JAK kinase inhibitors (JAKi) are a new therapeutic option in the treatment of rheumatoid arthritis, but they are not without risks, especially the incidence of herpes zoster (HZ).
Material and methodsSystematic literature review that evaluates the incidence of HZ published in the clinical trials of the different JAK is marketed or under study.
ResultsThe HZ rates ranged between 1.51 and 20.22. The results were expressed mainly as a percentage of events. The most recent studies better categorized the incidence of HZ and its severity.
ConclusionJAK is are associated with an increased risk of HZ. Although the HZ rates of the selective JAK1 JAK is are lower, more studies are needed to confirm these results.
El objetivo de esta revisión sistemática es evaluar la infección por herpes zoster (HZ) publicada en los ensayos clínicos fase II y fase III de pacientes con AR en tratamiento con inhibidores de JAK (iJAK).
Material y métodosRevisión sistemática de la literatura que evalúa la incidencia de HZ publicada en los ensatyos clínicos de los distintos iJAK comercializados o en estudio.
ResultadosLas tasas de HZ variaron entre el 1.51 y 20.22. Los resultados se expresaron principalmente en porcentaje de eventos. Los estudios más recientes categorizaron mejor la incidencia de HZ y su gravedad.
ConclusiónLos iJAK se asocian a mayor riesgo de HZ. Aunque las tasas de HZ de los iJAK sleectivos de JAK1 son menores, son necesarios más estudios que confirmen estos resultados.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that significantly impacts quality of life. The therapeutic goal in RA is to achieve remission or minimal inflammatory disease activity, and therefore early diagnosis and early initiation of treatment is recommended1,2.
A broad therapeutic arsenal is available to achieve remission or low disease activity, which includes the synthetic conventional disease-modifying antirheumatic drugs (scDMARDs), methotrexate being the leading drug; the biological DMARDs (bMARDs), such as monoclonal antibodies or fusion proteins targeting extracellular molecules, and the more recently introduced targeted synthetic DMARDs (tsDMARDs), the JAK kinase inhibitors (JAKi)1,2.
JAKi act at the intracellular level by reversibly inhibiting signalling of JAK, a dimeric protein, transducer, and activator of transcription (STAT) pathway that forms part of the immune response. There are four proteins that make up the receptors on which these drugs can act by inhibiting their function: JAK1, JAK2, JAK3, and TYK2.
Three JAKi have been approved to date for the treatment of RA with moderate to high inflammatory activity: tofacitinib, which inhibits JAK1, JAK3 and, to a lesser extent, JAK2, approved since 2012, baricitinib, which inhibits JAK1 and JAK2, approved since 2017, and upadacitinib, a selective JAK1 inhibitor, approved in 2019 in the United States, and in 2020 in Europe. Under development are: filgotinib, a selective JAK1 inhibitor, peficitinib, a pan-JAK inhibitor, and decernotinib, a selective JAK3 (and to a lesser extent JAK1) inhibitor3–5. Table 1 shows all the JAKi cited.
Available JAK kinase inhibitors and under study in rheumatoid arthritis.
| Drug | Commercial name | Study name | Target | Development |
|---|---|---|---|---|
| Tofacitinib | Xeljanz® | CP-690,550 | JAK1, JAK3 | Available |
| Baricitinib | Olumiant® | LY3009104 | JAK1, JAK2 | Available |
| Upadacitinib | Rinvoq® | ABT-494 | JAK1 | Available |
| Filgotinib | GLPG0634 | JAK1 | Phase III | |
| Peficitinib | ASP015K | JAK1, JAK3 | Phase III | |
| Decernotinib | VX-509 | Pan-JAK | Phase IIb completed |
Development studies of JAKi have identified common and differentiating adverse effects between the different molecules with respect to the scDMARDs and bDMARDs, such as thrombotic events and herpes zoster (HZ). Varicella zoster virus reactivation, with subsequent onset of HZ, has been confirmed with all JAKi, and although the incidence is known to be higher in the Japanese population, the mechanism by which JAKi increase the risk of HZ reactivation is unknown6.
Study objectiveThe aim of this systematic review was to evaluate published HZ infection in phase II and phase III clinical trials of RA patients treated with JAKi7,8.
