To update the recommendations on osteoporosis (OP) of the Spanish Society of Rheumatology (SER) based on the best possible evidence.
MethodsA panel of nine expert rheumatologists in OP was created, previously selected by the SER through an open call. The phases of the work were: identification of the key areas for updating the previous consensus, analysis and synthesis of the scientific evidence (using the SIGN levels of evidence) and formulation of recommendations based on this evidence and consensus techniques.
ResultsThis revision of the recommendations implies an update in the diagnostic evaluation and treatment of OP. It proposes some criteria to consider the high risk of fracture and some indications to start treatment. The recommendations also address issues related to the safety of treatments and the management of special situations such as inflammatory diseases and treatment with glucocorticoids.
ConclusionsWe present an update of SER recommendations on OP.
Actualizar las recomendaciones sobre osteoporosis (OP) de la Sociedad Española de Reumatología (SER) basadas en la mejor evidencia posible.
MétodosSe creó un panel formado por nueve reumatólogos expertos en OP previamente seleccionados por la SER mediante una convocatoria abierta. Las fases del trabajo fueron: identificación de las áreas claves para la actualización del consenso anterior, análisis y síntesis de la evidencia científica (utilizando los niveles de evidencia del SIGN) y formulación de recomendaciones a partir de esta evidencia y de técnicas de consenso.
ResultadosEsta revisión de las recomendaciones comporta una actualización en la evaluación diagnóstica de la OP y de su tratamiento. Propone unos criterios para considerar alto riesgo de fractura y unas indicaciones para iniciar tratamiento. Las recomendaciones abordan también cuestiones relativas a la seguridad de los tratamientos y al manejo de situaciones especiales como las enfermedades inflamatorias y el tratamiento con glucocorticoides.
ConclusionesSe presenta la actualización de las recomendaciones SER sobre OP.
Osteoporosis (OP) is a diffuse skeletal disorder characterised by a general reduction in bone strength leading to a higher risk of fracture due to fragility.
In Spain and in other countries the rate of fragility fractures is increasing, mainly due to higher life expectancies.1 One study reported that in 2010 2.4 million Spaniards (1.9 million women and 0.5 men) over 50 suffered from OP,2 and as a consequences of this there were 204,000 new fractures at a cost of 2842 million euros (2.8%of the total healthcare costs in Spain).3 It has been estimated that in 2025 there will be an 40% increase in the rate of fractures and a 30% increase in costs.3
Recent years have witnessed advances in the diagnosis and treatment of OP but only a minority of patients with high fracture risk are being accurately assessed and treated in keeping with the recommendations of clinical practice guidelines.4
This document contains the updated recommendations of the Spanish Society of Rheumatology (Spanish acronym: SER) on OP from the previous ones of 2011.5 These recommendations aim at helping physicians take decision for the management of post-menopausal OP and secondary OP (glucocorticoids, inflammatory diseases, OP of the male and other clinical forms).
MethodologyDesignQualitative synthesis of scientific evidence and consensus techniques have been used in this project which reflect the agreement by experts based on their clinical experience and scientific evidence.
Procedural stagesA series of steps were taken during the development of the recommendations document, which were:
- 1.
Creation of the work group. The document began with the formation of a panel of experts formed by 9 SER member rheumatologists. They were selected through an open call to all SER members. The Clinical practice Guideline and Recommendations Committee (Spanish acronym: GPC) of the SER assessed the curriculum vitae of all applicants, in accordance with objective criteria of contribution to knowledge on OP, taking into consideration publications in significant journals over the last 5 years. Coordination of clinical and methodological aspects was made, respectively, by one of these rheumatologists as the main researcher (MR) and a methodology specialist executive from the Research Unit of the SER.
- 2.
Identification of the key areas for updating of the previous agreement. All workgroup members were involved in giving shape to the document, and in establishing its content and key aspects. First they identified the clinical questions of research which could have the greatest impact in offering information on OP management: diagnosis, assessment, prevention, treatment and special circumstances. After this, they decided which of these required a response through the formulation of a PICO question (patient, intervention, comparison, and outcome). The methodology to be followed in the recommendation creation process was also defined.
- 3.
Literature review search. To respond to the non PICO clinical questions an updated systematic review (SR) search and GPC was made in MEDLINE and specialised sources in guidelines. The other clinical questions were reformatted in four questions with the PICO format. To respond to the PICO questions a search strategy was designed and a review of scientific evidence was made of studies published up until May 2017. The following databases were used: PubMed (MEDLINE), EMBASE and Cochrane Library (Wiley Online). The process was completed with a reference manual, posters and congress abstracts search which the reviewers and experts considered to be of interest.
- 4.
Analysis and synthesis of scientific evidence. The SR and GPC identified for the non PICO questions were assessed by the methodology coordinator. It was agreed that only those of high quality were considered apt for their incorporation as a source of evidence. Two rheumatologists from the workgroup of SER evidence reviewers were responsible for systematically reviewing the scientific evidence available for the PICO questions. After a critical reading of the complete test of the selected studies for each review, they drew up a summary using a homogenised formula including tables and texts to describe the methodology, results and quality of each study. They made in-depth notes on the reasons for exclusion of articles not included in the selection. The overall level of the scientific evidence was assessed using the Scottish Intercollegiate Guidelines Network (SIGN) (see Appendix 1) evidence levels.
- 5.
Formulation of recommendations. When the critical reading was completed, the MR and the panel of experts proceeded with the formulation of specific recommendations based on the available scientific evidence. This formulation was based on “formal assessment” or “reasoned judgement,” previously summarising the evidence for each of the clinical questions. They also took into account quality, quantity and consistency of the scientific evidence, the generality of the results, its applicability and its clinical impact. For the formulation of the recommendations two rounds of consensus were used. First, with the “reasoned judgement” system, where all of the experts drafted and discussed the recommendations. Then, in the presence of the methodologist, using the modified technique of nominal consensus, the level of agreement of the experts with the drafting of each recommendation was agreed upon. It was established that there was a high level of consensus when the percentage of experts who agreed with the recommendation draft was 75% or above. The agreement was evaluated using a dichotomous reply (yes: agreed with the wording; no: disagreed with the wording). Grading of the recommendations was made with the SIGN system (see Appendix 1). The recommendations were divided into four main areas: diagnosis and evaluation; treatment; special circumstances; new treatments.
- 6.
Public exposure. The draft of this document of SER recommendations was submitted to a process of public exposure by the members of SER and different interest groups (pharmaceutical industry, other scientific societies and patient associations) aimed at receiving scientific evaluation and discussion of its methodology and recommendations. The complete information of this process is found in the appendix to the SER web site (www.ser.es), in the section under SER Research and Recommendations.
The document includes all the formulated recommendations subdivided into the different above-mentioned areas. Management algorithms were formulated from the recommendations and these offer a summarised approach to the treatment of OP.
ResultsThere is a total of 28 formulated recommendations on OP (Table 1).
SER recommendations on osteoporosis.
| Recommendations | RR | AL |
|---|---|---|
| Diagnosis and evaluation | ||
| Recommendation: It is recommended that fragility fracture risk assessment should not be based exclusively on the measurement of BMD but should also take into account clinical risk factors. | B | 100% |
| Recommendation: The use of the FRAX®, tool is recommended, with or without BMD, to assess fracture risk. | √ | 78% |
| Recommendation: The classification of patients into high fracture risk is recommended when the risk quantified by FRAX®for hip fracture is ≥3%. | √ | 100% |
| Recommendation: To classify high risk patients using FRAX®for primary fracture it is recommended that the threshold used is ≥10% without BMD or ≥7.5% with BMD. | √ | 78% |
| Recommendation: the use of densitometry is recommended in the following cases: | D | |
| 1. Fragility fracture | 100% | |
| 2. Presence of two or more high fracture risk factors | 100% | |
| 3. FRAX® for primary fracture ≥5% | 78% | |
| 4. Treatment with aromatase inhibitor drugs, antiandrogen drugs and glucocorticoids | 100% | |
| 5. Disease associated with secondary OP | 100% | |
| Recommendation: A basic analytical study is recommended to rule out secondary causes of osteoporosis. | √ | 100% |
| Recommendation: The routine example of bone turnover markers is not established. However, they could be considered in the initial assessment and in follow-up of patients with osteoporosis. | B | 89% |
| Recommendation: Assessment of the presence of vertebral fractures is recommended in the high fracture risk patient or the patient with osteoporosis when kyphosis or significant height loss is perceived and when there is back pain or low back pain of recent onset. | D | 100% |
| Treatment | ||
| Recommendation: A healthy lifestyle is recommended for the prevention of primary and secondary osteoporosis and fractures, including a balanced diet and regular physical exercise, avoidance of tobacco, limitation of alcohol consumption and the use of preventative measures for falls. | √ | 100% |
| Recommendation: A daily intake of calcium between 1000 and 1200mg, most of which should be included in the regular diet. | D | 100% |
| Recommendation: The daily intake of 800UI of vitamin is recommended in the following cases: | D | 100% |
| 1. Patients with osteoporosis | ||
| 2. People over 65 years of age with fracture risk | ||
| 3. People with vitamin D deficiency | ||
| 4. People with limited exposure to sunlight | ||
| 5. People with inadequate calcium intake (under 700–800mg per day) | ||
| Recommendation: Initiation of pharmacological treatment is recommended to reduce the risk of osteoporotic fracture in the following situations. | √ | |
| 1. Fragility fracture of vertebra or hip in patients >50 years of age | 100% | |
