Systemic sclerosis (SSc) is a chronic multisystem autoimmune disease which involves the gastrointestinal tract in about 90% of cases. It may contribute to nutritional deterioration.
ObjectiveTo assess whether the application of a nutritional support protocol to these patients could improve their nutritional status and quality of life.
MethodsSingle center prospective study, performed on an outpatient basis, in a county hospital. The Malnutrition Universal Screening Tool (MUST) was used to screen risk for malnutrition. Health questionnaire SF-36 and the Hospital Anxiety and Depression Scale were used to assess quality of life and psychopathology respectively. Weight, height, energy and protein requirements, macronutrient intake and nutritional biochemical parameters were evaluated. Nutritional intervention was performed in patients at risk for malnutrition.
ResultsOf the 72 patients, 12.5% were at risk for malnutrition. Iron deficiency anemia (18.35%) and vitamin D deficiency (54%) were the most frequently observed nutritional deficits. The questionnaires on psychopathology and quality of life showed a high prevalence of anxiety and depression, and lower level poor quality of life in the physical and mental component. No significant improvements were observed in the weight, food intake, nutritional biochemical parameters, psychopathology and quality of life follow-up.
ConclusionsDietary intervention was able to maintain body weight and food intake. Iron deficiency anemia and vitamin D deficiency improved with iron and vitamine D supplements. No deterioration was observed in psychological assessment or quality of life. Studies with larger numbers of patients are needed to assess the efficacy of this intervention.
La esclerosis sistémica (ES) es una enfermedad autoinmune sistémica crónica que en cerca del 90% de los casos afecta al tracto gastrointestinal. Se estima que dicha alteración puede contribuir al deterioro nutricional.
ObjetivoEvaluar si la aplicación de un protocolo de soporte nutricional a dichos pacientes mejora su estado nutricional y su calidad de vida.
MétodoEstudio prospectivo unicéntrico realizado en consultas externas de un hospital comarcal. Se utilizó el test MUST para el cribado de malnutrición. El cuestionario de salud SF-36 y el de Hospital Anxiety and Depression Scale se utilizaron para la valoración de la calidad de vida y psicopatológica, respectivamente. Se determinaron: el peso, la talla, las necesidades energéticas y proteicas, la ingesta de macronutrientes y los parámetros bioquímicos nutricionales. Se realizó intervención nutricional a los pacientes con riesgo.
ResultadosDe los 72 pacientes, el 12,5% tenían riesgo de malnutrición. La anemia ferropénica (18,35%) y el déficit de vitamina D (54%) fueron los déficits nutricionales más observados. Los cuestionarios de psicopatología y calidad de vida indicaron elevada prevalencia de ansiedad y depresión, y puntuaciones más bajas en las dimensiones física y mental según el SF-36. No se evidenciaron mejoras significativas en la evolución del peso, en la ingesta alimentaria ni en los parámetros bioquímicos nutricionales, psicopatológicos ni de calidad de vida.
ConclusionesLa intervención dietética consiguió mantener el peso corporal y la ingesta energética y proteica. Los déficit de hierro y de vitamina D mejoraron con suplementación. No se observó un deterioro en la valoración psicológica ni en la calidad de vida. Se precisan estudios con mayor número de pacientes para valorar la eficacia de dicha intervención.
Systemic sclerosis (SSc) is a connective tissue disorder characterized by inflammation and fibrosis of the skin, blood vessels and multiple internal organ involvement. The gastrointestinal tract is the second most affected organ, found in almost 90% of patients, and the disorder can occur along its entire length: esophagus, stomach, small intestine, colon and anorectal portion.1 About half of the patients have nausea, postprandial fullness, bloating and changes in bowel habits, and may be associated with loss of body weight.2 The impact of this disease on the digestive tract may contribute significantly to the deterioration of the nutritional state.3
It has been reported that the prevalence of malnutrition in these patients ranges from 15 to 30% according to several case series.4–6 To overcome this deficit, a panel of experts from Canada drew up recommendations to detect malnutrition and malabsorption in these patients.6 Malnutrition, along with all the symptoms associated with SS, has been associated with a poor perceived quality of life,5,7 which is lower than that reported for the general population.8 In addition, this patient population referred a higher incidence of depressive symptoms,9 although symptoms related to anxiety did not receive the same attention and could be a result to be considered.10
It is therefore necessary to identify patients at risk of malnutrition to apply measures of nutritional support and to evaluate their effectiveness on a physical, mental and perceived quality of life level.
