The SUMAR project aimed to establish a consensus on the concept of remission in patients with rheumatoid arthritis (RA) that takes into account the different perspectives of patients, health care professionals and health care managers.
Materials and methodsThe scientific committee comprised a rheumatologist who acted as a national coordinator, 4 rheumatologists, 1 primary care physician, 1 nurse, 2 hospital pharmacists, 2 health care managers and 1 member of a patient advocacy group. The study was undertaken from 2020 to 2021 in three phases: (1) analysis of several perspectives on remission in RA with the participation of 275 patients, 160 rheumatologists and 31 health care managers; (2) an integrative definition of remission, which included two multidisciplinary workshops with 11 and 12 participants; and (3) extension and dissemination with up to 200 participants in 7 regional multidisciplinary meetings.
ResultsThe concept of remission in the different settings and by the different stakeholders was heterogeneous. It was agreed that, in addition to inflammatory activity, remission should include pain and functionality as well as the duration of remission. For the participants, the definition of remission varied depending on the clinical scenario, without or with structural damage, seeking to “normalize” the outcomes in the former and avoid progression in the latter. The implementation of the concept of comprehensive remission was considered less feasible, and the main barriers to implementation were the lack of time for consultation and the variability in information technology systems across the different autonomous communities.
Discussion and conclusionsThis definition of remission is not only based on the concept of the presence or absence of inflammatory activity based on existing indexes, but also includes variables directly reported by the patient that are related to their health and quality of life.
El proyecto SUMAR tuvo como objetivo establecer un consenso sobre el concepto de remisión en pacientes con artritis reumatoide (AR) reuniendo las diferentes perspectivas de los pacientes, los profesionales sanitarios y los gestores sanitarios.
Materiales y métodosEl comité científico estuvo formado por un reumatólogo (coordinador nacional), 4 reumatólogos, 1 médico de atención primaria, 1 enfermera, 2 farmacéuticos hospitalarios, 2 gestores sanitarios y 1 miembro de asociación de pacientes. El estudio se realizó de 2020 a 2021 en tres fases: 1) análisis de varias perspectivas sobre la remisión en la AR con la participación de 275 pacientes, 160 reumatólogos y 31 gestores sanitarios; 2) una definición integradora de remisión, que incluyó dos talleres multidisciplinarios con 11 y 12 participantes; y 3) extensión y difusión con hasta 200 participantes en 7 reuniones multidisciplinarias regionales.
ResultadosEl concepto de remisión en los diferentes marcos y por parte de los diferentes decisores fue heterogéneo. Se acordó que, además de la actividad inflamatoria, la remisión debería incluir el dolor y la funcionalidad, así como la duración de la remisión. Para los participantes, la definición de remisión varió según el escenario clínico, sin o con daño estructural, buscando «normalizar» los resultados en el primero y evitar la progresión en el segundo. La implementación del concepto de remisión integral se consideró menos factible y las principales barreras para su implementación fueron la falta de tiempo para la consulta y la variabilidad de los sistemas de tecnología de la información entre las diferentes comunidades autónomas.
Discusión y conclusionesEsta definición de remisión no sólo se basa en el concepto de presencia o ausencia de actividad inflamatoria en función de los índices existentes, sino que también incluye variables directamente comunicadas por el paciente que se relacionan con su salud y calidad de vida.