Material and methodsA systematic literature search was performed using Medline, Embase and Cochrane. A search deadline was set of 31 December 2019, and only English-language articles were included. We undertook a search of same terms and drugs in the 3 databases: “rheumatoid arthritis”, “herpes zoster”, “JAK inhibitor”, “tofacitinib”, “baricitinib”, “upadacitinib”, “filgotinib”, “peficitinib”, and “decernotinib”.
We decided to include phase II and phase III clinical trials and their extension studies to obtain as much homogeneity as possible between studies. Conference abstracts, case series and clinical registries were excluded.
The inclusion criteria for the available studies were as follows:
- •
Patients diagnosed with RA according to American College of Rheumatology and/or EULAR criteria9,10.
- •
Drugs evaluated: tofacitinib, baricitinib, upadacitinib, filgotinib, peficitinib, and decernotinib, all compared with placebo.
- •
Studies that included safety data on HZ infection.
The literature search was conducted by one investigator using a form containing specifications of previously defined selection and exclusion criteria; in the event of doubt, it was reviewed and decided by consensus with a second investigator. The GRADE (Grading the Quality of Evidence and Assessment of Recommendations) recommendations were used to analyse the quality of evidence of the studies. The study design, the follow-up time (at least 24 weeks) and how the onset of HZ was expressed (rate per 100 patients/year) were considered to assess the quality of evidence.
The main objective of our study was to evaluate the rate of HZ infection according to the doses of the drug administered, as well as placebo. If the study provided frequencies as a percentage, an estimate of the incidence rate was made using the number of HZ infections, the number of patients included, and the duration of the clinical trial.
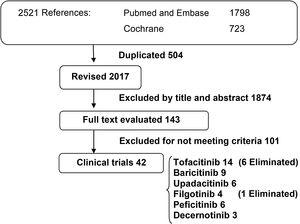
ResultsThe search yielded a total of 2521 publications. A total of 504 duplicate references were eliminated, and of the remaining 2017 references, those that did not meet the study criteria were removed by a review of title and abstract. A total of 143 full-text publications that met the selection criteria were reviewed and a total of 42 clinical trials were included (Fig. 1).
We selected 14 clinical trials of tofacitinib (4 phase II, 8 phase III, and 2 extension studies); 9 clinical trials of baricitinib (2 phase II, 5 phase III, and 2 extension studies); 6 clinical trials of upadacitinib (2 phase II and 4 phase III); 4 clinical trials of filgotinib (3 phase II and 1 phase III); 6 clinical trials of peficitinib (3 phase II, 2 phase III, and 1 extension study); and 3 phase II clinical trials of peficitinib. Clinical trials of these drugs have identified a higher number of opportunistic varicella zoster virus infections compared to placebo. Clinical trials that provided no or insufficient information on HZ infection were discarded from the final analysis. Six clinical trials of tofacitinib (3 phase II and 3 phase III), all published before 2013, and one phase II clinical trial of filgotinib published in 2017 were eliminated.
The tofacitinib clinical trials included a total of 4678 patients, excluding extension phases, with a maximum follow-up time of 9.5 years11–16. The baricitinib trials included 3899 patients, excluding extension phases, with a maximum follow-up time of 2.5 years17–22, and the upadacitinib trials included 4012 patients, with a maximum follow-up time of one year23–26. In addition, the filgotinib trials included 1325 patients, with a maximum follow-up time of 6 months27–28, and the peficitinib trials included 2587 patients, with a maximum follow-up time of one year29. The decernotinib trials included 605 patients and a maximum follow-up time of 6 months30.
Inhibition of the different JAK types can result in differences in safety profiles between these drugs, which could have clinical implications. We therefore analysed the results separately for each JAKi. The results for each JAKi are shown in Tables 2–5.