Only general dietary recommendations symptoms as per the need of the patients have been described to date, and there are no studies assessing the benefits of nutritional intervention.
Based on the above, we evaluate whether the application of a nutritional support protocol11 in SS patients who visited the outpatient department of a community hospital improves their nutritional status, emotional state and quality of life.
MethodsType of StudySingle-center, prospective and interventional year-long study. The protocol was reviewed and approved by the hospital ethics committee.
Study PopulationWe systematically included patients with SS, according to the criteria proposed by LeRoy and Medsger,12 who visited in the outpatient rheumatology and internal medicine clinics at the Hospital de Granollers, and who required nutritional support.
Inclusion CriteriaPatients of both sexes, aged 18 or older, who could read and write Catalan and/or Castilian and scoring with a score at or above 1 on the MUST screening test.13
Exclusion Criteria(a) Patients diagnosed with neoplastic processes or other conditions that interfere with the nutritional status of the patient, (b) patients who did not sign the informed consent and/or did not wish to participate, (c) those who had a mental, or cognitive psychiatric impairment that could alter the outcome of self-administered tests, though no psychosocial variables were assessed.
ProtocolOutpatient Visits to the Internist-rheumatologist. At each visit, the professional evaluating the involvement of various organs according to the Canadian survey designed for this purpose,6 determined the anthropometric and laboratory parameters, and applied the MUST method of screening. If the patient met the criteria described above, the food record sheet for the patient/caregiver's was completed over one week and was sent for assessment by the dietitian .In case of vitamin deficiencies, the patient was supplemented pharmacologically: 100mg Fe2+ per day in case of iron deficiency; 400–800IU/day of cholecalciferol when the deficit was moderate or 50000IU a week when there was a material or serious deficiency (vitamin D below 20ng/ml).14
Regarding the presenting symptoms, additional studies (endoscopy, pH-metry, manometry etc.) and assessment by certain specialists (gastroenterologist, otolaryngologist etc.) were made, and treatments applied.
Clinical Study Variables. Age, gender, weight, height, body mass index (BMI), blood count, vitamin A, serum folate, albumin, ferritin, vitamin B12 and 25 OH vitamin D3 were determined. In case of suspected malabsorption, plasma zinc levels and prothrombin time (PT) were determined.
Dietary Intervention. The dietitians–nutritionists carried out semi-structured interviews to collect the variables under study. At each visit, anthropometric parameters were determined and the previous weeks’ food record was collected, and energy and macronutrient intake was calculated. Energy requirements were estimated using the Harrison–Benedit formula and protein was established by direct estimation (1–1.5g/kg/day). The calculation of the energy and protein intake was estimated from the dietary record using a computer program of the Center d’ Ensenyament i Nutrició Human Dietetics (CESNID). With all these variables, the nutritional evaluation was performed and the diet adapted to individual needs, taking into account energy, macronutrient ratio and textures.
Patients were encouraged to eat a balanced diet according to their macro and micronutrient requirements (assuming there were no other medical contraindications). The basic tailored diet indication of enteral products and oral nutritional supplements was not different from that for other diseases associated with chronic malnutrition. Due to the involvement of multiple parts of the digestive tract and the fact that this is an evolutionary process, we were forced to perform highly customized dietary recommendations.
Patients were monitored biweekly for weight and, in case of a sudden and significant change in body weight (5% or more in a month), they contacted the dietitian.
Psychosocial Variables. Sociodemographic data determined were: age, gender, education level and profession. Hospital Anxiety and Depression Scale (HADS) Questionnaire15 assesses anxiety and depression on 2 subscales of 7 questions each, considered as a cutoff scores over 7. SF-36 Health Questionnaire16 consists of 36 questions grouped into 8 domains: physical function, social function, behavioral limitations, related physical problems, limitations in emotional behavior, related mental health, vitality, bodily pain and general health perception. Each domain is scored with a rating scale ranging from 0 (indicating worse health) to 100 (indicating better health). A score below 50 reflects a poorer quality of life compared with the average of the general population.
Statistical Analysis of the Variables. The data was analyzed using SPSS version 15. Quantitative variables were expressed as means and standard deviations and categorical variables as percentages. To compare quantitative variables, parametric tests for paired data were used. Significance was considered when P was less than .05.
