite this article as: Martínez-Barrio J, Martínez López JA, Galindo M, Ais A, Martínez Sánchez N, Cano L. Importancia de la planificación familiar en pacientes con enfermedades inflamatorias inmunomediadas: un abordaje multidisciplinar. Reumatol Clin. 2022;18:200–206.