The treatment of rheumatoid arthritis (RA) has evolved substantially in recent decades, and treatment goals have changed accordingly. Currently, with the treat-to-target strategy, clinical remission or, at least, low disease activity has become the main therapeutic target to prevent the progression of joint damage and optimize physical functioning, work and social participation.1 As such, it has been recommended by the European Alliance of Associations for Rheumatology (EULAR)2 and the American College of Rheumatology (ACR)3 guidelines for the management of RA. From a regulatory perspective, the European Medicines Agency considers remission the “ideal” endpoint reflecting a target disease state that should be selected as the primary endpoint for pivotal trials of treatments for RA, especially for the study of treatment-naïve patients. However, although the agency recognizes the limitations of some indices, it does not advocate for specific criteria for defining remission.4
To establish a standardized definition of remission, in 2011 a joint committee of the ACR and EULAR endorsed two provisional definitions of remission, a Boolean-based definition and an index-based definition (i.e., based on a cutoff of the Simplified Disease Activity Index [SDAI]) based on the same core domains, namely, tender joint count, swollen joint count, C reactive protein (CRP) and Patient Global Assessment (PtGA) of disease activity, adding the Physician Global Assessment (PhGA) of disease activity in the SDAI.5 These definitions are useful for evaluating treatment targets in clinical trials and clinical practice.6,7 However, there are several concerns regarding their use. A remission definition based on the 28-joint Disease Activity Score (DAS-28) is frequently used in clinical trials and clinical practice but has limitations. For example, the CRP could be impacted differently depending on the type of treatment, and the use of PtGA has been criticized because it is highly influenced by pain, fatigue and function, symptoms that are rarely related to the disease process responsible for structural damage.7 The DAS-28 definition of remission, regardless of the cutoff, is considered inadequate since it does not reflect true remission in terms of preventing radiographic and functional outcomes.7,8 Although the SDAI definition of remission has better performance than the DAS-28 definition, it has shown a sensitivity of 57% for detecting remission as examined by power Doppler ultrasonography.9 The different impact of treatments for RA on CRP could be addressed by using a different index that does not contain CRP, such as the Clinical Disease Activity Index (CDAI).7 For concerns about the use of PtGA, the exclusion of this item from the Boolean definition of remission has been explored10 with the important drawbacks of not taking into account patients’ perspective on remission and worsening the prediction of functional outcomes.11 Therefore, the ACR/EULAR has evaluated a new Boolean definition of remission with a less stringent criterium for PtGA (i.e., PtGA≤2) with good results regarding concordance with index-based definitions and without jeopardizing the prediction of functional and radiographic outcomes.11
Discordance between patient and physician global assessments of disease activity has been reported,12 which reinforces the importance of including patients’ perspective for the assessment of this outcome. Since 2010, experts from the Outcomes Measures in Rheumatology Trials (OMERACT) have been working to incorporate the patient perspective into the definition of remission.13–15 The main domains of remission from the patient's perspective, as identified by the OMERACT group, involve pain, independence and fatigue,14 but further investigation is needed on how to measure some of these domains, especially independence, which is poorly defined,15 and whether they can be incorporated into the ACR/EULAR definition of remission.14 Remission also has an impact on direct and indirect medical costs.16 Therefore, it is also likely that payers and other decision-makers could have a different perspective on how to define remission.
The SUMAR project aimed to establish a consensus on the concept of comprehensive remission that would take into account the different perspectives of patients, health care professionals and health care managers.
Material and methodsThe project involved the participation of a multidisciplinary scientific committee that comprised a rheumatologist who acted as a national coordinator, 4 rheumatologists, a primary care physician, a nurse, 2 hospital pharmacists, 2 health care managers and 1 member of a patient advocacy group. For the second phase of the project (see below), another 6 experts (3 rheumatologists, 1 hospital pharmacist and 2 health care managers) were included in the project.
The project was undertaken from 2020 to 2021 in three phases: (1) analysis of several perspectives on remission in RA; (2) integrative definition of remission; and (3) expansion and dissemination. The steps and objectives of the three phases are summarized in Fig. 1, and the methodology applied is explained in greater detail in the supplementary information.
ResultsPhase 1: analysis of several perspectives on remission in rheumatoid arthritisLiterature searchFrom the research perspective, although there is a large heterogeneity in the outcome measures used, the most frequent are DAS-28 and the ACR/EULAR Boolean definition. With regard to patient-reported outcomes (PROs), the most common are the Health Assessment Questionnaire (HAQ) and the Rheumatoid Arthritis Impact of Disease (RAID). Clinical practice guidelines recommend DAS-28, the ACR/EULAR Boolean definition and the SDAI and, except for the NICE guideline that recommends the HAQ, do not provide recommendations on PROs. There is also large heterogeneity in health outcome research, but DAS-28, the ACR/EULAR Boolean definition, HAQ and RAID are the most commonly used. In drug evaluation, the ACR criteria for response are the standard. Finally, we could not find a national strategic plan that includes the concept of remission. Details on the results of the literature search can be found elsewhere.17
Map of remissionTwo hundred seventy-five patients answered the questionnaire; 187 (68%) were female, their mean (±SD) age was 53.0 (±12.3), and the mean time since diagnosis was 11.6 (±8.4) years. One hundred eighty-five (67.3%) patients had not heard about the concept of remission. However, 133 (48.4%) understood remission as a significant improvement in symptoms, and 151 (54.9%) thought that effective remission should last forever (the remaining patients considered remission to last at least a mean of 13.4 [±11.6] months). Overall, 167 (60.7%) believed that they were not in remission, 44 (16.0%) believed that they were in remission, and 64 (23.3%) did not know whether they were in remission. The most frequent symptoms that had higher intensity and a greater impact on daily life were pain and inflammation (data not shown).