Interpretation of Results. It was established that the program had been effective if weight was maintained or improved, if there was no nutritional deficit and if an improvement was observed in the results obtained in the quality of life test.
Results72 ambulatory patients with SS (65 women and 7 men), were controled, 58 with limited SS and 14 with diffuse SS.
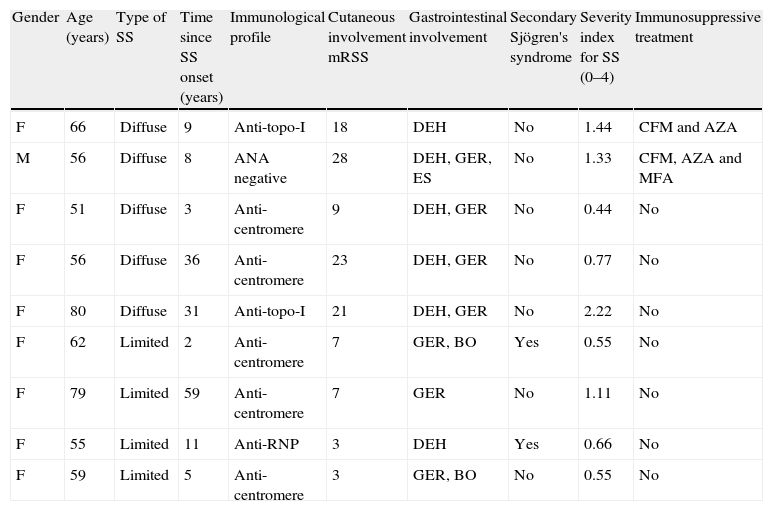
9 patients who met inclusion criteria (8 female and one male) with a mean age of 62.6±11.7 (51–80) years were included in the study. The clinical characteristics of the patients are described in Table 1. One-year follow-up was conducted. Of these, 6 patients had completed primary schooling and one, secondary studies. Four patients performed unskilled jobs, 2 had skilled jobs and one was a homemaker. Of the 9 patients, 4 had limited SS and 5 diffuse SS. Throughout the year follow-up one patient had pneumonia which resolved with antibiotics and 2 patients died from lung disease associated with SS.
Clinical Characteristics of Patients.
| Gender | Age (years) | Type of SS | Time since SS onset (years) | Immunological profile | Cutaneous involvement mRSS | Gastrointestinal involvement | Secondary Sjögren's syndrome | Severity index for SS (0–4) | Immunosuppressive treatment |
| F | 66 | Diffuse | 9 | Anti-topo-I | 18 | DEH | No | 1.44 | CFM and AZA |
| M | 56 | Diffuse | 8 | ANA negative | 28 | DEH, GER, ES | No | 1.33 | CFM, AZA and MFA |
| F | 51 | Diffuse | 3 | Anti-centromere | 9 | DEH, GER | No | 0.44 | No |
| F | 56 | Diffuse | 36 | Anti-centromere | 23 | DEH, GER | No | 0.77 | No |
| F | 80 | Diffuse | 31 | Anti-topo-I | 21 | DEH, GER | No | 2.22 | No |
| F | 62 | Limited | 2 | Anti-centromere | 7 | GER, BO | Yes | 0.55 | No |
| F | 79 | Limited | 59 | Anti-centromere | 7 | GER | No | 1.11 | No |
| F | 55 | Limited | 11 | Anti-RNP | 3 | DEH | Yes | 0.66 | No |
| F | 59 | Limited | 5 | Anti-centromere | 3 | GER, BO | No | 0.55 | No |
MFA: mycophenolic acid, AZA: azathioprine, CFM: cyclophosphamide, ES: esophageal stenosis, SS: systemic sclerosis; DEH: distal esophageal hypoperistaltism, F: female; mRSS: modified Rodnan skin score, GER: gastroesophageal reflux, BO: bacterial overgrowth; M: male.
Medsger TA to. Clin Exp Rheumatol. 2003; 21: S42–6.