| 2. Other fragility fractures in patients >50 years of age and with low bone mass (T-score <−1 SD) | 89% | |
| 3. OP defined by T-score ≤−25 SD in spine, femoral neck or hip, always considering age, BMD figures and other risk factors | 100% | |
| 4. Patients with high risk of hip fracture according to FRAX® with BMD ≥3% | 100% | |
| 5. Men being treated with androgen deprivation and T-score ≤−2.5 SD | 100% | |
| 6. Women in treatment with aromatase inhibitors and T-score ≤−2 SD or with a T-score <−1.5 SD and an additional risk factor, or with ≥2 risk factors without BMD | 78% | |
| 7. Patients who are going to receive glucocorticoids for over 3 months in the following cases: (a) initial dose ≥30mg/day of prednisone; (b) postmenopausal women and men >50 years of age with dose ≥5mg/day and previous fragility fracture or T-score ≤−1.5 SD or high risk of fracture: FRAX® for hip ≥3% or for primary fracture ≥10% without BMD or ≥7.5% with BMD | 100% | |
| Recommendation: When selecting treatment, among other factors, it is recommended to consider: | √ | 100% |
| 1. The efficacy and safety of the drugs | ||
| 2. The cost/effectiveness ratio | ||
| 3. The BMD value | ||
| 4. The presence of fractures, particularly vertebral or multiple fragility fractures | ||
| 5. Previous treatments and adherence to the same | ||
| 6. Age | ||
| 7. Comorbidities and polymedication | ||
| 8. Limitations for oral administration | ||
| Recommendation: It is recommended that fracture risk be reassessed after 5 years of treatment with oral bisphosphonates or after 3 years of treatment with zoledronic acid. | D | 100% |
| Recommendation: When treatment with denosumab is suspended an alternative treatment for the osteoporosis must be considered. | D | 100% |
| Recommendation: In high fracture risk patients, including those with previous fragility fracture, or with a T-score of proximal femur <−.5 SD, it is not recommended to discontinue the osteoporosis treatment. | D | 89% |
| Recommendation: A periodical clinical assessment is recommended to confirm adherence to and efficacy of treatment, incidence of fractures and the possible appearance of adverse effects. | √ | 100% |
| Recommendation: If the patient with OP has been subjected to any invasive dental procedure (extraction or implant) it is advised to postpone antiresorptive treatment initiation until the surgical wound has completely healed. | D | 100% |
| Recommendation: In patients treated with antiresorptives who are going to have a dental procedure it is not recommended to discontinue treatment with BP or denosumab. If risk factors additional to osteonecrosis of the jawbones exist or the surgical procedure is to be extensive temporary discontinuation of treatment with BP may be considered. | D | 78% |
| Recommendation: In patients with high fracture risk and prolonged treatment with bisphosphonates (>5 years for orals or >3 years for intravenous) it is recommended not to suspend treatment, since the risk of atypical femur fracture is very low and the benefits of fracture reduction greatly outweigh the risk of atypical fracture. | D | 100% |
| Special situations | ||
| Recommendation: It is recommended that all patients ≥50 years of age with a recent fragility fracture be assessed systematically to prevent further fractures. | D | 100% |
| Recommendation: The treatment of choice for an acute vertebral fracture is rest, rapidly scaled analgesics to control pain, and if necessary the use of a brace. | √ | 100% |
| Recommendation: Vertebroplasty and kyphoplasty are recommended for the treatment of vertebral fracture only in cases of serious refractory pain on a WHO analgesic scale, including opiates. | D | 100% |
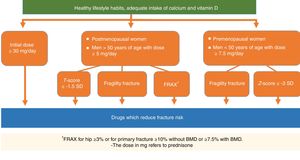
| Recommendation: In patients who receive or who are going to receive treatment with glucocorticoids fracture risk must be assessed and the initiation of treatment for osteoporosis as soon as possible. | D | 100% |
| Recommendation: It is recommended that pharmacological treatment be initiated to prevent OP in patients who are going to receive glucocorticoids for over 3 months in the following cases: | D | |
| 1. Patients with an initial dose ≥30mg/day of prednisone. In these cases it is recommended that treatment be imitated for OP immediately | 100% | |
| 2. Postmenopausal women and men >50 years of age with a prednisone dose ≥5mg/day who also present with some of the following conditions: | 100% | |
| a) Previous fragility fracture | 89% | |
| b) Low BMD (T-score ≤−1.5 SD in spine or hip) | 78% | |
| c) High fracture risk: FRAX®for hip ≥3% or for primary fracture ≥10% without BMD or ≥7.5% with BMD | 100% | |
| 3. Premenopausal women and men <50 years of age and daily dose ≥7.5mg/of prednisone who also present with one of the following conditions: | 78% | |
| a) Previous fragility fracture | 78% | |
| b) Low BMD (Z-score ≤−3 SD) | ||
| Recommendation: It is advisable to assess the risk of fracture and consider performing DXA in patients with rheumatoid arthritis, systemic lupus erythematosus and ankylosing spondylitis especially those over 50 years of age and who are being treated with glucocorticoids or with a severe or evolved disease. | √ | 100% |
| Recommendation: The study of the causes of secondary osteoporosis in all men with low bone mass or fragility fracture is recommended. | D | 100% |
| Recommendation: It is recommended that a study of secondary osteoporosis causes be made in all premenopausal women with a low bone mass or with a fragility fracture. | √ | 100% |
BMD: bone mineral density; FRAX®: Fracture Risk Assessment; AL: agreement level; RR: Recommendation rating (see Appendix 1).
OP is a diffuse skeletal disease characterised by a reduction in bone resistance which leads to a higher risk of fragility fractures. The concept of “bone resistance” encompasses factors relating both to bone mineral density (BMD) and the quality of the bone tissue.6
A fragility fracture is that brought on by a low impact trauma. A fall, standing up or being seated would be included in this concept. The most frequent and relevant fractures are those of the proximal femur, the spine and the distal forearm.7
OP may be defined in any of the following circumstances:
- a)
T-score in spine columnar, femoral neck or total hip ≤−2.5 SD.
- b)
Femoral fracture due to fragility, regardless of the BMD value, in postmenopausal women and in men >50 years of age.
- c)
Fracture due to fragility of the spine, proximal humerus or pelvis in postmenopausal women and in men >50 years of age, if there is a low BMD (T-score <−1.0SD).
The conceptual definition of OP by the World Health Organisation (WHO) is solely based on the results of the BMD. An individual has OP if the T-score in lumbar spine, femoral neck or total hip is equal to or under −2.5 standard deviation (SD) compared with the population peak bone mass.8,9
In normal clinical practice this definition fails to be operative due to many fragility fractures occurring in patients with a T-score >−2.5 SD. For this reason the National Bone Health Alliance has proposed the consideration diagnosing OP in postmenopausal women and in men >50 years of age with femoral fracture caused by fragility, regardless of the BMD value, and also when there is a low impact spinal fracture, of the proximal humerus or the pelvis, if the T-score <−1.0 SD.10 Doubts exist regarding forearm fracture, which in some cases could be included depending on age or the mechanism of the fracture. The authors also included OP diagnosis for the American population a quantified risk with the Fracture Risk Assessment (FRAX®) for the primary fracture ≥20% (proximal humerus, forearm, hip or clinical spine fracture) or a risk of femoral fracture ≥3%.10
In view of the recommendations of these groups of experts and in accordance with clinical experience, the panel of experts considered it appropriate to include postmenopausal women and men >50 years of age with fragility fracture of the femur (regardless of BMD) in the OP diagnosis, as well as spinal fracture, fracture of the humerus or the pelvis, if it was accompanied by low bone mass (AL: 89%).
Fracture risk factorsThe main aim in OP management is fracture prevention and it is therefore essential to identify those individuals with the highest risk of presenting with them.11
Table 2 describes high fracture risk factors (RF) (relative risk ≥2) and moderate RF (relative risk 1–2).5
Clinical fracture risk factors.
| Higher risk (relative risk ≥2) |
| Over 65 years of age |
| Low weight: body mass index <20kg/m2 |
| Personal history of fragility fracture |
| Maternal history of femur fracture |
| Glucocorticoids (>5mg/day of prednisone or equivalent for >3 months) |
| More than 2 falls in the last year |
| Moderate risk (relative risk between 1and 2) |
| Active smoker |
| Consumes >3units of alcohol per daya |
| Early menopause (<45 years of age), primary and secondary amenorrhea, hypogonadism in the male |
| Diseases which may reduce BMD: rheumatoid arthritis and other inflammatory arthropathies, intestinal pathology, celiac disease, malabsorption, hepatopathies, hyperparathyroidism, anorexia and bulimia, solid organ transplant, etc. |
| Drugs with the ability to reduce BMD/increases the risk of fractures: hydantoins, antiretrovirals, anticonvulsants, aromatase inhibitors, androgen deprivation drugs, etc. |
| Conditions related to falls: eye conditions, neurological diseases (stroke, Parkinson), use of psychodrugs |
Several risk factors, such as age, fracture history and the use of glucocorticoids, among others contribute to the risk of fracture regardless of the BMD. Patients with a recent fracture are at a particularly high risk of suffering from a new fracture.11
The term “imminent fracture risk” has recently been proposed to refer to patents with a short term highs risk, such as those with a recent fracture, elderly fragile people with frequent falls or people treated with glucocorticoids at high level doses.12,13
Recommendation:It is recommended that evaluation of fracture risk due to fragility should not be exclusively based on BMD figures but also bear in mind clinical risk factors (RR: B; AL: 100%).
The combination of the BMD with clinical RF provides the best estimation of fracture risk.14,15 This has led to the development of fracture risk calculation tools which are able to include many factors. Among the most popular and widely used is the FRAX®.9
Evaluation of fracture riskRecommendation:It is recommended that the tool FRAX®be used, with or without BMD, to assess the fracture risk (RR: √; AL: 78%).
The FRAX® calculates the probability of presenting a primary fracture (this include hip, clinical spine, humerus and forearm) or a hip fracture in the following 10 years, with the inclusion or non inclusion of the BMD score. When the BMD is included in the FRAX® tool the prediction of fracture risk is more precise and reliable.16,17
FRAX® limitations are mostly determined by insufficiencies in the data from which it was calculated. Thus the FRAX® does not consider the dose-response factor for several RF, nor lumbar BMD, nor the greater risk of fracture in patients with a recent fracture, and it does not encompass falls.9,16–18
The Spanish version of the FRAX® underestimates the risk of the primary fracture.19 However, the application of a decision algorithm with bone densimetry threshold indication (DXA) and treatment based on FRAX® has been shown to be cost-effective with regard to the exclusive use of T-score −2.5SD.20 Despite its limitations, the current version of the Spanish FRAX® may help to classify patients better with regard to fracture risk.