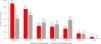
One hundred sixty rheumatologists answered the corresponding questionnaire. Of these, 75 (46.9%) were females, they had a mean age of 48.9 (±8.7) years, and they had 19.6 (±7.6) years of experience. Most rheumatologists (n=144, 90.0%) followed a clinical practice guideline (91 [63.2%] the Spanish guideline GUIPCAR-SER).18 Approximately half of the rheumatologists used DAS-28-based criteria for remission, 30 (18.8%) used the SDAI criteria, and 13 (8.1%) used the ACR/EULAR Boolean criteria (Fig. 2) and considered sustained remission to last 9.2 (±3.9) months. Ninety-four (60.3%) of 156 respondents used remission criteria at each clinical visit, and an estimated 43.9% (±20.9%) of patients attending their clinics were in remission. One hundred seven (66.9%) rheumatologists used PRO in routine clinical practice, mostly HAQ and pain assessment. The inclusion of remission as a quality indicator was considered useful or very useful by 119 (74.4%) of the rheumatologists.
Remission criteria used in clinical practice by rheumatologists. ACR, American College of Rheumatology; CDAI, Clinical Disease Activity Index; CRP, C reactive protein; DAS-28, 28-joint Disease Activity Score; ESR, erythrocyte sedimentation rate; EULAR, European Alliance of Associations for Rheumatology; SDAI, Simplified Disease Activity Index. Figures represent the relative (absolute) frequency of respondents among the rheumatologists surveyed (n=160).
Thirty-one health care managers participated in this step, mostly hospital pharmacists (n=11), pharmacy managers from the Autonomous Community (AC) health systems (n=6), hospital managers/directors (n=5), and other health care managers from ACs (n=8). All but one had heard about the concept of remission as a priority treatment goal. Among the 29 respondents, the most frequent criterion for decision-making was drug safety (n=23, 79.3%), but the criterion that they believed should be used was cost-effectiveness (23 of 30 respondents to this question, 76.7%). Among those who used efficacy/effectiveness or believed that it should be considered for decision-making (n=9), the most frequently used outcome measures were the DAS-28 (n=6, 66.7%) and the ACR criteria (n=5, 55.6%). Fourteen (45.2%) reported that there were plans/initiatives for monitoring patients with RA that recommended some remission criteria (DAS-28-PCR and DAS-28-ESR, in 64.3% and 50.0% of those cases, respectively).
Group dynamicsThe patients identified the most limiting moments and barriers to achieving remission as getting out of bed, working, resting, sleeping and personal hygiene/dressing. They also identified pain, stiffness/inflammation and fatigue as the main symptoms that affected living with RA. Health care professionals proposed that the concept of remission should include the “absence of clinical signs and extraarticular symptoms of the disease, without progression of the impairment of QoL, and must be persistent over time” and should be evaluated every 3–6 months. Finally, health care managers highlighted that the concept of remission must include the patient's perspective and must be simple, easy to implement, allow comparisons and be multidomain. A summary of the results of the group dynamics is presented in Table 1.
