The macrophage activation syndrome is a rare but potentially fatal complication of patients with autoimmune rheumatic diseases. This is a clinicopathological entity characterized by activation of histiocytes with prominent hemophagocytosis in the bone marrow and other reticuloendothelial systems. In patients with lupus it may mimic an exacerbation of the disease or infection. We report the case of a 7-year-old girl in whom the diagnosis of lupus erythematosus and macrophage activation syndrome was simultaneously made with response to the use of cyclophosphamide.
El síndrome de activación de macrófago es una complicación inusual pero potencialmente fatal de pacientes con enfermedades reumáticas autoinmunes. Esta es una entidad clínico-patológica caracterizada por la activación de histiocitos con hemofagocitosis prominente en la médula ósea y otros sistemas reticuloendoteliales. En pacientes con lupus, puede simular una exacerbación de la enfermedad o infección. Presentamos el caso de una paciente de 7 años de edad en la que el diagnóstico de lupus eritematoso sistémico y síndrome de activación de macrófago fue simultáneo con respuesta al uso de ciclofosfamida.
Autoimmune rheumatic diseases in children are rare, with a varied clinical presentation. Children represent 15%–20% of patients with systemic lupus erythematosus (SLE), with an incidence of 0.3–0.9 per 100000 and a prevalence of 3.3–8.8 per 100000 children.1 Unlike adults, children have more severe disease at diagnosis.2 One of the complications observed in patients with SLE is the macrophage activation syndrome (MAS). MAS is a severe and potentially fatal condition associated with excessive activation and expansion of T cells with macrophages and a high expression of cytokines, resulting in an uncontrolled inflammatory response, with high levels of macrophage colony-stimulating factor (M-CSF), TNF-α, IL-1β, IL-18, IL-6 and gamma interferon, with decreased expression of perforin in CD8 and NK cells. The cytokine profile of patients with MAS associated with SLE shows a predominance of TNF-α and M-CSF.3–6 However, MAS is not a complication but frequently presents a rapidly progressive course, so its early diagnosis and treatment are necessary. Its incidence has been increasing in patients with rheumatic diseases and especially associated with SLE.
The clinical presentation of MAS is usually acute, requiring admission to the intensive care unit, with unremitting high fever, pancytopenia, liver enlargement, generalized lymphadenopathy and elevated liver enzymes and, in some cases, alterations in coagulation tests, elevated ferritin over 500–10000ng/ml and CNS dysfunction in a third of cases.7 The pathognomonic feature is found in the bone marrow aspirate, showing numerous morphologically benign histiocytes with hemophagocytic activity. Its diagnosis is a challenge for the physician as it can mimic a relapse or an infectious complications.8
In the absence of controlled studies on the treatment of MAS, this is based on anecdotal experience. Usually high doses of steroids are used. Cyclosporin A (CsA) was used in the mid nineties for inherited forms of hemophagocytic syndrome (HS) and subsequently demonstrated effectiveness in patients with steroid-refractory MAS, and therefore is regarded as first-line therapy.9 Other treatments, such as intravenous immunoglobulin (IVIG), cyclophosphamide (CFM), anti-TNF, anakinra, plasmapheresis and etoposide, have shown conflicting results.
To date, 94 SLE cases have been reported associated MAS10; of these, 60 are in pediatric patients with a simultaneous presentation of SLE and MAS in 9 children.4,11–17
The reported mortality of MAS associated with juvenile SLE is 11% and organ dysfunction is more common compared with other rheumatic diseases.3
We report the case of a pediatric patient who presented MAS associated with the initial manifestations of SLE, with lack of response to steroids, IVIG and CsA, which finally answered with the use of CFM.