Recommendation:It is recommended that patients be classified as high fracture risk when the risk quantified by FRAX®for hip fracture is ≥3% (RR: √; AL: 100%).
Recommendation:To classify patients as high risk using the FRAX®for the primary fracture it is suggested that the threshold ≥10% without BMD be used or ≥7.5% with BMD (RR: √; AL: 78%).
The validity of the Spanish FRAX® was analysed in two prospective cohorts studies and in both the primary fracture risk was underestimated. The relationship between observed fractures and expected fractures was 2.2 in the FRIDEX20 study and 1.5 in the ECOSAP21 Study. However, the estimation of the Spanish FRAX® for hip fracture appears to be appropriate. A workgroup estimated the thresholds of the FRAX® in the Spanish population20,22,23 defining high primary fracture risk as a FRAX®, with or without BMD, ≥7.5% and intermediate risk between 5% and 7.5%. This was a single study which defined risk thresholds in Spain and had methodological limitations such as not being validated in regions other than Catalonia, including only 8.9% of subjects with intermediate risk and 8.5% with high fracture risk, as well as overlapping in the confidence interval of the high risk threshold. In this sense, the panel considers that a women aged around 70 years with a prior fracture (is approximately equivalent to as Spanish FRAX® for the principal fracture without BMD of 10%) is an example of high fracture risk.
From the scarce evidence and low level of agreement in classifying the patient as high fracture risk based on the FRAX®, the panel of experts agreed to consider a high risk fracture as FRAX® ≥3% for a hip fracture. Since no further studies on the FRAX® thresholds and/or national official positioning were available, the panel of experts also considered high fracture risk a FRAX® for primary fracture ≥10% without BMD or ≥7.5% with BMD.
The panel of experts also considered as high fracture risk, but without reaching an acceptable level of agreement (AL: 55%), the combination of two or more high RF. Should the FRAX® tool not be used, the presence of two high risk factors may serve as the starting point for assessing the BMD.
Bone densimetryThe technique of choice today for measuring BMD is the DXA dual energy X-ray absorptiometry.6,10,11 The measurement is made in the lumbar spine and the proximal femur (femoral neck and total hip). According to the experts, measurement using DXA of the distal third of the forearm should only be performed when measurement in the lumbar spine or the proximal femur is not feasible and/or in specific pathologies such as hyperparathyrodism.24
The DXA has a high specificity for the reduction of fracture risk, but a low sensitivity. The risk increases exponentially as the BMD descends.25 However, the majority of fragility fractures occur in patients with a T-score above −2.5 SD. Studies made in patients diagnosed with OP using DXA and without other RF of fractures conclude that it could be appropriate to estimate risk using FRAX® before evaluating treatment.26,27
Different groups of experts agree that the BMD determined in the peripheral skeleton using DXA of the phalanx and heel spur and the ultrasound technique of the heel spur may be useful for prediction of fracture risk but not for diagnosing OP.28,29
Bone densitometry indicationsCriteria for requesting a DXA vary widely nationally and internationally. The majority of international guidelines on OP recommend evaluation of BMD in postmenopausal women ≥65 years of age, regardless of other RF.30–32 There is controversy regarding the screening age in males. The National Osteoporosis Foundation (NOF) and the International Society for Clinical Densitometry (ISCD) recommend measurement in males ≥70 years of age, whilst the Canadian guidelines put the threshold at ≥65 years of age.31
According to the conclusions of a cross-sectional study the aim of which was to evaluate DNX indication criteria in postmenopausal Spanish women based on the FRAX® tool, the indication of the test should be based on clinical criteria to allow for patients to be selected where its evaluation is more efficient.33 The authors conclude that the strategy should begin with calculation of the fracture risk according to clinical RF, and in this setting using FRAX®33 could be helpful.
Based on the accepted international recommendations on DXA indications, the panel of experts considers that its main applications in clinical practice are: OP diagnosis, evaluation of fracture risk, evaluation of treatment and monitorisation of response to treatment.
In patients with an imminent risk of fracture performing a DXA should not impede treatment initiation. Thus patients with a high fracture risk may be treated without the need to carry out a DXA, although it seems appropriate to know the baseline BMD for evaluation of treatment effectiveness at a later date. The panel of experts agree that when the fracture risk is low no DXA should be performed and that a DNA should not be recommended to all women going through the menopause.
Recommendation:It is recommended to perform a densitometry in the following cases (RR: D):
- 1.
Fragility fracture (AL: 100%).
- 2.
Presence of two or more high risk fracture factors (AL: 100%).
- 3.
FRAX®for primary fracture ≥5% (AL: 78%).
- 4.
Treatment with aromatase inhibitor drugs, antiandrogens and glucocorticoids (AL: 100%).
- 5.
Diseases associated with secondary OP (AL: 100%).
Based on these international studies, the panel of experts considered the use of FRAX® without BMD as initial assessment of fracture risk. When the risk calculated using the Spanish FRAX® without BMD, for the principal fracture is ≥5% it is advisable to perform DXA.34 Low risk patients are therefore not recommended to have DXA.
The panel also considered measuring in BMD, regardless of age, in the population which present with RF for fragility fractures such as: previous fragility fracture, women who begin treatment with aromatase inhibitors for breast cancer,35 males treated with androgen deprivation therapy for prostate cancer and patients treated with glucocorticoids. The recent guideline of the American College of Rheumatology (ACR) for the prevention and treatment of OP using glucocorticoids recommends performing DXA in patients who start chronic treatment with glucocorticoids in the following cases: (1) adults <40years of age when there is a background of fracture or high RF of fracture and (2) adults ≥40 years of age in all cases and calculate the FRAX® with BMD.36 the oncology guidelines and those of the International Osteoporosis Foundation (IOF) recommend DXA to decide whether to initiate treatment for OP in women receiving aromatase inhibitors35 and in males in androgen deprivation therapy.37
In cases of recent fracture due to fragility or treatment with high doses of glucocorticoids, studies concluded that performing a DXA should not delay initiation of treatment to prevent new fractures.12,13
Other patients who are eligible for DXA due to presenting with high OP and fragility fracture risks are those suffering from diseases associated with secondary OP, such as chronic inflammatory arthropathies, inflammatory bowel disease, intestinal malabsorption syndromes, chronic liver disease or advanced chronic kidney disease. In these cases the panel considers BMD reasonable in order to establish prevention measures and appropriate treatment for each individual patient.
Trabecular Bone ScoreThe Trabecular Bone Score (TBS) is an analysis of the bone texture obtained from the DXA which evaluates parameters related to the bone micro architecture of the lumbar spine and has good correlation with computerised tomography.38,39 Low TBS values are associated with an increased risk of primary osteoporotic fracture, regardless of BMD.38 The combination of TBS and lumbar BMD increases the reduction of the risk of developing fractures.39 Furthermore, the introduction of the TBS in the FRAX® algorithm enables a better prediction of the development of future fractures.38,40,41 As a result, according to studies, the TBS could be an additional tool for the evaluation of fracture risk in patients with OP38–40 and in some causes of secondary OP (diabetes, hyperparathyroidism, glucocorticoids). However, its usefulness in the monitorisation of the therapeutic response has not yet been established.38
To sum up, the panel of experts consider that although TBS could have several advantages in the evaluation of fracture risk, further studies are required to recommend its use in clinical practice.
AnalysisRecommendation:It is recommend that a basic analytical study be performed to rule out secondary causes of osteoporosis (RR: √; AL: 100%).
Basic laboratory tests to identify secondary OPs and make the differential diagnosis with other bone disease include30,42,43: haemogram, serum calcium and phosphate tests, alkaline phosphatase, test, proteingram, albumin, creatinine, liver function tests, calciuria and 25-hydroxy vitamin D tests.
Different scientific societies30,42,43 have produced documents with the following recommendations:
- •
Additional tests will be carried out if there is suspicion of an associated condition:
- 1.
PTH (primary or secondary hyperparathyroidism).
- 2.
TSH (hyperthyroidism).
- 3.
Immunoelectrophoresis (myeloma).
- 4.
Antitransglutaminase antibodies (celiac disease).
- 5.
Urinary cortisol (Cushing's).
- 6.
Serum tryptase (mastocytosis).
- 7.
Sexual hormone study (young people).
- 8.
Genetic study (imperfect osteogenesis, hypophosphatase and others).
- 1.
Recommendation:The routine use of bone turnover markers has not been established. However, they could be considered in initial assessment and in follow-up of patients with osteoporosis (RR: B; AL: 89%).
The reductions of the greatest magnitude in markers of bone turnover (MBT) are significantly associated to more marked reductions in the non vertebral fracture risk,44 and the initial changes in a formation marker predict the efficacy of the bisphosphonates (BP) in the reduction of vertebral fracture risk.45 However, there is not sufficient evidence to conclude that MBT are able to identify a reduction in risk fracture unequivocally.46
The majority of GPC recommend the consideration of the use of MBT in the initial evaluation and in follow-up, as an additional test. In the initial evaluation, the high levels may predict a faster loss of bone mass and a higher risk of fracture. But their main indication is in follow-up, since they can contribute to assessing treatment adherence and efficacy, and also contribute to monitoring the duration of therapeutic holidays.30,42,43,47 The groups of international experts conclude that further research studies are required prior to making a recommendation based on the evidence.43 To conclude, although the use of bone turnover markers has not been established as a routine, the panel of experts suggest that they could provide additional information in the initial evaluation and in the patient follow-up with OP.
The two main serum references of MBT which are recommended to use by the IOF and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) are the precollagen type I N-terminal propeptide (PINP), bone turnover marker and the Carboxyl-terminal telopeptide of type I collagen (CTX), a reabsorption marker.48
Vertebral fracture screeningRecommendation:It is recommended that the presence of vertebral fractures be assessed in the patient with a high fracture risk or with osteoporosis, when kyphosis or significant loss of height has presented and when there is back pain or low back pain of recent onset (RR: D; AL: 100%).