Main results of the group dynamics for delineating the remission map.
| Group | Patients | Health care professionals | Health care managers |
|---|---|---|---|
| # of participants | 12 | 11 | 4 |
| Characteristics | 10 females, mean (SD) age 51.5 (9.2) years, time since diagnosis 15.6 (12.8) years | 5 females; 6 rheumatologists, 1 nurse, 3 hospital pharmacists, 1 primary care physician | 2 females; 1 director of socio-health coordination of the regional health service, 1 head of pharmacy service of a regional health service, 1 former managing director of a regional health service, 1 hospital manager |
| Main conclusions | • Most limiting times of the day due to RA: getting out of bed, work, rest, sleep and personal hygiene/dressing.• Main barriers to achieving remission (taking into account the worst condition caused by the disease and what you want to achieve to cope appropriately with it): getting up, personal hygiene/dressing, work, rest and sleep.• Main symptoms and signs that affect living with the disease: pain, stiffness/inflammation, fatigue and the emotional component (in this order); the first three are the key ones to achieve remission. | • The concept of remission should include the “absence of clinical signs and extraarticular symptoms of the disease, without progression of the impairment of QoL, and must be persistent over time.”• Remission should be evaluated with clinical criteria (using composite indices) and PROs that include the most important issues for the patient.• The frequency of evaluation should be every 3–6 months.• The multidisciplinary approach in evaluation is relevant. | • The concept of remission must include the patient's perspective.• The impact on the quality of life as perceived by the patient is, and should be, an important aspect in decision-making.• The appropriateness of adopting lax or strict criteria for remission will depend on the objective (access, evaluation, planning) and the type of patient (naïve or long-term evolution).• The development of initiatives that include objectives related to the concept of remission is necessary.• The concept of remission must be simple, easy to implement, standardized or allow comparisons and multidomain. |
In the first workshop (11 participants), after two rounds of voting separated by a discussion of the initial results, it was agreed that two patients’ profiles should be distinguished: those without and with structural damage. For the former, all participants believed that remission was achievable, while for the latter, they believed that remission was achievable in 20% of patients. After evaluating the 11 optional domains, regardless of the patient's profile, there was consensus that in addition to inflammatory activity (mandatory), the following domains should be assessed: PhGA, pain, fatigue, stiffness, functionality, emotional status, and the duration of remission (Fig. 3).
In the second workshop (12 participants), in the first stage established by the scientific committee (“Preparation”; see explanation in supplementary information), because it was not considered feasible in clinical practice to evaluate all the previously cited domains, it was determined that pain and functionality were the most important domains and that the duration of remission should be taken into account. In the second stage (“Where are we”), the participants suggested that the main strength for the implementation of the concept of remission was the willingness to empower patients’ decision-making, and the main weakness was resistance to change by health care professionals and the variability of the Spanish regional health systems. The assessment tools and thresholds, actions, roles and timing for the assessment agreed upon in stage 3 (“Designing the road map”) are presented in Table 2. In stage 4 (“The way ahead”), the participants identified two key concepts to achieve the implementation of the concept of integrative remission: training (of the patients and health care professionals) and team.
Designing the road map for implementing the integrative definition of remission.
| Objective | Recommendation | |
|---|---|---|
| What | Assessment tools and thresholds for each patient's profile | Tools:-Inflammatory activity: one of the validated index-based measures-Pain: numerical rating scale or visual analog scale-Functionality: the HAQ questionnaireThresholds:-Without structural damage: a score close to normality (“0”)-With structural damage: no progression |
| How | Genal actions for the implementation | i. Clinical practice: training nurses and patients and integrating the PROs into the electronic health record (EHR)ii. Research: carry out cost-effectiveness studies in clinical practice and establish remission as the treatment goal both in clinical trials and in real-world studiesiii. Evaluation of health outcomes: prioritize the evolution of information systems and scorecards, integrate comprehensive remission into the overall concept of evaluation of health outcomes, incorporate global costs into the system with health outcomes, focus on the qualitative rather than the quantitative, collect relevant health outcomes that include both the patient's perspective and the proportion of patients in remission, link the concept of remission to persistence and establish indicators to measure health outcomesiv. Drug evaluation: linking health outcomes with drug prioritization and analyzing overall costs, taking into account indirect costsv. Health care planning: include the objective of comprehensive remission in documents and health plans, introduce the percentage of patients in remission in management agreements, incorporate the goal of comprehensive remission in the strategic plans and reach a consensus on a proposal for objectives and evaluation |
| Who | People involved and their roles in implementation | Rheumatologists (lead the implementation), nurses (train the patient and help to complete PROs), pharmacy (part of the multidisciplinary team and promote adherence), managers (provide necessary resources and highlight the importance of health outcomes) and other agents (scientific societies, primary care physicians, psychologists, physiotherapists, social services and patient advocacy groups) |
| When | Timing of assessments | Make at least one consultation every three months with the nursing team to assess the PROs and another every six months with rheumatology to assess inflammatory activity (67% of votes (n=8)). Through teleconsultation, it is possible to improve the frequency of assessment of remission using questionnaires such as the RAID or the RAPID3 |
Up to 200 participants from 15 ACs in 7 meetings answered 17 questions on the project. Regarding the first 5 questions on the proposal and the methodology, over 95% of respondents agreed that a concept of comprehensive remission was needed, the methods used in the project were adequate, and the set of core domains for defining remission could be implemented in their clinic/setting. They believed that the most important domains to be included in the concept in the future were health-related quality of life (37.1%) and the PhGA (25.8%) and that the main contribution of the proposal would be to establish a homogenous definition of remission (68.2%).