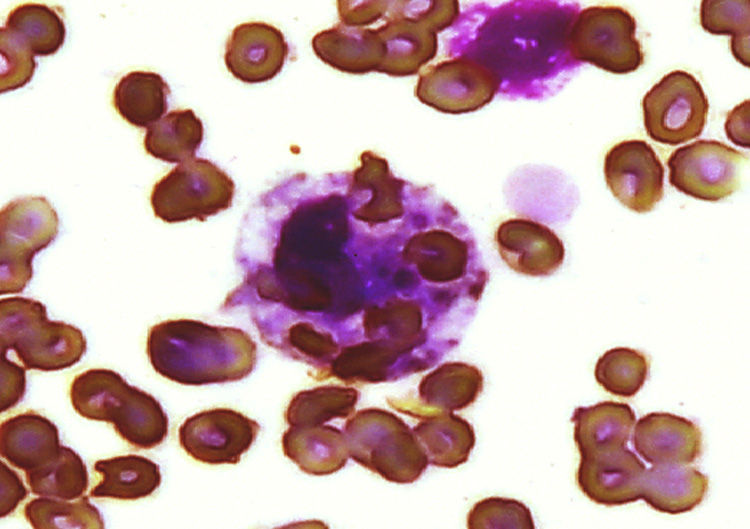
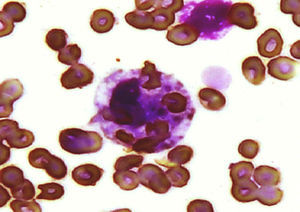
Clinical CaseThe patient was a 7 year-old girl with a presentation 4 months prior characterized by fatigue, weakness and decreased appetite, bipalpebral facial edema and pain in the right upper quadrant, with progression to anasarca, dyspnea of great efforts and hypertension, seen at the hospital where laboratory tests were performed showing nephrotic/nephritic syndrome; due to this she was sent to our hospital. On admission, she had generalized pallor, fever of 40°C, petechiae, arthritis of the hands and elbows, bilateral pleural effusion l, holosystolic murmur 3/6, ascites and hepatosplenomegaly. The blood count showed 1980 white blood cells/μL (VN 4000–13500/μL), neutrophils 820/μL, 910 cells/μL, hemoglobin (Hb) 6.3g/dL (11.5–14 VN, 5g/dL), platelets 44000/μL (VN 150000–400000/μL), prolonged activated partial thromboplastin time, uncorrected after 1:2 addition of normal plasma (control 30s), fibrinogen 368mg/dL (VN 200–400mg/dL), antinuclear antibody CLIA 5 (VN 0.5–1.5) for IFI 1:160 homogeneous pattern and anti-DNA antibodies 82 (VN 0–20), C3 38mg/dL (VN 90–177mg/dL), C4 3.3mg/dL (VN 15–45mg/dL), positive lupus anticoagulant with Russell's viper venom test and anticardiolipin IgG over 280μ/ml and IgM 162μ/ml (high positive), false positive VDRL, negative fluorescent treponemal antibody, serum creatinine of 1.4mg/dL and creatinine clearance ml/min/1.34, 73m2 (VN 70–150ml/min/1.73m2) and C reactive protein 50.4mg/L (NV 0–5mg/L). With these data, we reached the diagnosis of SLE with renal, cutaneous, articular and hematologic involvement with secondary antiphospholipid syndrome. Treatment was started with methylprednisolone pulses (30mg/kg/day) for 5 days, with poor response; persisting with involvement of the 3 cell lines in the blood count, elevated triglycerides to 287mg/dL (VN 120–200mg/dL). Given the lack of response to steroids, IVIG was initiated at a dose of 400mg/kg/day for 5 days and filgrastim; laboratory tests showed leukocytes 1890/μL, neutrophils 0/μL, lymphocytes 1760/μL, platelets 57000/μL, Hb 7.2g/dL with negative direct Coombs test. An infectious process was ruled out with viral panel for cytomegalovirus, herpes 1 and 2, rubella, Epstein–Barr virus, toxoplasma, human immunodeficiency virus, hepatitis B and C viruses, and bacterial cultures, which were negative. A bone marrow aspirate showed decreased cellularity and numerous histiocytes in the entire smear with a conglomerate showing phagocytosis of erythrocytes and platelets, leading to the diagnosis of SH (Fig. 1). Serum ferritin was 1.058ng/ml. We began CsA at doses of 1mg/kg/day (renal failure), for 4 days with no response, leukocyte 660/μL, neutrophils 0/μL, lymphocytes 580/μL and platelets 52000/μL, so we switched to CFM 1g/m2 body surface area, with monthly dose. 15 days after the first dose of CFM, frank hematologic recovery was observed with leukocytes 14970/μL, neutrophils 12800/μL (in the absence of infection), 490 cells/μL, Hb 11.6g/dL and 90000 platelets/μL. During the evolution the patient did not merit mechanical ventilation or vasopressors. The patient was sent home with prednisone 1mg/kg/day and monthly pulses of CFM. Three months later, the renal biopsy showed lupus nephritis class IV according to World Health Organization. After 14 CFM monthly pulses, she presented Fisher Evans syndrome (autoimmune hemolytic anemia and primary immune thrombocytopenia), peripheral neuropathy, once again treated with steroid pulses, IVIG, and rituximab CFM. She received a total of 18 monthly pulses of CFM and continued treatment with mycophenolate mofetil and low-dose steroid. The patient, after 3 years, leads a normal life and goes to school.