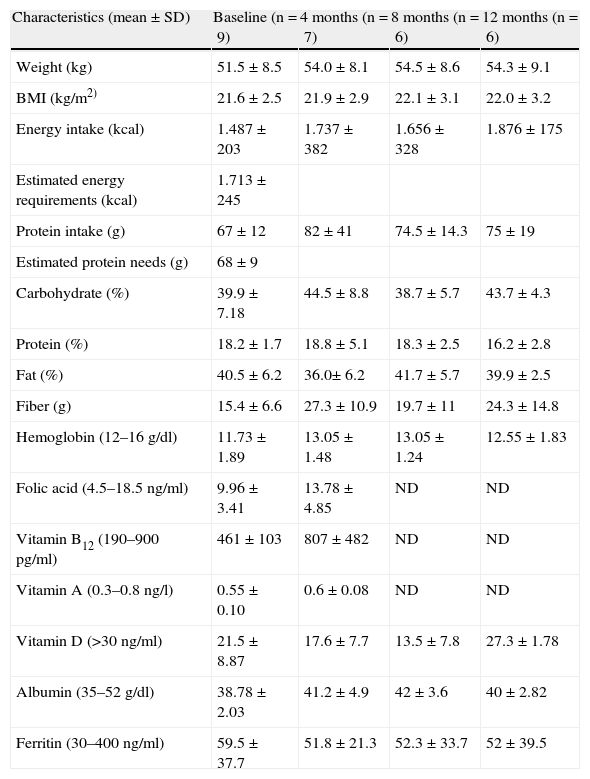
Of the 9 patients included on initial visit 3 patients (33.3%) had a BMI below 20kg/m2, but none of them presented a BMI of less than 18.5kg/m2. Weight gain and BMI throughout the year of intervention was not statistically significant. One patient always maintained a BMI of 19kg/m2, and the BMI of the remaining patients was higher than 20 during the study. Table 2 shows the evolution of weight, BMI, energy and protein intake, estimation of nutritional requirements and the evolution of nutritional biochemistry.
Evolution of Anthropometric Variables, Energy and Protein Intake, Estimation of Nutritional Requirements and Biochemical Parameters.
| Characteristics (mean±SD) | Baseline (n=9) | 4 months (n=7) | 8 months (n=6) | 12 months (n=6) |
| Weight (kg) | 51.5±8.5 | 54.0±8.1 | 54.5±8.6 | 54.3±9.1 |
| BMI (kg/m2) | 21.6±2.5 | 21.9±2.9 | 22.1±3.1 | 22.0±3.2 |
| Energy intake (kcal) | 1.487±203 | 1.737±382 | 1.656±328 | 1.876±175 |
| Estimated energy requirements (kcal) | 1.713±245 | |||
| Protein intake (g) | 67±12 | 82±41 | 74.5±14.3 | 75±19 |
| Estimated protein needs (g) | 68±9 | |||
| Carbohydrate (%) | 39.9±7.18 | 44.5±8.8 | 38.7±5.7 | 43.7±4.3 |
| Protein (%) | 18.2±1.7 | 18.8±5.1 | 18.3±2.5 | 16.2±2.8 |
| Fat (%) | 40.5±6.2 | 36.0±6.2 | 41.7±5.7 | 39.9±2.5 |
| Fiber (g) | 15.4±6.6 | 27.3±10.9 | 19.7±11 | 24.3±14.8 |
| Hemoglobin (12–16g/dl) | 11.73±1.89 | 13.05±1.48 | 13.05±1.24 | 12.55±1.83 |
| Folic acid (4.5–18.5ng/ml) | 9.96±3.41 | 13.78±4.85 | ND | ND |
| Vitamin B12 (190–900pg/ml) | 461±103 | 807±482 | ND | ND |
| Vitamin A (0.3–0.8ng/l) | 0.55±0.10 | 0.6±0.08 | ND | ND |
| Vitamin D (>30ng/ml) | 21.5±8.87 | 17.6±7.7 | 13.5±7.8 | 27.3±1.78 |
| Albumin (35–52g/dl) | 38.78±2.03 | 41.2±4.9 | 42±3.6 | 40±2.82 |
| Ferritin (30–400ng/ml) | 59.5±37.7 | 51.8±21.3 | 52.3±33.7 | 52±39.5 |
SD: standard deviation, ND: not determined.
At the initial visit, patients ate 86.8%±18.3% of their daily energy estimate and 97.5%±17.7% of protein needs. 33% of the patients ate less than 75% of their estimated energy requirements and protein intake in all was above 75%. At the initial visit 3 patients needed oral nutritional supplements, but we preferred to prescribe a basic tailored diet and evaluate them later. Thus, during follow-up visits 3 patients were prescribed oral nutritional supplements 500ml daily of a complete, polymer, and normoporteic normocaloric diet, which were dismissed by one patient.