Regarding the implementation of the proposal in the ACs, 65.4% of the respondents considered it feasible and identified the main barriers as the lack of time for consultation (67.4%) and the variability across regions in the information technology systems (55.6%). Implementation in clinical practice was considered important by 96% of the respondents and feasible by 66.4%. The main actions for implementation were including PROs in electronic health records and training the nursing team; the former was considered feasible by 31.9% and the latter by 59.4%. Similarly, 91.9% of the respondents considered it important to include remission in drug evaluation/access, but 51% considered this feasible in the short term. Regarding inclusion in health care plans, 93.8% considered this issue important while 40.6% considered it feasible. Finally, 94.7% of participants considered it important to include comprehensive remission in the evaluation of health outcomes in their Acs; this was considered feasible by 30.1%
DiscussionOur results show the heterogeneity of the concept of remission in different settings and by different stakeholders. Nevertheless, all of them consider the concept of comprehensive remission important and support the value of the SUMAR project. Furthermore, they agree that in addition to inflammatory activity, remission should include pain and functionality as well as the duration of remission. The definition of remission varies depending on the clinical scenario and the absence or presence of structural damage, seeking to “normalize” the outcomes in the former and avoid progression in the latter. The implementation of the concept of comprehensive remission is considered less feasible, especially within health planning and the evaluation of health outcomes in ACs. The main barriers to implementation are the lack of time for consultation and the variability in the information technology systems across different ACs.
As expected, the literature search showed heterogeneity in the concept of remission as reflected by the number of criteria found, although criteria based on DAS-28 continue to be the most frequently used in most settings. Importantly, in the Spanish setting of evaluation of drugs, ACR response criteria are the standard measure of efficacy. This may reflect the requirement of this endpoint by the US Food and Drug Administration (FDA) for the evaluation of drugs for the treatment of RA. It also reinforces the importance of raising awareness of the concept of remission among health care managers when they evaluate treatment effectiveness.
In the map of remission, two findings are notable. Two-thirds of patients had not heard of the concept of remission. Patient-centered care requires a better understanding of patient goals, better methods for engaging patients in their care, and better measures of outcomes that have meaning for patients.19 Therefore, informing and training patients about remission as a treatment target is imperative. To further support this need, we found differences between patients and health care professionals in the proportion of patients they considered in remission: only 16% of patients reported being in remission, while rheumatologists estimated that a mean of 44% of patients attending their clinics were in remission. This difference is remarkable if we bear in mind that from the perspective of patients, remission meant a significant improvement in symptoms.