DiscussionSH is rare but potentially fatal in patients with autoimmune rheumatic diseases. This is a clinicopathologic entity characterized by activation of histiocytes with prominent hemophagocytosis in the bone marrow and other reticuloendothelial systems.18 It occurs in primary and secondary forms. The primary type includes a group of genetic disorders that result in an altered function of the immune cells. Secondary or acquired may be associated to malignancy, infections or autoimmune disease.8 In patients with autoimmune diseases it is called MAS and can occur as the initial manifestation at the time of diagnosis or form part of an exacerbation or an infectious process.4 Autoimmune diseases associated with MAS are rheumatoid arthritis, Sjögren's syndrome, dermatomyositis, Kawasaki disease, systemic sclerosis, mixed connective tissue disease (MCTD) and SLE.18 Although there are no specific data on the prevalence of MAS in SLE, it is estimated to range from 0.9% to 4.6%.7
The diagnosis of MAS is difficult because it can simulate an infectious complication or an exacerbation of an underlying disease.18 We report the case of a patient with concurrent diagnosis of SLE and MAS. When presented in parallel, the difficulty for diagnosis increases because the clinical manifestations of both entities overlap.
The patient met the criteria of the American College of Rheumatology criteria for SLE and the Histiocyte Society,8 Kumakura et al.,19 as well as the criteria recently reported Parodi et al. for MAS.7 The criteria proposed by the Histiocyte Society have high specificity but the sensitivity is not satisfactory, since approximately 33% of patients with MAS do not meet these criteria.7 In 2004, Kumakura et al. proposed diagnostic criteria for SH associated with autoimmune diseases, and the clinical manifestations are different from those of other reactive hemophagocytic syndromes.19 In 2009, Parodi et al. developed preliminary guidelines for the diagnosis of MAS in patients with juvenile SLE using 5 clinical criteria (fever, hepatomegaly, splenomegaly, hemorrhagic manifestations CNS dysfunction) and 6 laboratory criteria (cytopenias affecting two or more cell lines, increased aspartate transaminase, DHL, triglycerides and ferritin, decreased fibrinogen) and histopathological criteria (evidence of hemophagocytosis). With one or more clinical criteria and 2 or more laboratory criteria, the diagnosis can be established; a bone marrow aspirate is reserved for doubtful cases. The laboratory criterion which showed higher sensitivity (96%) and specificity (100%) was hyperferritinemia.7
In the systematic review by Atteritano et al. related to SH in rheumatic diseases, they found 94 patients with SLE and SH.10 A more detailed review of the cases associated with SLE showed that of the 94 cases, 60 belonged to pediatric patients and 29 had simultaneous SLE presentation with SH with 9 of these being pediatric patients. Our case is the tenth reported with a concurrent diagnosis in the pediatric population and the youngest one.