Tofacitinib studies.
| Author, year | Study phase, population | Study duration | Patients, no. | Dose of drug in mg (no. of patients), placebo | Herpes zoster (%) | Rate of events per 100 patients /year | Quality of evidence | |
|---|---|---|---|---|---|---|---|---|
| Mean RA duration (years) | ||||||||
| 1 | Kremer, 2009 | IIb, International | 12 weeks | 264 | 5 (61) | 1 (1.6) | 6.55 | High |
| 8.7−10.2 | 10 (69) | 2 (2.9) | 11.6 | |||||
| 30 (69) | 2 (2.9) | 11.6 | ||||||
| Placebo (65) | 1 (1.5) | 6.15 | ||||||
| 2 | Van Vollenhoven, 2012 | III, International | 52 weeks | 717 | Placebo (108) | 1 (1) | 1 | High |
| ORAL-STANDARD11 | 6.9−9.0 | 5 (204) | 6 (3) | 6 | ||||
| 10 (201) | 12 (6) | 12 | ||||||
| Adalimumab (204) | 5 (2.5) | 5 | ||||||
| 3 | Kremer, 2013 | III, International | 52 weeks | 792 | Placebo (159) | 0 | 0 | High |
| ORAL-SYNC12 | 8.1−10.2 | 5 (315) | 0 | 0 | ||||
| 10 (318) | 1 (.3) | .31 | ||||||
| 4 | Lee, 2014 | III, International | 24 months | 956 | 5 (373) | 13 (3.5) | 6.97 | High |
| ORAL-START13 | 2.7−3.4 | 10 (397) | 18 (4.5) | 9.07 | ||||
| Methotrexate (186) | 2 (1.1) | 2.15 | ||||||
| 5 | Fleishmann, 2017 | III, IV, International | 12 months | 1.152 | 5 (386) | 5 (1) | 5.18 | High |
| ORAL-STRATEGY14 | 5.4−6.1 | 5+Mtx (378) | 10 (2) | 10.58 | ||||
| Ada + Mtx (388) | 6 (1) | 6.18 | ||||||
| 6 | Li, 201815 | OLE, China | 12 months | 408 | 5 (86) | 8 (5.2) | 1.72 | High |
| 6.4−9.5 | 10 (86) | 3 (7.7) | 1.51 | |||||
| Placebo (44) | 0 | 0 | ||||||
| 7 | Van der Heijde, 2019 | III, International | 24 months | 797 | 5 (321) | 24 (7) | 14.95 | High |
| 8.8−9.5 | 10 (316) | 33 (10) | 20.88 | |||||
| Placebo (160) | 11 (6) | 13.75 | ||||||
| 8 | Wollenhaupt, 201916 | OLE, International | 9.5 years | 4481 | 5 (1123) | 119 (10.6) | 2.56 | High |
| 7.7−8.6 | 10 (3358) | 386 (11) | 3.36 |
Studies on baricitinib.
| Author, year | Study phase, population | Study duration | Patients, no. | Dose of drug in mg (no. of patients), placebo | Herpes zoster (%) | Rate of events per 100 patients /year | Quality of evidence | |
|---|---|---|---|---|---|---|---|---|
| Mean duration of RA (years) | ||||||||
| 1 | Keystone, 2015 | IIb, International | 24 weeks | 299 | Placebo (98) | 0 | 0 | High |
| 5.3−6.6 | 1 (49) | 0 | 0 | |||||
| 2 (52) | 0 | 0 | ||||||
| 4 (52) | 0 | 0 | ||||||
| 8 (50) | 0 | 0 | ||||||
| 2 | Tanaka, 2016 | IIb, Japan | 52 weeks | 145 | Placebo (49) | 0 | 0 | High |
| 5.06−6.32 | 1 (24) | 0 | 0 | |||||
| 2 (24) | 0 | 0 | ||||||
| 4 (24) | 0 | 0 | ||||||
| 8 (24) | 0 | 0 | ||||||
| 3 | Genovese, 2016 | III, International | 24 weeks | 527 | Placebo (176) | 2 (1) | 2.27 | High |
| RA-BEACON17 | 14 | 2 (174) | 2 (1) | 2.3 | ||||
| 4 (177) | 7 (4) | 7.91 | ||||||
| 4 | Taylor, 2017 | III, International | 52 weeks | 1.307 | Placebo (488) | 2 (.4) | .41 | High |
| RA-BEAM18 | 10 | 4 (487) | 11 (2) | 2.25 | ||||
| Adalimumab (330) | 5 (1.5) | 1.51 | ||||||
| 5 | Fleishmann, 2017 | III, International | 52 weeks | 584 | Mtx (210) | 2 (.9) | .95 | High |