Chest and lumbar spine X-rays (focused on T7 and L3, respectively) are the method of choice for diagnosis of a vertebral fracture. A side projection is usually sufficient, thus minimising the radiation the patient receives.49 These are useful in the initial evaluation of all patients diagnosed with OP when there is a significant loss of height or a change in relevant statics (especially thoracic kyphosis) and dorsolumbar pain of recent onset with or without a history of trauma50 in a patient with a risk of OP or fracture. Height loss which is significantly associated with the presence of vertebral fractures is not defined, varying between 2cm compared with measurement in previous years to 4cm of historic loss since youth.30,51 The presence of a vertebral fracture, the number of them and their degree of severity are highly relevant indicators of the risk of new vertebral fractures, even those of the hip.52
The panel of experts has thus considered that the presence of vertebral fractures should be assessed in the evaluation of the patient with OP or with a high risk of fracture when kyphosis or significant loss of height is present and there are clinical features of vertebral fracture. Some DXA teams also use the Vertebral Fracture Assessment (VFA) to assess fractures.
TreatmentNon pharmacological therapiesRecommendation:Included in the prevention of primary and secondary osteoporosis and fractures is the recommendation for a healthy lifestyle, consisting of a balanced diet and regular physical exercise, not smoking, limiting alcohol consumption and using preventative measures against falls (RR: √; AL: 100%).
Several studies have concluded that the first step to the prevention of OP and the avoidance of fractures is to maintain healthy life habits.53–57 Evidence suggests that exercise has a modest effect on reducing fractures and preventing falls.57Table 3 contains a summary of the most important measures to be taken into consideration.
Main habits of a healthy lifestyle.53–57
| Cover nutritional requirements with a healthy diet which includes an adequate intake of proteins (0.8g per kilo of body weight), calcium, fruit and vegetables |
| Limit caffeine intake |
| Prudent exposure to sunlight |
| Avoid consumption of tobacco and limited intake of alcohol to under 3 units per daya |
| Encourage physical activity with regular exercise both own weight bearing (examine: walking, dancing, practising taichi 30–40min per session) and muscle and postural strengthening, 3–4 days per week |
Studies show that the adoption of fall prevention measures, in which exercise programmes and actions on environmental safety and minimisation of the use of drugs such as hypnotics, diuretics, antihypertensives and others which impair balance, also contribute both to primary and secondary fragility fracture prevention.57,58
In contrast, exercises which require great effort or are abrupt and those which involve repeated flexion or twisting of the trunk and abdominals are ill-advised.
Regarding specific measures for the patient who has kyphosis, studies have proven the effectiveness of exercise, and training in muscular strength and balance.59
The effectiveness of hip protectors in the prevention of fractures is inconclusive, and adherence to their use is very low.60 Finally, vibratory platforms have not demonstrated any consistent efficacy.61
Calcium and vitamin DRecommendation:It is recommended that a daily intake of calcium between 1000 and 1200mg be taken and that it should mainly originate from a regular diet (RR: D; AL: 100%).
Recommendation:In the following cases a daily intake of 800UI of vitamin D is recommended (RR: D; GA: 100%):
- 1.
Patients with osteoporosis.
- 2.
People over 65 years of age with fracture risk.
- 3.
People with a vitamin D deficit.
- 4.
People with limited exposure to the sun.
- 5.
People with an insufficient calcium intake (under 700–800mg daily).
Studies coincide in the need to ensure an adequate provision of calcium as an essential feature in any OP treatment programme.47 Thus patients in pharmacological treatment for OP must use calcium and vitamin D supplements because practically all clinical trials which have demonstrated the efficacy of the antiosteoporotic drugs routinely include calcium supplements and cholecalciferol (vitamin D3). When the calcium provided in the diet is insufficient, it is recommended that calcium and vitamin D supplements be taken. In postmenopausal women with OP the international expert groups advise an intake of 800–1.200mg/of calcium per day and 800UI/of vitamin D per day.62,63
In primary prevention a daily intake of calcium of 1000–1200mg for women >50 years of age and men >70 years of age is advised, preferably in the diet, and 1000mg/of calcium per day in women <50 years of age and men aged between 51 and 70.
Some foods are rich in calcium, such as milk products (milk, cheese, and yoghurt) tinned oily fish, beans and almonds. A list of foods and an online calculator are available in the IOF website.64,65 if supplements are used, the experts advise bearing in mind the optimum dose for calcium absorption which is situated in 500mg of calcium, and if larger doses are administered they should be spread over time.66
With regard to the possible side effects of calcium supplements, constipation and dyspeptic upsets are the most notable. An increased risk of nephrolithiasis was demonstrated (RR 1.17) in the Women's Health Initiative67 study, attributed to a diet rich in calcium of the women studied. However, a systematic review concluded that the calcium supplements in OP treatment, alone or in combination with other types of treatment, did not significantly increase the risk of nephrolithiasis or renal colic.68 Controversy exists regarding the possible increased cardiovascular risk associated with calcium supplements, particularly if the recommended maximum dose is exceeded. The available evidence suggests that a total daily intake of calcium (obtained by adding up dietary intake plus supplements) under 2000mg did not increase cardiovascular risk.69,70
Regarding vitamin D, in OP patients, the conclusion reached by the experts is to maintain minimum serum concentrations of 25-hydroxy cholecalciferol (calcidiol) of 30ng/ml. They therefore recommend measuring the calcidiol levels in patients with a risk of vitamin D deficiency and in patients with OP. Given the essential role of sunlight as a source of vitamin D, they advise an exposure to the sun of 10–15min per day.62,63 When necessary; they advise vitamin D supplements with a dose between 800 and 2000UI/day, depending on baseline levels. In patients with hepatopathies, malabsorption syndromes, treatment with anticonvulsives or other situations where the vitamin 25D hydroxylation may be compromised, administration of the metabolite calcidiol is recommended.
Current scientific evidence enables us to state that neither the increase of dietary calcium nor the isolated intake of calcium supplements is able to protect against the presence of fractures.71,72 Vitamin D administered as a monotherapy is not effective in reducing fragility fractures either, in non institutionalised elderly people.72,73 Calcium and vitamin D supplements have been show to be effective in the population >65 years of age who reside in care homes, reducing the risk of non vertebral fracture and marginally of hip fracture.72,74 This effect depends to a large extent on the vitamin D dose (≥800UI daily) and is more obvious at a greater age and with lower vitamin D levels.72 Calcium and vitamin D supplements do not reduce the risk of vertebral fracture.72
The efficacy of calcium and vitamin D supplements in the non institutionalised population >65 years of age (residents in the community) is questionable, as evidenced by the high number required to treat (NNT) to prevent fracture in this low risk population group74 and the results of a recent meta-analysis.75
There are contradictory data on the efficacy of vitamin D in the prevention of falls. Some results indicate that vitamin supplements, with and without calcium, would be effective in the prevention of falls,76 and especially in the elderly with vitamin D deficit.77 Other studies do not show this beneficial effect of vitamin D,78 and even the administration of annual high doses could increase the risk of falls.79
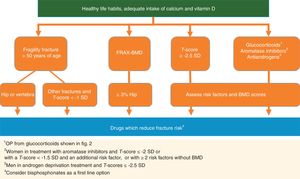
Treatment to prevent fracturesRecommendation:It is recommended that pharmacological treatment be initiated to reduce the Risk of osteoporotic fracture in the following situations (RR: √):
- 1.
Fragility fracture of vertebra or hip in patients >50 years of age (AL: 100%).
- 2.
Other fragility fractures in patients >50 years of age and with low bone mass (T-score <−1 S) (AL: 89%).
- 3.
OP defined by T-score ≤−2.5 SD in spine, femoral neck or total hip, always evaluating age, BMD figures and other risk factors (AL: 100%).
- 4.
Patients with a high hip fracture risk according to FRAX®with BMD ≥3% (AL: 100%).
- 5.
Males in androgen deprivation treatment and T-score ≤−2.5 SD (AL: 100%).
- 6.
Women in treatment with aromatase inhibitors and T-score ≤−2 or with a T-score <−1.5 SD and an additional risk factor, or with ≥2 risk factors without BMD (AL: 100%).
- 7.
Patients in treatment with glucocorticoids and: (a) initial dose ≥30mg/day of prednisone; (b) postmenopausal women and men >50 years of age with a dose of ≥5 mg/day and previous fragility fracture or T-score ≤−1.5 SD or high risk of fracture: FRAX®for hip ≥3% or for primary fracture ≥10% without BMD or ≥7.5% with BMD (AL: 78%).
The drugs used for the treatment of OP have been shown to be more effective in patients with a high risk of fracture and in those with an existing fracture or with a T-score ≤−2.5 SD.80
The NOF recommendations and those of other expert groups support the use of FRAX® in selecting candidates for treatment, including cases without OP using DXA.30,80
The panel thus considers that treatment is indicated in patients with fragility fracture, OP from DXA T-score ≤−2.5 SD, high fracture risk (FRAX® for hip fracture with BMD ≥3%)23,34 and in sub-groups of patients who take glucocorticoids, aromatase inhibitors35 or antiandrogens.37 The specific circumstances where osteoporotic antifracture treatment should be assessed is commented upon in detail in other sections.
Regarding patients with fragility fractures, the NOF recommends treating all hip and vertebra fractures, whilst the other fractures would be treated depending on the results of the DXA and the risk of new fractures calculated using the FRAX®.30
Regarding the evaluation of treatment based on the DXA outcome with a T-score ≤−2.5 SD, the panel wishes to highlight that in these cases clinical risk factors and the FRAX® should also be considered when decision are to be taken.
Prophylaxis and treatment of OP induced by glucocorticoids are dealt with in a separate section.
Several international guidelines, including those on oncology, advise that treatment be indicated for OP in women who receive aromatase inhibitors and have a T-score below or equal to −2 SD or with a T-score <−1.5 SD with an additional risk factor, or with ≥2 risk factors without BMD.35 For men in androgen deprivation therapy, the IOF fixes the threshold for treating OP in a T-score of −2.5 SD or a raised fracture risk by FRAX®37 (Fig. 1).