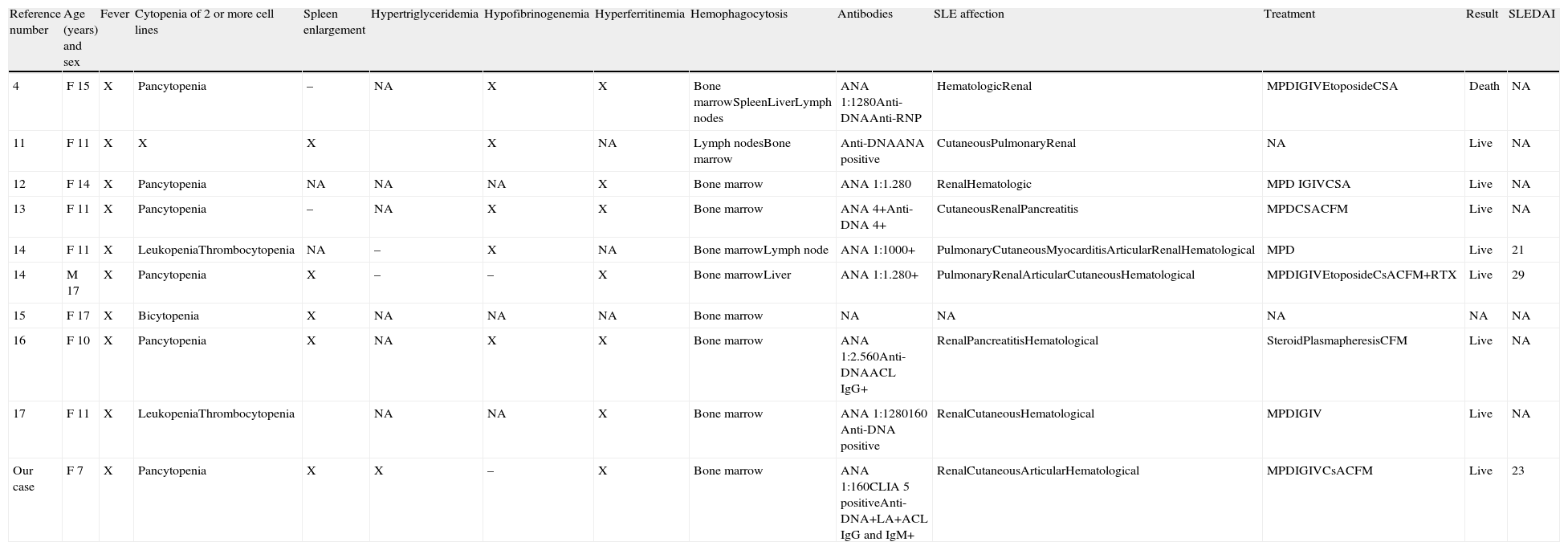
The review of pediatric cases in which the presentation of SLE was simultaneous to that of MAS, including ours, are shown in Table 1.14,11–17 We found that fever, cytopenias of at least 2 cell lines and hemophagocytosis were presented in 100% of cases, hyperferritinemia in 70% of cases (30% in the data is found to be available from case series) and hypofibrinogenemia and splenomegaly in 50%. Hemophagocytosis was demonstrated in bone marrow in 100%, lymph node 30%, liver 20% and 10% spleen.
Characteristics of Patients Reported in the Literature With Simultaneous Diagnosis of SLE and MAS.