| RA-BEGIN19 | 1.3−1.9 | 4 (159) | 4 (2.5) | 2.51 | ||||
| 4+mtx (215) | 5 (2.3) | 2.36 | ||||||
| 6 | Dougados, 2017 | III, International | 24 weeks | 684 | 2 (229) | 4 (1.7) | 3.49 | High |
| RA-BUILD20 | 7−8 | 4 (227) | 3 (1.3) | 2.64 | ||||
| Placebo (228) | 0 | 0 | ||||||
| 7 | Keystone, 201821 | OLE, International | 128 weeks | 201 | 4 (169) | 7 (4) | 2.84 | High |
| 5.3−6.6 | 8 (32) | 3 (9) | 1.22 | |||||
| 8 | Tanaka, 2018 | III, International | 24 weeks | 353 | 4 Begin 104 | 8 (7.7) | 15.38 | High |
| 1.3−10 | 4 Beam 249 | 7 (2.8) | 5.62 | |||||
| 9 | Tanaka, 201822 | OLE, Japan | 64 weeks | 142 | 4 (71) | 5 (7) | 5.63 | High |
| 5.5−5.9 | 8 (71) | 6 (9) | 6.76 |
Studies on upadacitinib and filgotinib.
| Author, year | Study phase, population | Study duration | Patients, no | Dose of drug in mg (no. of patients), placebo | Herpes zoster (%) | Rate of events per 100 patients /year | Quality of evidence | |
|---|---|---|---|---|---|---|---|---|
| Mean RA duration (years) | ||||||||
| Upadacitinib | ||||||||
| 1 | Kremer, 2016 | IIb, International | 12 weeks | 276 | Placebo (56) | 2 (4) | 14.28 | High |
| BALANCE I | 10.9−12.3 | 3 (55) | 1 (2) | 7.27 | ||||
| 6 (55) | 0 | 0 | ||||||
| 12 (55) | 1 (2) | 7.27 | ||||||
| 18 (55) | 1 (2) | 7.27 | ||||||
| 2 | Genovese, 2016 | IIb, International | 12 weeks | 299 | Placebo (50) | 0 | 0 | High |
| BALANCE II | 3.9−9.3 | 3 (50) | 1 (2) | 8 | ||||
| 6 (50) | 0 | 0 | ||||||
| 12 (50) | 0 | 0 | ||||||
| 18 (50) | 0 | 0 | ||||||
| 24 (49) | 2 (4) | 16.32 | ||||||
| 3 | Genovese, 2018 | III, International | 24 weeks | 499 | Placebo (169) | 2 (1) | 2.37 | High |
| SELECT-BEYOND23 | 12.4−14.5 | 15 (165) | 3 (2) | 3.63 | ||||
| 30 (165) | 6 (3.6) | 7.27 | ||||||
| 4 | Burmester, 2018 | III, International | 12 weeks | 661 | Placebo (221) | 1 (.4) | 1.81 | High |
| SELECT-NEXT24 | 7.2−7.3 | 15 (221) | 1 (.4) | 1.81 | ||||
| 30 (219) | 2 (.9) | 3.65 | ||||||
| 5 | Smolen, 2019 | III, International | 14 weeks | 648 | Mtx (216) | 1 (.5) | 1.85 | High |
| SELECT-MONOTHERAPY25 | 5.8−7.5 | 15 (217) | 3 (1.4) | 5.53 | ||||
| 30 (215) | 6 (2.8) | 11.16 | ||||||
| 6 | Fleishmann 2019, | III, International | 48 weeks | 1.629 | Placebo (651) | 3 (.5) | .92 | High |
| SELECT-COMPARE26 | 8 | 15 (651) | 5 (.8) | 1.53 | ||||
| Adalimumab (327) | 1 (.3) | .61 | ||||||
| [0,1–9] | ||||||||
| Filgotinib | ||||||||
| 1 | Westhovens, 2017 | IIb, International | 24 weeks | 594 | Placebo (86) | 1 (1.2) | 2.32 | High |
| DARWIN I | 7−8 | 50 (168) | 1 (.6) | 1.19 | ||||
| 100 (170) | 0 | 0 | ||||||
| 200 (170) | 3 (1.8) | 3.53 | ||||||
| 2 | Kavanaugh, 2017 | IIb, International | 24 weeks | 283 | Placebo (72) | 0 | 0 | High |
| DARWIN II27 | 9−10 | 50 (72) | 1 (1.4) | 2.77 | ||||
| 100 (60) | 0 | 0 | ||||||
| 200 (59) | 0 | 0 | ||||||
| 3 | Genovese, 2019 | III, International | 24 weeks | 448 | 200 (147) | 2 (1.4) | 2.72 | High |
| FINCH 228 | 9.8−10.3 | 100 (153) | 2 (1.3) | 2.61 | ||||
| Placebo (148) | 0 | 0 | ||||||
Studies on peficitinib and decernotinib.