Antifracture treatment choiceRecommendation:When selecting treatment, among other factors, it is recommended to take the following into consideration (RR: √; AL: 100%):
- 1.
The efficacy and safety of the drugs.
- 2.
Cost-effectiveness ratio.
- 3.
The value of the BMD.
- 4.
The presence of fractures, particularly vertebral or multiple fractures due to fragility.
- 5.
Treatments prior to this and adherence to them.
- 6.
Age.
- 7.
Comorbidities and polymedication.
- 8.
Limitations for oral administration.
The final aim of OP treatment is the prevention of fractures. Table 3 contains the efficacy in primary and secondary prevention of vertebral and non vertebral fractures of the drugs used in Spain.81–94
In the main studies conducted with drugs in patients who only had osteopenia without prevalent fracture (primary prevention) no fracture reduction occurred in primary outcome.
In primary fracture prevention in patients with OP, the drugs which demonstrated a reduction of vertebral fracture were: alendronic acid, zoledronic acid, raloxifene, bazedoxifene and denosumab, whilst denosumab also reduced non vertebral fracture and hip fracture in this group.
In patients with OP and vertebral fracture, alendronic acid, risedronic acid and zoledronic acid reduced new vertebral, non vertebral and hip fractures, whilst teriparatide reduced vertebral and non vertebral fractures and denosumab reduced vertebral fractures. For their part, ibandronic acid, raloxifene and bazedoxifene reduced vertebral fractures in patients with previous fracutre.81–94 With regard to forearm fractures, alendronic acid demonstrated a significant reduction of new wrist fractures in meta-analysis in patients with previous vertebral fracture.81
Tables 4–7 contain details of the risk reduction for different fractures in the published studies.83–94
Efficacy of the drugs to reduce vertebral, non vertebral and hip fracture.
| Vertebral | Non vertebral | Hip | |||||
|---|---|---|---|---|---|---|---|
| Osteopenia | Osteoporosis (no previous vertebral fracture) | Established Osteoporosis (with previous vertebral fracture) | Osteoporosis (no previous vertebral fracture) | Established Osteoporosis (with previous vertebral fracture) | Osteoporosis (no previous vertebral fracture) | Established Osteoporosis (with previous vertebral fracture) | |
| Alendronic acid83,84 | ND | SI | SI | ND | SI | SG | SI |
| Risedronic acid85,86 | ND | PH | SI | ND | SI | ND | SI |
| Zoledronic acid87 | ND | SI | SI | ND | SI | ND | SI |
| Denosumab88 | ND | SI | SG | SI | ND | SI | ND |
| Teriparatide89 | ND | ND | SI | ND | SI | ND | ND |
| Ibandronic acid90 | ND | NA | SI | ND | PH | ND | ND |
| Raloxifene91 | PH | SI | SI | ND | PH | ND | ND |
| Bazedoxifene92 | ND | SI | SI | PH | PH | ND | ND |
ND: not demonstrated; PH: post hoc analysis by subgroups (designed after knowledge of the trial outcome); SG: subgroup analysis designed prior to knowing the trial outcome; SI: ITT (intention to try) analysis.
Efficacy of treatments to reduce vertebral fracture. Reference studies used for the approval of each drug.
| Study (duration) | Drug | Baseline risk profile | Mean age (years) | RRR (%) | RRA (%) | NNT |
|---|---|---|---|---|---|---|
| FIT-183 (3 years) | Alendronic acid | Vertebral Fr | 71 | 47 | 7 | 14 |
| FIT-284 (3 years) | Alendronic acid | Without Vertebral Fr | 68 | 44 | 1.7 | 60 |
| BONE90 (3 years) | Ibandronic acid | Vertebral Fr | 69 | 62 | 4.9 | 27 |
| VERT NA85 (3 years) | Risedronic acid | Vertebral Fr | 69 | 41 | 5 | 20 |
| VERT MN86 (3 years) | Risedronic acid | Vertebral Fr | 71 | 49 | 10.9 | 10 |
| HORIZON87 (3 years) | Zoledronic acid | T <−1.5 SD+Vertebral Fr | 73 | 70 | 7 | 14 |
| or | ||||||
| T <−2.5 SD±Vertebral Fr | ||||||
| MORE91 (3 years) | Raloxifene | Vertebral Fr or | 67 | 35 | 3.5 | 16 |
| T <−2.5 SD | ||||||
| Silverman et al.92 (3 years) | Bazedoxifene | Sin Vertebral Fr T <−2.5 SD | 67 | 38 | 1 | 82 |
| and >−4 SD | ||||||
| With Vertebral Fr T >−4 SD | ||||||
| FPT89 (2 years) | Teriparatide | Vertebral Fr | 70 | 65 | 9.3 | 11 |
| FREEDOM88 (3 years) | Denosumab | T between −2.5 and −4 SD | 72 | 67 | 4.9 | 20 |
SD: standard deviation; RF: risk factor; Fr: fracture; NNT: necessary number to treat; ARR: absolute risk reduction; RRR: relative risk reduction; T: T-score.
Efficacy of treatments to reduce non vertebral fracture. Reference studies used for the approval of each drug.
| Study (duration) | Drug | Baseline risk profile | Mean age (years of age) | RRR (%) | ARR (%) | NNT |
|---|---|---|---|---|---|---|
| FIT-1 (3 years)83 | Alendronic acid | Vertebral Fr | 71 | 20 | 3 | – |
| FIT-2 (3 years)84 | Alendronic acid | Sin Vertebral Fr | 68 | 12 | 1.8 | – |
| VERT NA (3 years)85 | Risedronic acid | Vertebral Fr | 69 | 40 | 3.2 | 31 |
| VERT MN (3 years)86 | Risedronic acid | Vertebral Fr | 71 | 33 | 5.1 | – |
| HIP (3 years) | Risedronic acid | Group 1 | 74 | 20 | 1.8 | 55 |
| T <−4 SD <−3 SD and RF | ||||||
| HORIZON (3 years of age)87 | Zoledronic acid | T <−1.5 SD+Vertebral Fr | 73 | 28 | 2.7 | 37 |
| or | ||||||
| T <−2.5 SD±Vertebral Fr | ||||||
| HORIZON-R93 | Zoledronic acid | Hip Fr | 75 | 27 | 3.3 | 30 |
| FPT (2 years)89 | Teriparatide | Vertebral Fr | 70 | 35 | 2.9 | 34 |
| FREEDOM (3 years)88 | Denosumab | T between −2.5 and −4 SD | 72 | 20 | 1.5 | 66 |
SD: standard deviation; RF: risk factor; Fr: fracture; NNT: necessary number to treat; ARR: absolute risk reduction; RRR: relative risk reduction; T: T-score.
Efficacy of treatments to reduce hip fracture. Reference studies used for the approval of each drug.
| Study (duration) | Drug | Baseline risk profile | Age (years) | RRR (%) | ARR (%) | NNT |
|---|---|---|---|---|---|---|
| FIT-183 (3 years) | Alendronic acid | Vertebral Fr | 71 | 51 | 1 | 90 |
| HIP94 (3 years) | Risedronic acid | Group 1 | 74 | 40 | 1.3 | 77 |
| T <−4 SD or <−3 SD and RF | ||||||
| HORIZON87 (3 years) | Zoledronic acid | Vertebral Fr+T< | 73 | 41 | 0.9 | 109 |
| −1.5 SD | ||||||
| or | ||||||
| T <−2.5 SD±Fr | ||||||
| vertebral | ||||||
| FREEDOM88 (3 years) | Denosumab | T <−2.5 and >−4 SD | 72 | 39 | 0.4 | 230 |
SD: standard deviation; RF: risk factor; Fr: fracture; NNT: necessary number to treat; ARR: absolute risk reduction; RRR: relative risk reduction; T: T-score.
In patients with at least two moderate vertebral fractures or at least one severe fracture teriparatide was significantly more effective than risedronic acid for reducing new morphometric and clinical vertebral fractures after 24 months of treatment. There were no significant differences in the rate of new non vertebral fractures.95
Strontium renalate has not been included in these recommendations because its production has been suspended, nor calcitonin, because of the limitation its technical record has in treatment indication and duration. However, therapy with oestrogens reduces vertebral and hip fractures, but is currently not used due to its poor risk/benefit profile and because safer treatments exist.
The following aspects have to be taken into consideration when integrating trial outcomes into clinical practice: (1) the patient profile included is a postmenopausal woman with a high baseline risk due to age and the presence of previous fractures; (2) the populations of the trials are different to one another, and therefore may not be directly compared; (3) the studies were conducted with calcium and/or vitamin D supplements and (4) real treatment adherence is lower than that reported in the trials. Moreover, treatment should be individualised according to the circumstances of each patient.
Another aspect to take into account is the cost/effectiveness ratio of treatment. The studies undertaken in other countries fix the threshold of FRAX® for primary fracture, so that the cost-effectiveness of the treatment is 8.8% (Portugal),96 13.8% (Switzerland)97 and 10%–15% (Greece).98 For estimation the cost of alendronic acid was considered (Portugal and Switzerland) and the average cost of treatment for OP (Greece). When drugs other than alendronic acid were considered, the FRAX® threshold for cost effectiveness was higher.96–98 Despite no cost-effectiveness studies existing for Spain based on fracture risk, the panel of experts advised taking into account BP as first line option in OP treatment.
The panel of experts therefore consider that when choosing treatment to prevent fractures the efficacy and safety of the drugs, cost-effectiveness ratio, BMD figures, and presence of previous features (location, number and time passed since fracture) and oral administration route limitations should be contemplated. Other factors which also were to be taken into account were previous treatments and adherence to them, co morbidities and patient preferences. Thus, in patients with several fractures, oral intolerance, dementia, malabsorption and poor adherence, parenteral therapy could be considered.
Sequential and combined therapyThe risk of long-term complications in OP treatments99–101 and the increase in the risk of fracture after their suspension102,103 often obliges physicians to put forward a sequential treatment strategy.