| Reference number | Age (years) and sex | Fever | Cytopenia of 2 or more cell lines | Spleen enlargement | Hypertriglyceridemia | Hypofibrinogenemia | Hyperferritinemia | Hemophagocytosis | Antibodies | SLE affection | Treatment | Result | SLEDAI |
| 4 | F 15 | X | Pancytopenia | – | NA | X | X | Bone marrowSpleenLiverLymph nodes | ANA 1:1280Anti-DNAAnti-RNP | HematologicRenal | MPDIGIVEtoposideCSA | Death | NA |
| 11 | F 11 | X | X | X | X | NA | Lymph nodesBone marrow | Anti-DNAANA positive | CutaneousPulmonaryRenal | NA | Live | NA | |
| 12 | F 14 | X | Pancytopenia | NA | NA | NA | X | Bone marrow | ANA 1:1.280 | RenalHematologic | MPD IGIVCSA | Live | NA |
| 13 | F 11 | X | Pancytopenia | – | NA | X | X | Bone marrow | ANA 4+Anti-DNA 4+ | CutaneousRenalPancreatitis | MPDCSACFM | Live | NA |
| 14 | F 11 | X | LeukopeniaThrombocytopenia | NA | – | X | NA | Bone marrowLymph node | ANA 1:1000+ | PulmonaryCutaneousMyocarditisArticularRenalHematological | MPD | Live | 21 |
| 14 | M 17 | X | Pancytopenia | X | – | – | X | Bone marrowLiver | ANA 1:1.280+ | PulmonaryRenalArticularCutaneousHematological | MPDIGIVEtoposideCsACFM+RTX | Live | 29 |
| 15 | F 17 | X | Bicytopenia | X | NA | NA | NA | Bone marrow | NA | NA | NA | NA | NA |
| 16 | F 10 | X | Pancytopenia | X | NA | X | X | Bone marrow | ANA 1:2.560Anti-DNAACL IgG+ | RenalPancreatitisHematological | SteroidPlasmapheresisCFM | Live | NA |
| 17 | F 11 | X | LeukopeniaThrombocytopenia | NA | NA | X | Bone marrow | ANA 1:1280160 Anti-DNA positive | RenalCutaneousHematological | MPDIGIV | Live | NA | |
| Our case | F 7 | X | Pancytopenia | X | X | – | X | Bone marrow | ANA 1:160CLIA 5 positiveAnti-DNA+LA+ACL IgG and IgM+ | RenalCutaneousArticularHematological | MPDIGIVCsACFM | Live | 23 |
ACL: anticardiolipin; Anti-DNA: anti-DNA antibodies; ANA: antinuclear antibodies; CFM: cyclophosphamide; CsA: cyclosporin A; IgG: immunoglobulin G; IgM: immunoglobulin M; IVIG: intravenous immunoglobulin; LA: lupus anticoagulant; SLE: systemic lupus erythematosus; MPD: methylprednisolone; NA: not available; RTX rituximab.
The main symptoms of MAS include prolonged fever, hepatosplenomegaly and cytopenias. The presence of cytopenia is common in SLE, but isolated pancytopenia occurs in less than 10% of cases, and if it occurs, usually is mild. Leukopenia is detected similarly in patients with SLE and MAS, but severe leukopenia (less than 2000 cells) obliges the clinician to consider the presence of MAS. Thrombocytopenia is the best indicator of MAS compared with7,19 leukopenia and anemia. In the cases of simultaneous SLE and MAS reported, pancytopenia occurred in 60%, leukopenia and thrombocytopenia in 80%. Our case presented leukopenia of less than 2000 cells, and what drew our attention was neutropenia, which became absolute, something atypical to the usual presentation of juvenile SLE, and an indicator of impaired primary bone marrow and a guide in the diagnosis of MAS.
The review, by Parodi et al.,7 of 38 patients with juvenile SLE and MAS compared with a group of 29 patients with active SLE and a group of 387 patients from a cohort of SLE with organ damage, showed that patients who developed MAS had a higher frequency of nephritis, arthritis, serositis and hematological involvement at the time of diagnosis of MAS. In the 10 pediatric patients with SLE and concurrent diagnosis of MAS, renal disease was found in 90%, 70% had hematologic affection, skin was affected in 60%, lungs and joints in 30% and cardiac manifestations found in 10%, and called attention to pancreatic disease in 20%, as this is a rare condition of pediatric SLE and in the literature there are only case reports and small series relating to it. In the study by Wang et al. in adult and pediatric patients with SLE and pancreatitis, we found that the average SLEDAI of pediatric patients at the time of pancreatitis was 21, so a high disease activity could be related to the occurrence of MAS.20 Supporting this is the series reported by Lambotta of 15 cases of MAS associated with SLE, where the mean SLEDAI was 22.14 Kim et al. conducted a case–control study of 15 patients with SLE associated with MAS, compared with SLE patients without MAS, finding in the first group a higher SLEDAI (14 vs 8) and deeper cytopenias, being statistically significant.21 Of the 10 cases with simultaneous diagnosis of SLE and MAS, 2 patients had a SLEDAI over 20 and in our patient it was 23. Taken together these observations indicate that a high value of SLEDAI with hematologic and kidney disease may be a risk factor for the development of MAS.