| Author, year | Study phase, population | Study duration | Patients, no. | Dose of drug in mg (no. of patients), placebo | Herpes zoster (%) | Rate of events per 100 patients /year | Quality of evidence | |
|---|---|---|---|---|---|---|---|---|
| Mean RA duration (years) | ||||||||
| Peficitinib | ||||||||
| 1 | Takeuchi, 2016 | IIb, Japan | 12 weeks | 281 | Placebo (56) | 0 | 0 | High |
| 6.92−8.03 | 25 (55) | 2 (3.6) | 14.54 | |||||
| 50 (57) | 0 | 0 | ||||||
| 100 (55) | 2 (3.6) | 14.54 | ||||||
| 150 (58) | 0 | 0 | ||||||
| 2 | Genovese, 2017 | IIb, International | 12 weeks | 289 | Placebo (51) | 1 (2) | 7.84 | High |
| 9.8−11 | 25 (59) | 0 | 0 | |||||
| 50 (57) | 0 | 0 | ||||||
| 100 (58) | 0 | 0 | ||||||
| 150 (64) | 0 | 0 | ||||||
| 3 | Kivitz 2017 | IIb, International | 12 weeks | 378 | Placebo (72) | 0 | 0 | High |
| 7.2−8.1 | 25 (67) | 0 | 0 | |||||
| 50 (78) | 0 | 0 | ||||||
| 100 (84) | 2 (2.4) | 9.52 | ||||||
| 150 (78) | 1 (1.3) | 5.13 | ||||||
| 4 | Genovese, 2019 | OLE, International | 24 months | 611 | Pbo-25 (116) | 2 (1.8) | .86 | High |
| 7.2−11 | 25−25 (112) | 2 (1.8) | .89 | |||||
| 50−25 (124) | 4 (3.2) | 1.61 | ||||||
| 100−25 (128) | 2 (1.6) | .78 | ||||||
| 150−25 (131) | 5 (3.8) | 1.9 | ||||||
| 5 | Takeuchi, 201929 | III, Japan | 52 weeks | 519 | Placebo (170) | 2 (1.2) | 3.2 | High |
| 4.30−4.41 | 100 (175) | 13 (7.5) | 8.3 | |||||
| 150 (174) | 6 (3.5) | 3.8 | ||||||
| 6 | Tanaka, 2019 | III, Japan | 52 weeks | 509 | Placebo (102) | 0 | 0 | High |
| 6.98−10.4 | 100 (104) | 5 (4.8) | 5.8 | |||||
| 150 (102) | 4 (3.9) | 4.4 | ||||||
| Etanercept (201) | 5 (2.5) | 2.6 | ||||||
| [0,1–9] | ||||||||
| Decernotinib | ||||||||
| 1 | Fleishmann, 2015 | IIa, International | 12 weeks | 204 | Placebo (41) | 0 | 0 | High |
| 6.3−10 | 25 (41) | 0 | 0 | |||||
| 50 (41) | 0 | 0 | ||||||
| 100 (40) | 3 (7.5) | 30 | ||||||
| 150 (41) | 0 | 0 | ||||||
| 2 | Genovese, 201630 | IIb, International | 24 weeks | 358 | Placebo (71) | 0 | 0 | High |
| 6.5−8.1 | 100 (71) | 0 | 0 | |||||
| 150 (72) | 0 | 0 | ||||||
| 200 (72) | 0 | 0 | ||||||
| 100/12 h (72) | 1 (1.4) | 2.77 | ||||||
| 3 | Genovese 2016 | IIb, International | 12 weeks | 43 | Placebo (12) | 0 | 0 | High |
| 6.8−10.8 | 100 (11) | 0 | 0 | |||||
| 200 (10) | 0 | 0 | ||||||
| 300 (10) | 0 | 0 | ||||||
Although all the studies were of a high standard, largely because they were randomised clinical trials, some stood out as having a longer follow-up time and presented their HZ results in rates15,16,21,29. In addition, higher doses of JAKi, not marketed, included in the clinical trials were associated with a higher risk of HZ; the risk was doubled in some cases23–25.