Although there are currently no outcomes on fracture reduction with sequential treatment,104,105 the bone forming sequence (teriparatide) followed by antiresorptive sequence (denosumab or BP)104–106 is the most effective in terms of gaining BMD. Teriparatide followed by denosumab appears to be more effective than teriparatide followed by BP.107
The administration of teriparatide after an antiresorptive drug is also effective, although its anabolic effect may slow down (less change in bone markers and lower increase of BMD),108 and if administered after denosumab it has been described as a loss of initial BMD in the hip.106 Sequential treatment with romosozumab followed by an antiresorptive drug is dealt with in the section relating to new treatments.
Little information exists on the use of one antiresorptive drug followed by another, especially if the first was administered for years. A review of 11 prospective studies of patients previously treated with alendronic acid or risedronic acid showed that the change to ranelate from strontium or denosumab achieved an additional increase in BMD.109 However, no data were available on fracture reduction either.109 In one study denosumab showed increases in BMD in patients who had been treated with zoledronic acid.110
There are studies which show greater efficacy in combined therapy (bone formation and antiresorptive) compared with monotherapy. The combination of zoledronic acid and teriparatide has been associated with a higher increase in femoral BMD one year after treatment compared with teriparatide in monotherapy.104,111 The combination of denosumab and teriparatide has been associated with a greater increase in lumbar spine and proximal femur BMD, compared with both drugs in monotherapy.112 Notwithstanding, there is no evidence of reduction of fractures with these combinations. After a review of the literature, the panel concluded that combined (bone formation and antiresorptive) treatment did not appear to be an option to recommend in general, although its use could be justified in highly selected serious cases of OP.
Treatment durationOP is a chronic disease, and treatment must therefore be maintained indefinitely or at least for several years. However, there is no recommended maximum treatment for each drug used.42,100
The antiresorptive drugs (selective oestrogen receptor modulators, SERM, BP and denosumab) generally reduce fracture risk from 12 to 18months88,91,113 and have no maximum approved duration.
In the case of BP, its efficacy, in terms of BMD and fracture risk reduction is maintained for at least 3 years of treatment.114,115 Some studies show that from the 4th or 5th year BMD slightly rises or remains stable and the risk of some complications increases so that after 5 years of treatment with oral BP or 3 years with zoledronic acid it is advisable to evaluate the risk/benefit of continuing with treatment.116 The Groups of experts agrees in recommending maintaining treatment (up to 10 years for oral BP or up to 6 years for zoledronic acid) in patients with a high fracture risk (fractures prior or during treatment, age >75 years or T-score ≤−2.5 SD in femoral neck or total hip).42,100,117,118
Denosumab produces a progressive increase of BMD for at least 10 years of treatment, maintaining its ant fracture efficacy over time.119 Although there is no consensus regarding the duration of this treatment, some authors indicate that it could be established according to a predefined objective (treat to target strategy), i.e. after reaching a certain BMD value.120 however, they should not suspend treatment with denosumab without establishing other treatment for OP (see section “Suspension or discontinuation of treatment”).
Although data exist on efficacy and safety of SERM (raloxifene and bazedoxifene) up to 8 years, maximum duration of the treatment is conditioned by the risk of complications such as thromboembolism, which is more frequent in patients over 70.121
Regarding patients who receive glucocorticoids, aromatase inhibitors or androgen deprivation therapy, the panel of experts consider that antiresorptive treatment should be maintained at least whilst the patient receives the inductor drug of OP (see “Osteoporosis by glucocorticoids”).
Teriparatide has been shown to reduce the risk of fracture after 6–12months of treatment initiation. However, to achieve maximum efficacy, both in trabecular and cortical bones, different groups of experts agree that it is recommendable to maintain it for 2 years, which is the maximum period approved.42,100,108
Treatment suspension or discontinuationRecommendation:It is recommended that fracture risk be reassessed after 5 years of treatment with oral bisphosphonates or after 3 years of treatment with zoledronic acid (RR: D; AL: 100%).
Recommendation:When treatment with denosumab it suspended an alternative treatment for osteoporosis should be contemplated (RR: D; AL: 100%).
Recommendation:In patients with high fracture risks like those with a previous fragility fracture, or with a T-score of proximal femur <−2.5 SD, it is not recommended to discontinue osteoporosis treatment (RR: D; AL: 89%).
OP treatment implies that the patient will receive medication for years, although in some cases it is possible to have interruptions. The so-called “therapeutic holidays” are a temporary suspension of treatment with BP to reduce the presence of adverse effects, because they accumulate in the bone tissue and maintain their effect even years after having been suspended.115,122–124 This residual effect is not observed with other drugs such as SERM, denosumab or teriparatide.
Although the decision to discontinue treatment with BP must be individualised, the consensus of experts advise reassessing treatment in patients with low fracture risk and who have not presented with incident fractures after 5 years of treatment with oral BP or 3 years with zoledronic acid.100 The duration of this discontinuation is unknown and has been calculated to be between 2 and 3years, depending on the BP used.100,125 Although there is little evidence, the changes in BMD and bone turnover markers could help with decision-making.100
The majority of international guidelines advise not to suspend treatment with BP in high fracture risk patients, like those with previous fragility fractures, a low T-score in hip or in those who present with a fragility fracture during treatment.100,126,127 Regarding the value of the BMD capable of predicting a high fracture risk on suspending treatment, the post hoc analysis of the FLEX and HORIZON studies state this as a femoral T-score <−2.5 SD.122,124 If treatment with BP is prolonged, it seems reasonable to reassess after 5 years of treatment with oral BP or 3 years with zoledronic acid.100,126
According to different scientific societies and groups of experts, the concept of treatment suspension should not be applied to other drugs such as denosumab, since its removal may lead to an increase in bone remodelling, with a decrease in BMD and an increase in fracture risk, including multiple fractures after its suspension.127–130 For this reason, they concluded that if it is decided to suspend treatment for any reason with denosumab, an alternative treatment should always be considered.128 The panel of experts, despite little evidence regarding the consequences of suspension of denosumab and its alternative treatment efficacy, recommend that when treatment is suspended with denosumab an alternative OP treatment be sought.
Follow-up, monitorisation and adherenceRecommendation:Periodical clinical evaluation for confirming compliance and efficacy of treatment, incidence of fractures and the possible presence of adverse effects is recommended (RR: √; AL: 100%).
AdherenceLow OP treatment adherence is a frequent problem, after 12 months of treatment less than half of the patients stick to the therapy they were prescribed.131 Low adherence is also associated with an increased fracture risk.132
The efficacy of different measures has been studied to improve adherence, such as telephone calls or meetings with patients, but none of them appear to have been truly effective.133 In a systematic review of 20 studies it was observed that the simplification of posology, electronic prescription and the intervention of the pharmacist improved adherence or persistence of OP treatment. Patient education has demonstrated its effectiveness in several studies, but not in all of them, whilst monitorisation and supervision had no significant impact on adherence.134 However, the Fracture Liaison Service (FLS) units for secondary fracture prevention reported an adherence >70% at 1–2 years, which was related partly to the educational efforts made by the nurse.135,136
MBT could help with follow-up and adherence. The IOF and European Calcified Tissue Society (ECTS), in a workgroup for analysing the usefulness of the MBT in oral BP adherence, recommended measuring PINP and CTX at the beginning of treatment and after 3 months, to verify whether there was a greater reduction to the minimum significant change of the MBT.137 The same workgroup considered a significant change to be a reduction that was higher than 38% for PINP and above 56% for CTX, whilst absence of change suggested the need for reassessment to identify problems with treatment, mainly low adherence.137 In one meta-analysis of 6 studies which assessed the efficacy of the MBT to improve treatment adherence, compliance was a high average, and it was difficult to assess the repercussion of the act of informing of the outcomes of the MBT.46 In the meta-analysis one study stood out where higher persistence was observed if the patient was informed of their response to treatment (HR for discontinuation 0.71; 95% CI: .53–.95), whilst informing of the poor response to the drug reduced it (HR for discontinuation 2.22; 95% CI: 1.27–3.89).138 The reinforcement using the outcomes of the MBT thus had an influence on persistence, depending on the response of the MBT.
MonitorisationOP treatment monitorisation includes the analytical determinations and the DXA, as well as asking the patient about new fractures. Several studies conclude that the bone remodelling markers could be useful for early monitoring of compliance and response to treatment,139 but the panel of experts consider that they cannot recommend their systematic determination in patient follow-up. The panel recommends monitoring the response to treatment through central DXA, bearing in mind patient characteristics.
There are no controlled, good quality studies on the frequency with which measurement of the BMD should be repeated during treatment.140 Possible variation in BMD with current treatments is slow to occur and usually of little magnitude. Given the risk of DXA measurement error, international experts concluded that measurement should be made when the expected change is equal to or higher than the minimum significant difference.32 For this reason, in general, it is not advised to repeat measurement of the BMD before 2–3 years.5,141 Other guidelines advise lengthening this time period to 3 years for zoledronic acidy and up to 5 years for oral BP.126,142,143 In situations of high fracture risk,5 in treatment with high doses of glucocorticoids or when there is a suspicion of failure in therapy, such as when a new fracture occurs, the period for repeating DXA may be shortened.144 The aim is to detect the patients who, despite treatment, suffer from a significant drop in BMD. It is also advised to carry out a DXA when planning temporary suspension of the drug for “therapeutic holidays”.124 Although the greatest changes in BMD are detected in the lumbar spine, it is useful to also monitor the hip, as it is less dependent on artefacts impacted by degenerative changes.
Several authors propose using a treat to target strategy in OP where the objectives would be to reach a certain value of the T-score (for example, T-score: −2.5 SD) or BMD. If this occurred, monitorisation of the BMD would enable the knowledge of whether the therapeutic aim had been reached. However, not all drugs lead to an improvement of BMD proportional to the reduction of fracture risk, and the real value of this strategy in clinical practice is also yet to be defined.117
Determination of TBS provides information on the changes which treatment may produce on bone microarchitecture,145 but insufficient data is available to recommend its use in clinical follow-up.