In each of the evaluations of food logs we observed that 16.6% had an energy intake of less than 75% of daily energy estimate, not always in the same patient. Protein intake was less than 75% in one patient at 9 and 12 months. An increase in protein and energy intake during the dietary intervention was observed, but the differences were not statistically significant.
Regarding the biochemical parameters evaluated, 6 patients (54.5%) had low hemoglobin levels, but just 2 patients had low ferritin levels (18.3%). The mean hemoglobin levels improved during the intervention, although the differences were not significant. Two patients received oral iron supplementation. It was observed that the average levels of ferritin decreased, though not statistically significant, due to a patient who always had low values.
Of the 9 patients, none had to start folic acid, vitamin B12, vitamin A, zinc due to coagulation disorder or due to deficiency. 54% were deficient in vitamin D, and in 18% this had not been determined. Of the patients with deficiency of vitamin D, 16.6% were severe (<10ng/ml), in 66.6% it was important (10–20ng/ml) and 16.6% were moderate (20–30ng/ml).
Three patients received supplementation with calcium and vitamin D (600–800IU/day) and 3 patients were prescribed higher doses of the same. The evolution of the average levels of vitamin D improved slightly, but at the end of the study all had insufficient vitamin D.
Two patients of the 9 who had albumin determinations showed low levels: one at the initial visit (11%), which improved during the dietary intervention, and in the other patient this was detected in the 3 month control.
The psychological evaluation was possible in 5 of the 9 patients, and evaluation of quality of life, in 7 of the 9 patients. At baseline, the mean anxiety score was 8.00±2.71, and depression, 8.8±8.19 Of the patients evaluated, 60% had a score greater than 7 for anxiety and at 40% for depression score.
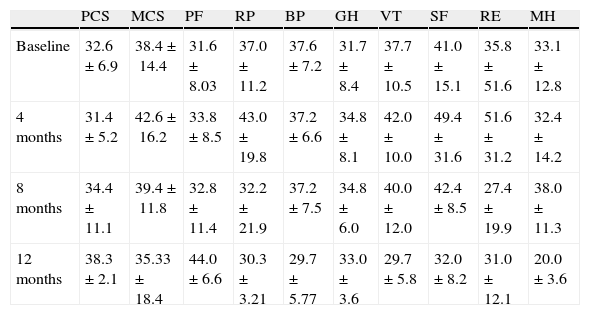
Table 3 shows the mean scores of the SF-36, where it is noteworthy that all patients assessed scored below the criteria of normality in all dimensions, both at baseline and at the end of the intervention. Throughout the operation, no statistically significant differences were observed.
Evolution of the SF36 Questionnaire Score.
| PCS | MCS | PF | RP | BP | GH | VT | SF | RE | MH | |
| Baseline | 32.6±6.9 | 38.4±14.4 | 31.6±8.03 | 37.0±11.2 | 37.6±7.2 | 31.7±8.4 | 37.7±10.5 | 41.0±15.1 | 35.8±51.6 | 33.1±12.8 |
| 4 months | 31.4±5.2 | 42.6±16.2 | 33.8±8.5 | 43.0±19.8 | 37.2±6.6 | 34.8±8.1 | 42.0±10.0 | 49.4±31.6 | 51.6±31.2 | 32.4±14.2 |
| 8 months | 34.4±11.1 | 39.4±11.8 | 32.8±11.4 | 32.2±21.9 | 37.2±7.5 | 34.8±6.0 | 40.0±12.0 | 42.4±8.5 | 27.4±19.9 | 38.0±11.3 |
| 12 months | 38.3±2.1 | 35.33±18.4 | 44.0±6.6 | 30.3±3.21 | 29.7±5.77 | 33.0±3.6 | 29.7±5.8 | 32.0±8.2 | 31.0±12.1 | 20.0±3.6 |
BP: bodily pain, GH: general health, MCS: mental health component, MH: mental health, PCS: physical component function, PF: physical functioning, RE: Emotional behavior, RP: physical behavior, SF social function VT: vitality.
This work evaluates the impact of a nutritional intervention in patients with SS at risk of malnutrition and their nutritional status and quality of life.
Several studies have shown the high prevalence of malnutrition in these patients,4–6 but there are no studies that assess the impact of a structured nutritional intervention after one year of follow-up. In the present series, 12.5% of patients with SS were candidates for nutritional intervention, using the MUST screening method. The prevalence is similar to that described in the literature.