DiscussionIn general, all JAKi entail an increase in the number of opportunistic HZ infections as a side effect, as a probable class effect of this therapeutic group. It should be noted that the rate of HZ infection is strongly influenced by the endemic nature of VZV according to the geographical area of the study population, and therefore it was clearly higher in Asia and India, and was lower in Europe and North America.
After the first two JAKi (tofacitinib and baricitinib) were marketed, recent studies have focused on more selective drugs with the ability to inhibit the activity of only one JAK, JAK1, with the aim of improving the safety profile by minimising the effects of JAK2 and JAK3. For most of the JAKi, HZ incidence data were similar between the different doses of each drug, although we could only confirm this through a statistical study, which is beyond the scope of our study. However, a higher incidence is shown with upadacitinib 30 mg compared to the marketed daily dose of 15 mg23–26.
Although our study was not intended to compare the quality of HZ collection as a complication of HZ treatment, it is noteworthy that early published clinical trials (not included in the final evaluation for this reason) did not specify the presence of herpes or classify its severity. However, more recently published studies, aware of the risk of HZ caused by JAKi, are more detailed about HZ cases, in many cases classifying them by severity. This aspect clearly influences to a greater or lesser extent the difference in reported cases.
However, we must bear in mind that there are other factors that may influence the development of HZ in these patients, such as concomitant treatment with high-dose corticosteroids, a history of HZ infection, the state of immunosuppression associated with the disease itself and possible associated diseases, among others. In fact, in the upadacitinib trials, the use and dose of corticosteroids was recorded and no influence on the onset of HZ was shown, probably because low doses of less than 7.5 mg/day were used23–26.
The data on the safety profile of JAK1 in relation to HZ infection appear to be consistent with those previously known and observed with tofacitinib and baricitinib11–22. Although the results of clinical trials of JAK1 selective drugs seem to suggest a lower rate of HZ, ideally clinical trials should be designed that directly compare the various available JAKi, along with indirect real-life comparisons and registries, to help better understand the real potential benefit of selective JAK1 inhibition compared to blocking other JAKs.
Vaccinating patients before starting treatment is another aspect to consider. This vaccination is not included in the current vaccination schedule, and most patients are not vaccinated against HZ. The authors of some of the studies included mention this aspect, concluding that it does not seem to influence the subsequent development of HZ. This is very relevant but also beyond the scope of our study.
Our study is not without limitations. Because this is a systematic review of clinical trials, the external validity of the results expressed here may be limited, as no data from registries are included. Furthermore, the included studies were selected by a single investigator, although any doubts were discussed between two investigators. The results of HZ incidence were presented disparately across the different studies, mostly in percentages and some in rates, which, together with the variability in the duration of each study, means we should use caution in comparing between studies. Finally, we did not perform a meta-analysis of the included studies as HZ data are reported heterogeneously, which limits the interpretation of the results.
ConclusionsJAKi are associated with the onset of opportunistic HZ infection, which varies according to the study population, but appears consistent among the different JAKi. It has been suggested that selective inhibition of JAK1 is associated with a lower risk of HZ compared to other JAKi, but more studies, clinical trials and registries are needed to support this conclusion.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez González CO, Nieto González JC. Inhibidores de receptores JAK quinasa e infección por virus varicela zoster en pacientes con artritis reumatoide. Revisión sistemática de la literatura. Reumatol Clín. 2022;18:453–458.