Therapy failureThe definition of therapeutic failure in the treatment of OP is complex and there are no established criteria. Experts agree that it is advisable to ensure that treatment adherence is correct, confirm an adequate supply of calcium and vitamin D and rule out secondary OP causes. With regard to treatment failure, after one year of treatment with any antiosteoporotic drug, a group of international experts suggest the following criteria of assessment for treatment changes144:
- 1.
Two or more fragility fractures during treatment.
- 2.
One fragility fracture together with one of the following factors:
- -
Significant loss of BMD (>5% in lumbar spine or >4% in proximal femur).
- -
Absence of significant changes in the MBT (reduction of PINP or CTX >25% with antiresorptive treatment, or increase >25% with bone turnover treatment after 6 months).
- -
- 3.
Absence of significant changes in the MBT together with a significant loss of BMD.
In general the drugs approved for OP treatment are safe and present with good tolerability.146 Oral BP may lead to a series of adverse digestive effects (pyrosis, dyspepsia, esophagitis, dysphagia or abdominal pain) and oral effects (conjuntivitis, anterior uveitis), of minor intensity in most cases, which disappear when the drug is withdrawn. After the first infusion of zoledronic acids symptoms of unclarified origin have been described characterised by fever, myalgias and bone pains and this occurs in between 20% and 30% of patients. In subsequent infusions the symptoms tend to disappear. This effect may occur rarely with oral BP.
Treatment with BP does not increase cardiovascular risk. No association has been demonstrated between the development of auricular fibrillation and treatment with oral BP. In one study an increased risk of auricular fibrillation was observed with the use of intravenous zoledronic acid.147 Isolated cases of cancer of the oesophagus have been published in patients on oral BP, but the most recent studies have not confirmed this relationship.148 Osteomuscular pain, kidney damage and hepatotoxicity from BP are exceptional and will rarely lead to withdrawal of the drug.149 Use of BP is not advised in patients with glomerular filtration rate <30ml/min, since there are no available studies that have demonstrated a reduction in fractures in these patients and on the contrary, they increase the risk of impairment of a reduced remodelling osteodystrophy.
Denosumab, in the guideline approved for the treatment of OP, is a drug which is generally well tolerated. A higher rate of infections has been observed, and in particular cutaneous88 and urinary,150 but their overall rate is very low.151 However, due to their structure, they are not eliminated by the kidney, which is an advantage for patients with kidney diseases where a higher risk of hypocalcaemia must be monitored. As with BP, denosumab should not be used when there is clinical suspicion or histomorphometric evidence of reduced remodelling osteodystrophy.
Adverse reactions to teriparatide are generally not serious. Muscle pain, cramps and dizziness are frequent. Treatment with teriparatide may raise calcaemia and calciuria, and it is therefore advisable to determine the levels of calcium in the blood and urine prior to initiating treatment.
In recent years complications have appeared which are associated with prolonged treatment with antiresorptives (BP and denosumab) and which have generated uncertainty about safety. The relative importance of these rare adverse effects (osteonecrosis of the jawbones and atypical femur fracture) in the treatment of OP is a highly controversial issue.
The fear of patients in suffering these complications may be an impediment for adherence and therapeutic compliance, although the benefit–risk balance of maintaining treatment is highly favourable for high fracture risk patients. A brief analysis will now be made of both complications, based on the systematic reviews carried out to complete this document.
Osteonecrosis of the jawbonesRecommendation:If any invasive dental procedures has been indicated to the OP patient (dental extraction or implant) it is recommended that antiresorptive treatment initiation be postponed until the surgical wound has completely healed (RR: D; AL: 100%).
Recommendation:In patients treated with antiresorptives who are going to carry out a dental procedure it is not recommended that they discontinue treatment with BP or denosumab. If other additional osteonecrosis of the jawbone risks prevail or the surgical procedure is to be extensive, temporary suspension of the treatment with BP may be considered (RR: D; AL: 78%).
Osteonecrosis of the jawbones has been associated with the prolonged use of BP or denosumab, although its rate in patients with OP is very low (between 1/10,000 and 1/100,000).152–154
According to the outcomes of a previous review, published in 2013, available evidence on BP is scarce and of low quality.154 There is insufficient data to confirm that oral BP or intravenous BP used for OP treatment confers any significant risk of osteonecrosis of the jawbones. There have not been enough studies to assess the role of denosumab in the development of osteonecrosis of the jawbones (level of evidence 3).152,153 Several systemic factors such as previous treatment with BP or dental extractions are associated with the development of osteonecrosis of the jawbones in patients treated with denosumab (level of evidence 3).153
Several medical associations have published recommendations to reduce the risk of this complication. In general, guidelines include correct dental hygiene in their proposals and dental check-up if poor dental health is observed. If any invasive dental procedures has been indicated (dental extraction or implant) it is recommended that treatment initiation be postponed until the surgical wound has completely healed.155 The use of CTX marker thresholds have been suggested to assess the baseline risk of osteonecrosis of the jawbones. However, available evidence does not endorse its use.156
There is some controversy regarding the which approach to take in patients who are already taking BP or denosumab. The majority of guidelines advise not to suspend antiresorptive treatment in non oncological patients. In 2011, the American Dental Association guide recognised that the risk of osteonecrosis of the jawbones was very low in patients with OP, and it was therefore not necessary to suspend an oral BP prior to a dental procedure.157 However, and according to the recommendations made by the international panel of experts, in patients who have undergone extensive oral surgery and with associated risk factors (diabetes, periodontal disease, immunodeficiency, tobacco habit), clinical judgement may suggest temporal suspension of treatment.155 In this situation, and given that withdrawal of denosumab leads to a bone remodelling and the risk of appearance of multiple vertebral fractures, the panel considers that treatment with denosumab should not be interrupted.
During OP treatment, invasive dental procedures may be made only after careful consideration of their necessity. In the case of denosumab, and whenever possible, surgery must be avoided in periods close to drug administation.158 In this situation a prolonged unnecessary suspension should be avoided, especially in patients treated with denosumab. Should a relevant adverse event occur such as osteonecrosis of the jawbones, and although no scientific evidence demonstrates withdrawal of the drug will help the process evolve, the same document considers it prudent to suspend treatment and assess drug indication with a different mechanism of action.155
Atypical femur fractureRecommendation:In patients with high fracture risk and prolonged treatment with bisphosphonates (>5 years for orals or >3 years for intravenous) it is recommended not to suspend treatment, since the risk of atypical femur fracture is very low and the benefits of fracture reduction greatly outweigh the risk of atypical fracture (RR: D; AL: 100%).
Atypical fracture of the femur is a potential complication from prolonged treatment with BP or denosumab, although real incidence is very low.
According to the definition proposed by the American Society for Bone and Mineral Research (ASBMR),159 the atypical fracture of the femur is located between the lesser trochanter and the suprachondylar crest and should present with a minimum of 4 out of the following 5 criteria: (1) minimum or absent trauma; (2) origin of the fracture in the external cortical and directed cross-sectionally or obliquely; (3) without comminution or, if this exists, it must be minimal; (4) periostic or endostic thickening of the external, localised cortical, and (5) involvement of the external side cortical (incomplete fracture) or both corticals (complete fracture). Minor criteria are not essential for diagnosis, but they increase it: (a) cortical thickness increased diffusely in femoral diaphysis; (b) pain in thighs or groin area, prior to fracture; (c) bilateral fracture of similar characteristics, and (d) delay in consolidation.
Apart from prolonged treatment with antiresorptives, the atypical fracture of the femur has also been associated with several comorbidities or with taking medication such as proton pump inhibitors or glucocorticoids.
According to the results of a previous 2011 review, the rate of atypical fracture of the femur was very low and it could not be confirmed that there was a significant increase in the risk of atypical fracture of the femur in patients with Op treated with BP. Since then several poor quality studies have been published which show that the rate of atypical fracture of the femur as very low. Two of the 3 published meta-analyses,160,161 based on randomised clinical trials (RCT), cohort, case and control studies, with a high degree of overlapping, demonstrated a significant increase in the risk of subtrochanteric or diaphysary fracture with the use of BP ranged between 1.70 and 1.99, although with a high level of heterogeneity. In a third meta-analysis which only explored alendronic acid,162 the relative risk of atypical fracture of the femur with this drug was 3.23 (95% CI: .88–11.84), but the differences were not significant. When a stratified analysis was made with the studies to assess the effect of alendronic acid over 5 years, heterogeneity was insignificant. Using a model of fixed effects, the relative risk was 2.55 (95% CI: 2.26–2.88), concluding that the alendronic acid did not increase the risk of atypical fracture of the femur short-term, but it did in the long term (>5 years).162
A recent systematic review162 concluded that the rate of atypical fracture of the femur associated with BP was very low, but that the relative risk (RR) increased with prolonged use (especially if it was over 3 years). According to the authors, the benefits of BP in reduction of OP fractures greatly outweigh the risk of atypical femur fracture. A limiting feature highlighted was that almost all studies were retrospective and of variable duration (4–9 years), limiting the interpretation of long term exposure.
Regarding the RCT,163,164 isolated cases have been reported on atypical femur fractures, but these studies present with relevant limitations, such as the short duration of the same, the non-inclusion of this outcome as a main variable or the losses in follow-up, which impede the obtainment of reliable outcomes. If we analyse observational studies,165,166 the incidence and absolute risk of atypical fracture of the femur in patients treated with BP have very low rates (between 10.8 and 19.1 per 100,000 patients per year).
The rate of atypical femur fracture represents 0.3% of the total of femur fractures or 5% of the total of subtrochanteric/diaphyseal fractures. Women represent 80% of all diaphyseal fractures and 95% of the atypical fractures of the femur, with a relative risk which is higher in men. The outcome of recent review studies and meta-analyses suggest a favourable benefit/risk ratio to maintain treatment up until 10 years with antiresorptives.
