Osteomalacia is defined as a defect in mineralization of the bone matrix. We describe the case of a patient with chronic hepatitis B infection in whom treatment with adefovir induced renal phosphate loss with intense and sustained hypophosphatemia which derived in symptomatic osteomalacia.
La osteomalacia se define como un defecto en la mineralización de la matriz ósea. Describimos el caso de un paciente con infección crónica por virus de la hepatitis B en el que el tratamiento con adefovir indujo una pérdida renal de fosfato con hipofosfatemia intensa y mantenida que derivó en osteomalacia sintomática.
Adefovir is an antiretroviral drug used for the treatment of patients with chronic infection with hepatitis B. Among its adverse effects one finds nephrotoxicity, although this usually occurs when high doses (60–120mg/day) are employed.1 The currently recommended dose is approximately 10mg/day PO, which minimizes the risk of nephrotoxicity in patients without a history of renal disease.2–4 The case presented is that of a patient treated with adefovir at a dose of 10mg/day which caused abnormalities in the proximal renal tubule, leading to5,6 hypophosphatemic osteomalacia.
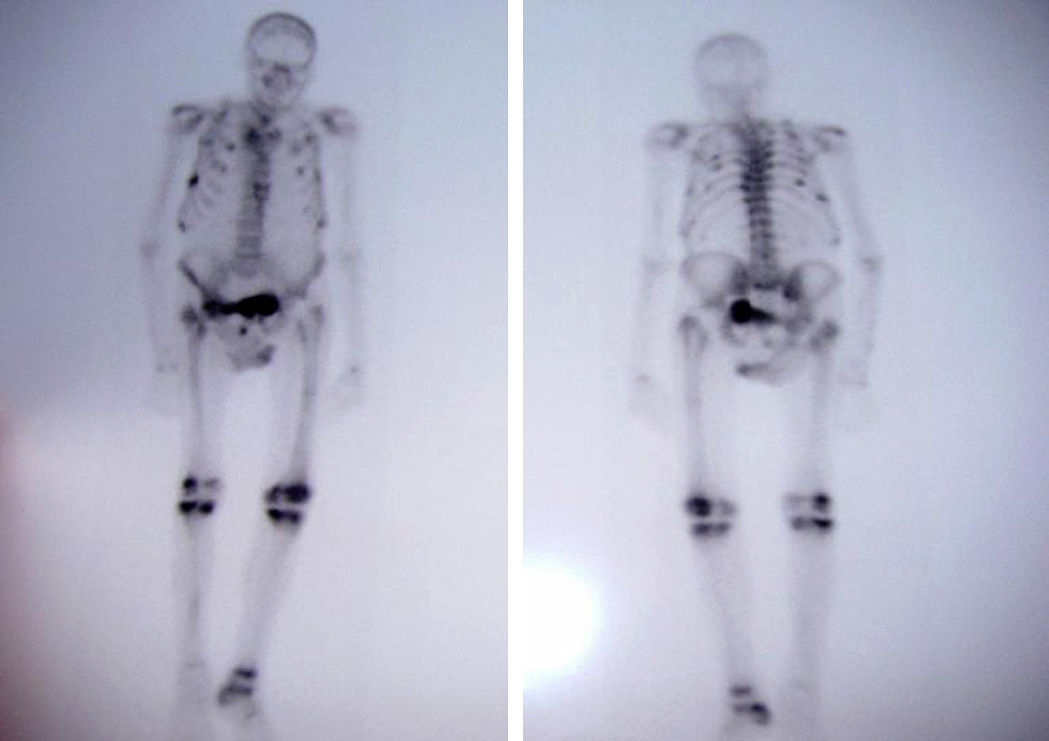
Case PresentationThe patient was an 81 year old male with a single kidney, with chronic hepatitis B treated with adefovir for four years approximately at a dose of 10mg/day. The patient had poor overall condition, fatigue, weakness, bone pain, muscle atrophy and weight loss which had lasted for a year. He had elevated alkaline phosphatase (386U/l; normal <140U/l) and a bone scan showed multiple pathological uptake areas (Fig. 1). With the presumptive diagnosis of Paget's disease, treatment was begun with bisphosphonates (etidronate 30mg/day for 2 months), calcium and vitamin D, with no clinical improvement, so the patient was referred to the Rheumatology department. Laboratory results showed elevated alkaline phosphatase (329U/L), hypoproteinemia (5.5g/dl), hypoalbuminemia (3.3g/dl), hypouricemia (1.5mg/dl) with a fractional excretion of urate of 50% and hypophosphatemia (1.2mg/dl) with tubular reabsorption of phosphate 9% (normal >80%). PTH levels were normal as was 25-hydroxycholecalciferol. Regarding renal function, creatinine was 1.13mg/dl and the estimated glomerular filtration rate was 53ml/min. The patient had proteinuria (1178mg/day) and glucosuria (>100mg/day). A chest X-ray showed rib fractures on both sides and bone scintigraphy showed multiple foci of uptake in the ribs, right sacrum, knees and right tibia.
A historical review of the analysis showed that the onset of disturbances, in particular decreasing phosphate levels, coincided with the beginning of the administration of adefovir (4 years prior). Adefovir was discontinued and the patient was treated with intravenous phosphate, presenting an increase in phosphate values to 4.1mg/dl. He was discharged with oral phosphate at a dose of 7g/day. In the next 6 months, there was a clear, albeit slow, clinical improvement.
DiscussionIn the case presented, laboratory abnormalities coincided with the start of the administration of adefovir and were associated with an improvement in phosphate values after drug withdrawal. Adefovir is associated with nephrotoxic effects, which are dose-dependent, leading to dysfunction of the proximal renal tubule and glomerular filtration rate.7 The lowering effect of the plasma phosphate levels of adefovir has been observed in 22%–50% of patients treated with doses of 30mg/day for at least 6 months and manifests itself as a progressive increase in serum creatinine, hypophosphatemia or both.8 While use of 10mg/day orally is not associated with significant renal dysfunction, in this case a low dose caused a decrease in tubular phosphate reabsorption and resulted in hypophosphatemia, leading to clinically manifested osteomalacia and bone pain, functional impairment and generalized muscle pain.5
Predisposing factors were the presence of a single kidney, and a moderately low glomerular filtration rate which contributed to a greater impact of the drug on renal function, as seen in previous cases.9
The pathophysiology of renal proximal tubule dysfunction caused by adefovir is due to its concentration in the mitochondria, resulting in mitochondrial toxicity and inhibition of ATP-dependent transporters in proximal tubule cells, leading to altered phosphate reabsorption, decreasing its concentration in plasma and ultimately leading to10,11 osteomalacia.
In conclusion, although low doses of adefovir are not usually associated with renal toxicity and hypophosphatemia due to renal phosphate loss, the comorbid conditions present in this case led to a situation of increased susceptibility.
Ethical ResponsibilitiesProtection of people and animalsThe authors state that no experiments were performed on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients and all patients included in the study have received sufficient information and gave their written informed consent to participate in this study.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Rivas Zavaleta MN, et al. Osteomalacia inducida por adefovir en paciente con hepatitis B. Reumatol Clin. 2014;10:120–121.