Intestinal perforation, a rare complication of interleukin (IL)-6 therapy for immune-mediated diseases (mainly rheumatoid arthritis), typically manifests in the lower gastrointestinal tract, often in association with prior history of diverticulitis. Patients may present with acute abdominal pain and suspicion for this complication should remain high even in the absence of elevated C-reactive protein. We describe a 69-year-old female patient with a history of resistant seropositive palindromic rheumatism treated with sarilumab who developed a nontraumatic terminal ileal perforation.
La perforación intestinal, complicación poco frecuente del tratamiento con inhibidores de interleucina-6 para enfermedades inmunomediadas (principalmente artritis reumatoide), generalmente se manifiesta en el tracto gastrointestinal inferior, a menudo en asociación con antecedentes de diverticulitis. Los pacientes pueden presentar dolor abdominal agudo y la sospecha de esta complicación debe permanecer alta incluso en ausencia de elevación de proteína C reactiva. Describimos a una paciente de 69 años con antecedentes de reumatismo palindrómico seropositivo refractario tratada con sarilumab que desarrolló una perforación ileal terminal no traumática.
The association between interleukin (IL)-6 inhibitors and perforation of the lower gastrointestinal (GI) tract in patients with a history of diverticulitis is well known. However, perforations can also occur at different sites. We present a patient with palindromic rheumatism (PR) treated with sarilumab who developed a spontaneous terminal ileal perforation complicated by faecal peritonitis.
Clinical observationWe present the case of a 69-year-old woman with a history of atypical seropositive PR with polyarticular inflammatory flares and fever (genetic study negative for autoinflammatory syndromes). Despite 10 years of conventional treatment (hydroxychloroquine, salazopyrin, methotrexate [MTX], and colchicine), the patient continued to experience flares without progression to chronic arthritis. As a result, treatment with sarilumab was initiated, with optimal clinical response. The patient reported no previous episodes of diverticulitis or relevant GI manifestations.
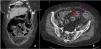
Fifteen months later, the patient experienced a spontaneous terminal ileal perforation, diagnosed by CT (Fig. 1), complicated by faecal peritonitis and bacteraemia (Bacteroides fragilis). The clinical presentation included vomiting and acute abdominal pain with elevated CRP. Ileal resection with lateral-lateral anastomosis was performed with subsequent conversion to terminal ileostomy due to anastomotic dehiscence and favourable postoperative recovery. Histopathological analysis revealed inflammation, vascular congestion, and fibrosis in the ileal wall without evidence of malignancy. Sarilumab was discontinued before surgery and colchicine was restarted, with no further flares for 18 months. However, the patient subsequently progressed to rheumatoid arthritis (RA) and was started on MTX and low-dose glucocorticoids (GC). From that time to the present, and over a follow-up period of 2 years, the patient has remained clinically asymptomatic, with no new episodes of abdominal pain.
Extensive diffuse pneumoperitoneum associated with a significant amount of free fluid predominantly in left parietocolic gutters (white arrow). In the distal ileum (approximately 10–15 cm from the ileocecal valve) there is a 5 mm parietal solution of continuity, suggestive of perforation (red arrow).
Lower GI perforations, although rare, are a serious complication associated with IL-6 inhibitors. A higher incidence has been demonstrated with IL-6 inhibitors in patients with RA compared to conventional disease-modifying (cDMARDs) and biological (bDMARDs) antirheumatic drugs,1–3 in relation to mechanisms involving the role of IL-6 in maintaining the intestinal barrier by supporting epithelial proliferation, angiogenesis, and wound healing.4,5
One risk factor associated with lower GI perforation is a history of diverticulitis. However, an increased risk has also been described in the absence of diverticulitis.1 In addition, IL-6 inhibitor therapy itself has been associated with a higher rate of diverticulum development, possibly due to the role of IL-6 in colonic contraction.6 Additional risk factors should be considered, such as cumulative doses of GCs and non-steroidal anti-inflammatory drugs (NSAIDs).
Most lower GI perforation events have been reported in patients treated with tocilizumab, probably due to more extensive use, while few have been associated with sarilumab.7,8 In a safety analysis of several clinical trials of sarilumab in RA involving 2343 patients, only 4 cases of lower GI perforation were observed.9
Our case is unique in several ways. First, it is a seropositive PR with atypical findings without progression to RA at the time of GI perforation, despite a long follow-up. To our knowledge, this is the first case of PR treated with sarilumab. Second, the patient had no history of diverticulitis or inflammatory bowel disease.
Before the advent of the bDMARDs, GI perforations in patients with RA were mainly associated with NSAIDs.10 However, these complications predominantly affect the proximal small bowel, and our patient was not taking them.
Despite these atypical features, treatment was discontinued.
ConclusionsThe presence of an acute abdomen in patients treated with IL-6 inhibitors should alert the clinician to the possibility of intestinal perforation, and imaging studies are recommended for diagnosis, bearing in mind that the systemic response may be masked by treatment. Additional risk factors such as the use of NSAIDs and GCs further increase the likelihood of this complication. Rapid diagnosis is essential due to the life-threatening nature of the condition.
The authors have no conflict of interest to declare.