For the health care professionals involved in the SUMAR project, the concept of remission is multidomain; in addition to inflammatory activity, it should include physician global assessment, pain, fatigue, stiffness, functionality, emotional status, and the duration of remission. However, to be feasible, which is a key characteristic according to health care professionals and managers, the participants agreed that pain, functionality and the duration of remission are the key domains. Pain and functionality are consistent with the most important domains reported by patients in the literature. As mentioned above, the OMERACT identified pain, independence and fatigue as the main domains of remission from the perspective of patients.14 Pain is easy to measure, and several evaluation tools exist for this purpose. In our project, the most straightforward visual analog scale and numerical rating scale were selected by the participants. Independence is more difficult to define and to measure. The OMERACT identified several components of independence, namely, a return to a state before arthritis, being physically and functionally able, a sense of freedom without needing to rely on others and having control over the organization of one's life.15 Functionality, as measured with the HAQ, the recommended tool in our project, addresses only the component of being physically and functionally able. This is a key component and, as such, was identified by the patients participating in the SUMAR project. However, more research is needed on the importance of the other components of independence for patients and how to incorporate them into an evaluation tool. Regarding the measure of the mandatory domain, inflammatory activity, this project does not recommend a specific tool since the priority was to measure this domain. However, it is important to note that measures based on the DAS-28, which is most frequently used in most settings, have important limitations and are not recommended by experts, including the Spanish clinical practice guidelines.7,8,18 Instead, the CDAI or SDAI are preferred. Identifying two patients’ profiles for defining remission is somewhat consistent with current clinical practice guidelines.2,3 However, in the SUMAR project, profiles are defined by the absence or presence of structural damage. In the multidisciplinary workshops, a relevant proportion of participants (i.e., 60%) also considered important to include in the integrative concept of remission the evaluation of structural damage and systematic manifestations of the disease. In addition, some potential domains related to remission were not included in the project, such as treatment adherence, and should be further explored. Thus, higher adherence to csDMARDs is associated with an increased likelihood of clinical remission.20
Although the concept of comprehensive remission is considered important by all stakeholders, the feasibility of the implementation is reduced, especially with regard to its incorporation in health plans and the evaluation of health outcomes in the ACs. An important barrier to implementation among rheumatologists, not unexpectedly, is time for consultation. A proposal to overcome this issue is the creation of a nurse practitioner consultation devoted to patients with RA. In addition, improving collaboration between rheumatologists and primary care physicians would improve the follow-up of these patients. Moving forward to an integrative care model is of paramount importance in all medical disciplines. Finally, to incorporate comprehensive remission in health planning, scientific societies may play an important role.
This project has several strengths, including a robust methodology and the involvement of more than 500 stakeholders throughout Spain. It also has several limitations, the most important of which is that we are unable to propose a specific index or cutoff points for evaluating comprehensive remission. A project on this issue is currently being conducted.
In conclusion, this definition of remission is not only based on the concept of the presence or absence of inflammatory activity based on existing indexes, but also includes variables directly reported by the patient that are related to their health and quality of life.
Clinical trial registrationNot applicable.
Authors’ contributionsAB was responsible for the coordination of the project and writing this manuscript. JC was responsible for the design and analysis. All authors have contributed to the acquisition and interpretation of data, critically review the manuscript, approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Informed consentAll the participants in this project expressed their agreement to participate by signing a contract. Their responses or feedback are presented in an aggregate and anonymous manner.
Ethics approvalNor applicable.
Role of the funding sourceThe funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
FundingThe project was funded by AbbVie company.
Conflict of interestAB declares fees for consulting/speaker's fees and/or grant support from Pfizer, AbbVie, Bristol-Myers Squibb, UCB, Novartis, Galapagos, Nordic, Lilly. SR-Y has received speaker honoraria from Astra Zeneca, Abbvie, Galapagos, Roche, and UCB; has received support for attending meetings and/or travel from Lilly, Astra Zeneca, Abbvie, Pfizer, and Sandoz. RS is Member of the Advisory Board of the la MHDA Evaluation Committee, Cat-Salut, Generalitat de Catalunya; has provided/participated conferences/advisory boards and research projects sponsored by Abbott/Abbvie, BMS, MSD, Roche, Pfizer, Lilly; Roche and Gebro. JLA, JCH-H, JL-N, JNP, JJPV, AITG-P, JCV-G, and JC declare no conflict of interest.
Data availabilityData are available from the corresponding author upon reasonable request.
The authors thank Fernando Rico-Villademoros from COCIENTE S.L. (Madrid, Spain) for the assistance with the medical writing. This assistance was funded by AbbVie.