The therapeutic strategy is not well established in MAS complicating SLE. As infections are a common trigger, their exclusion is important to establish an appropriate treatment. In case of infection, a decrease in the dose of immunosuppressants becomes important. As supportive therapies, IVIG and G-CSF are used in case of neutropenia.18
Immunosuppressive therapy is indicated when the trigger is the disease activity. Bennet et al. and Parodi et al. found that the treatments most commonly used are high-dose steroids, IVIG, CsA, CFM, mycophenolate mofetil, azathioprine, rituximab, plasmapheresis or anti-TNF.2,3,21 In the review of pediatric cases with concurrent diagnosis of SLE and MAS, steroids were used in 100% of cases, IVIG, CsA in 50%, 40% used CFM and rituximab and plasmapheresis in 10%. The favorable response to the use of steroids alone was 10%, with 40% seen with CsA with CFM, 100% (one case associated with rituximab) and in the case of IGIV, 20%. Our patient was treated with high dose steroids, and CsA as well as IVIG, with no response, so she was finally treated with CFM, obtaining a successful response. Pancytopenia did not worsen with the use of CFM. Although this treatment has shown conflicting results,9 based on the effectiveness shown in these cases it can be assumed that CFM is a therapeutic option that should be considered in the treatment of MAS associated with the onset of SLE.
Anti-TNF therapy has been used for refractory cases. Kikuchi et al.22 and Takahashi et al.23 reported two cases resistant to steroids therapy, IVIG and CsA, and one case resistant to methotrexate that responded to etanercept. Ideguchi et al.24 and Henzan et al.25 reported two steroid-refractory cases, who also showed a lack of response to cyclosporine, plasmapheresis and etoposide, which finally responded with infliximab. One of them was treated with a single dose of CFM 500mg, which influenced the fact that the desired response was not obtained. Our patient responded to doses of 1g/m2 body surface area intravenously without undesirable immediate effects (profound pancytopenia).
Another therapeutic option is anakinra, which showed utility in patients with MAS associated to vasculitis and systemic juvenile idiopathic arthritis. In these diseases, IL-1, IL-6 and IL-1826–28 predominate. Because the treatment of MAS also focuses on treating the underlying disease and in patients with SLE-associated MAS the cytokine profile is different, the use of CFM in our case resulted more suitable.
MAS associated with SLE should be considered a severe complication that puts the patient's life at risk, as their mortality is 11% and the number of patients requiring PICU admission, mechanical ventilation and cardiovascular dysfunction (63%, 53% and 47%, respectively) is high.3,10 The review of the 10 cases reported a mortality of 10%, consistent with other publications. Our patient had a favorable outcome and is currently 10 years old and leads a normal life.
ConclusionMAS is a rare but potentially fatal complication of SLE, so early diagnosis and treatment are essential to improve survival. MAS should be suspected in patients with high levels of activity in SLE. CFM in our case was effective for the treatment of clinical manifestations. It should be considered for use in patients with SLE associated with MAS despite profound cytopenias, without fear of worsening the presentation. Evidence as to the best treatment is scarce, given the rarity of the entity, so it is necessary to identify cases to determine appropriate therapy in these patients.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that no experiments have been performed on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained informed consent from patients and/or subjects referred to in this article. This document is in the possession of the corresponding author.
Conflict of InterestThe authors declare no conflicts of interest.
To the research unit of the Mexican College of Rheumatology and Dr. Luis Javier Jara Quezada, unit coordinator, for their support in the workshop “How to write a scientific article step by step.”
Please cite this article as: Torres Jiménez A, Solís Vallejo E, Zeferino Cruz M, Céspedes Cruz A, Sánchez Jara B. Síndrome de activación de macrófago como manifestación inicial de lupus eritematoso sistémico severo de inicio juvenil. Respuesta favorable a ciclofosfamida. Reumatol Clin. 2013;10:331–335.