At the initial visit, when the nutritional assessment was performed, we found that three patients had an energy intake of less than 75% of estimated needs. In all cases the intake covered over 50% of the nutritional requirements. In our sample, the prevalence of inadequate energy intake was highly lower than that reported by Krause et al.,17 as was the prevalence of patients requiring nutritional support, either through oral intake or artificial nutrition. The discrepancy could be explained by the fact that both studies have used different methods to estimate the requirements and energy intake. It has been reported that if the food is enriched, this improves energy and protein intake between 23 and 26%, approximately, without affecting hunger before the meal or the desire to eat, leading us to prescribe oral nutritional supplements when intake is less than 75% of requirements.18 Although these 3 patients had such an indication, we preferred to start a basic tailored diet and evaluate the patient at the next control visit. In the follow-up visit we prescribed an oral nutritional supplement to 3 patients, although one of them refused.
The recommendations of the panel of American experts did not include the determination of plasma albumin routinely in nutritional screening, since the type of malnutrition observed in these patients is mainly caloric.6 In our series, only two patients had low plasma albumin levels. One of them was detected in the second follow-up visit; it was a patient who breached the recommendations given and did not come for further control visits. The other patient had, without a context of infection, low albumin at the initial visit, reflecting protein-energy malnutrition. This patient improved with nutritional intervention, but died after 18 months from the start of the intervention due to multisystem involvement. In patients with SS, the existence of an association between mortality and BMI less than 18.519kg/m2 has been described, as it has with specific body composition determined by bioelectrical impedance.17 However, the detection of hypoalbuminemia surely confers an increased morbidity and thereby leads to death, as suggested above.4 In this sense, another patient died of respiratory infection in the setting of severe pulmonary involvement secondary to SS and showed initial albumin levels at the lower limit of normal (36mg/dl).
Iron deficiency anemia and vitamin D deficiency were attributable to complications associated to SS with nutritional deficit observed, such as gastrointestinal vascular ectasia and malabsorption syndrome. No vitamin A, vitamin B12, folic acid deficits or bleeding disorders were observed. The prevalence of vitamin D deficiency observed is similar to that described in a cross-sectional study in patients with SS performed in Spain.20 At the end of the period of nutritional intervention, all had insufficient vitamin D. This finding could be explained by other factors such as malabsorption, physical inactivity and impaired dermal activation; low hydroxylation of vitamin D could contribute21 as well as polypharmacy characteristic of such patients, preventing both the prescription and its completion.
At the end of the intervention, according to the new diagnostic criteria for malnutrition22 and with the techniques available in our environment (body weight gain, food intake and the presence of fluid retention), only one patient had malnutrition, and had specifically rejected oral nutritional supplements.
The results observed in the psychopathology and quality of life questionnaires showed a high prevalence of anxiety and depression, lower than that reported in the general population2,8 for physical and mental items. Although the patients reported feeling better, statistical analysis of the results of the tests and questionnaires did not show significant improvement throughout the intervention. These results may be due to the small sample size, the high incidence of anxiety and depression that can interfere with proper adhesion to therapeutic measures associated to the loss of 2 patients during follow-up, the presence of concomitant diseases, clinical worsening during cold periods reported by the patients and items not reflected in the questionnaires, among other possibilities.
ConclusionsThe risk of malnutrition in patients with SS affects a striking percentage of patients. In our series it reached 12.5% of patients. The dietary intervention was able to maintain or increase body weight, and energy and protein intake. The iron and vitamin D deficiency improved with supplementation. The psychological assessment and questionnaire of life test did not reflect a statistically significant improvement, although the patients reported feeling better. Longitudinal studies are needed with larger numbers of patients to assess the efficacy of this intervention beyond nutritional outcomes.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that no experiments have been performed on humans or animals.
Data privacyThe authors declare that they have followed the protocols of their workplace on the publication of data from patients and all patients included in the study have received sufficient information and gave written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained informed consent from patients and/or subjects referred to in the article. This document is in the possession of the author of correspondence.
Conflict of InterestThe authors have no conflicts of interest.
Please cite this article as: Ortiz-Santamaria V, Puig C, Soldevilla C, Barata A, Cuquet J, Recasens A. Soporte nutricional a pacientes con esclerosis sistémica. Reumatol Clin. 2014;10:283–287.