Regarding denosumab, the systematic review results on the risk of atypical femur fracture are very limited due to the scarcity of high quality studies. The studies included were one RCT,147 two follow-up studies167,168 and one cohort study.169
According to observational studies, long-term treatment with denosumab (between 7 and 10 years) is associated with a nil168 or very low rate of atypical fracture of the femur (.8 per 10,000 patients per year).167 A clinical trial of postmenopausal women with OP who had switched from BP to denosumab was unable to demonstrate that the rare cases of atypical fracture of the femur which appeared early on could be attributable to the use of denosumab, in contrast they appeared to be derived from prior use of BP.160
Although cases of atypical femur fracture have been described in patients treated with denosumab, the panel of experts decided not to make a specific recommendation on the risk of atypical femur fracture with denosumab due to low evidence and the need for further studies with a higher number of cases and longer time period.
To reduce the clinical impact of atypical femur fracture, in patients under prolonged treatment with BP or denosumab attention must be paid to the presence of pain in the thighs. In these cases it is recommended that X-rays be performed to detect stress fractures or cortical thickening and for early determination of atypical femur fracture. It may also be useful to use magnetic resonance or a bone scan to confirm diagnosis.
Treatment of an atypical femur fracture is surgical. When this complication arises, studies conclude that antiresorptive treatment should be withdrawn, the pain treated, an adequate provision of calcium and vitamin D ensured and a drug with a different mechanism of action be indicated.170 No control studies exist On the efficacy of teriparatide in patients who have suffered an atypical femur fracture. In a short series of cases, treatment with teriparatide increased the bone mass and the bone remodelling markers, but with no consistent effect on fracture consolidation.170
Special situationsFragility fractureRecommendation:It is recommended that all patients’ ≥50 years of age with a recent fragility fracture be assessed systematically to prevent further fractures (RR: D; AL: 100%).
Patients with fragility fracture have a significant increase in the risk of further fractures. However, it is normal that fewer than 25% of them start treatment for OP after a fracture.4 The term “imminent fracture risk” has recently been introduced to refer to patients with recent fractures, fragile elderly people with frequent falls and fracture patients who have started treatment with high doses of glucocorticoids.12
In keeping with the EULAR171 recommendations the panel of experts suggested that all patients with recent osteoporotic fracture aged ≥50 years of age should be systematically assessed to prevent new fractures.
The FLS model, or Fracture Unit appears to be the most effective172 in terms of evaluation and treatment initiation. It is also cost-effective173 and positive experiences have been had with it in Spain.136 The central element of a Fracture Unit is the coordinator (in Spain this is usually a medical bone metabolism expert), who works in close collaboration with a specialised nurse. The keys to success in the Fracture Unit are: (a) identification of all cases, usually from a list of emergencies and of hospitalised patients; (b) study of the patients, including determination of risk factors, DXA and analysis; (c) the advice of the nurse on non pharmacological measures and adherence to medication; (d) early initiation of antifracture treatment and (e) the drawing up of a report with precise indications for the primary care physician.
In patients with fragility fracture the panel of experts concludes that it would be useful to assess fracture risk factors and falls and identify secondary OP. They advise the carrying out of DXA and assessment of the presence of vertebral fractures.
Vertebral fractureRecommendation:The treatment of choice for an acute vertebral fracture is rest, rapidly scaled analgesics to control pain, and if necessary the use of a brace (RR: √; AL: 100%).
Acute vertebral fracture may lead to serious pain and disability, lasting for several months.174 General measures that have been shown to be useful and shorten the recovery period are bedrest (for 4 days or less) and rapidly scaled analgesics (including the use of powerful opiates when necessary).175
Ortheses (rigid or semi-rigid brace) are useful for acute episode in combination with analgesic treatment, as they help to immobilise the fracture, reduce load and improve spinal alignment in patients who can tolerate them. The type of orthesis will depend on fracture location. Prolonged use is not advisable. It has not been demonstrated that the rate of new fractures will decrease.176
In patients with persistent and highly intense pain (visual analogue scale [VAS] ≥7), after 4–6 weeks of treatment as described above, some studies concluded that facet infiltrations could be considered or radiofrequency ablation of the lumbar medial branch,177 before prescribing a vertebroplasty or kyphoplasty. According to other studies, in the sub-acute and chronic phase it would be beneficial to have physical therapy using heat applied techniques, ultrasound or hydrotherapy and a progressive initiation of an exercise programme to strengthen the paravertebral and thoracoabdominal muscles.178 Weight-bearing exercises and balance training prevent falls and fractures and improve the patient's quality of life.59,176
Vertebroplasty and kyphoplastyIn which cases would vertebroplasty and kyphoplasty be indicated?
Recommendation:Vertebroplasty and kyphoplasty are recommended for the treatment of vertebral fracture only in cases of serious refractory pain on a WHO analgesic scale, including opiates (RR: D; AL: 100%).
Systematic reviews of the clinical efficacy of vertebroplasty in patients with fractures due to OP, with pain which is refractory to analgesic treatment, conclude that in open trials vertebroplasty has better results than an optimum analgesic treatment in improving quality of life and reducing pain and disability.179,180 However, in double blind trials there is no evidence that vertebroplasty is superior to placebo with local anaesthesia. Also, after a month, the average pain, according to the VAS, was 5 points in the placebo group and 4.3 points in the vertebroplasty group, with an absolute reduction in pain of 7% and a relative reduction of 10%.179 Evidence of its clinical efficacy is not consistent. The complications associated with the procedure are rare, but may be serious. Notwithstanding, it cannot be determined with certainty whether vertebroplasty leads to an increased risk of new fractures in adjacent vertebra.179,180
Systematic reviews of the clinical efficacy of kyphoplasty conclude that in open studies it has better outcomes than conservative treatment in improving quality of life and reducing pain and disability, but the evidence of its clinical efficacy is not consistent as there are no available double blind studies to compare with placebo.179,181
Reduction in pain associated with kyphoplasty analysed through VAS, compared with conservative treatment, was −1.82 after 30 days, −1.45 after 3 months and −1.48 after 6 months, but −.84 and −.69 after 12 and 24 months, respectively.181 The authors conclude that vertebroplasty and kyphoplasty could improve quality of life and reduce pain in vertebral fractures refractory to analgesic treatment, with a doubtful increase in the rate of new fractures in the adjacent vertebra. There is therefore not sufficient evidence for their recommendation in standard clinical practice.
Osteoporosis from glucocorticoidsRecommendation:In patients who receive or who are going to receive treatment with glucocorticoids fracture risk must be assessed and the initiation of treatment for osteoporosis as soon as possible (RR: D; AL: 100%) (Fig. 2).
Recommendation:It is recommended that pharmacological treatment be initiated to prevent OP in patients who are going to receive glucocorticoids for over 3 months in the following cases (RR: D):
- 1.
Patients with an initial dose ≥30 mg/day of prednisone. In these cases it is recommended that treatment be imitated for the OP immediately (AL: 100%).
- 2.
Postmenopausal women and men >50 years of age with doses of prednisone ≥5mg/day who also present with some of the following conditions:
- a)
Previous fragility fracture (AL: 100%).
- b)
Low BMD (T-score ≤−1.5 SD in spine or hip) (AL: 89%).
- c)
High fracture risk: FRAX®for hip ≥3% or for primary fracture ≥10% without BMD or ≥7.5% with BMD (AL: 78%).
- a)
- 3.
Premenopausal women and men <50 years of age and dose ≥7.5mg/day of prednisone who also present with some of the following conditions:
- a)
Previous fragility fracture (AL: 100%).
- b)
Low BMD (Z-score ≤−3 SD) (AL: 78%).
- a)
OP induced by glucocorticoids is the most frequent cause of secondary OP, and is associated with the presence of fractures in up to 30%–50% of cases.182 The risk of fracture depends on factors such as age, prior BMD, daily accumulated dose of glucocorticoids and baseline disease. The loss of BMD is fast, especially in the first 6–12 months, even with low doses of glucocorticoids, with trabecular bone being the most affected.182 According to studies, the fractures associated with treatment with glucocorticoids appear with BMD which is not too low, due to the fact that the glucocorticoid mainly affects the whole bone micro architecture, and the TBS and FRAX® adjusted with TBS could have a certain additional usefulness in the assessment of the risk of these patients.183 However, the application of the FRAX® in OP through glucocorticoids has certain limitations and underestimates the real risk of fracture, since it does not adjust from accumulated dose from daily dose or treatment duration.
ACR recommendations consider that in patients who receive or are going to receive treatment with glucocorticoids the fracture risk must be assessed (preferably using FRAX®) and treatment initiation as soon as possible should be assessed. Furthermore, general preventative OP measures would have to be used in all patients, such as healthy life habits for bones and diet (see section on non pharmacological treatment, calcium and vitamin D). Furthermore, the lowest possible dose of glucocorticoid should be used and for as short a time as possible, and using immunosuppressants if necessary.36
In patients with an intermittent corticoid regime and who have an accumulated dose of prednisone >5g/year or those with annual losses of BMD above 10% the panel of experts consider that treatment for OP would also be indicated.
Premenopausal women or men <50 years of age are at a lower fracture risk. There are also fewer data on efficacy and safety or OP treatments for this population group and treatment indications appear to be more restrictive.
The pharmacological treatment of choice is oral BP (risedronic acid and alendronic acid) or intravenous zoledronic acid since they stabilise or increase the BMD and reduce the risk of vertebral fractures in comparison with control groups.184 According to the technical specifications, the drugs which currently have an glucocorticoid OP indication in Spain are teriparatide, risedronic acid and zoledronic acid.
In women with established OP, teriparatide has been demonstrated to be more effective than risedronic acid in reducing vertebral fractures and clinical fractures, regardless of having received previous treatment with BP.185 In fact, teriparatide has demonstrated superiority against alendronic acid in both gain in BMD and reduction in morphometric vertebral fractures, in premenopausal, postmenopausal women and men treated with glucocorticoids after 18 months of follow-up.186 As a result, the panel of experts consider that high risk patients or those refractory or who have contraindications to antiresorptive treatment could evaluate the treatment with teriparatide.47
In patients who start or have already received glucocorticoids, the administration of denosumab for 12 months has shown an increase in BMD which is significantly higher than with risedronic acid,187 and the European Medicines Agency (EMA) has recommended that denosumab be added into the technical specification as a new indication: for treatment of the loss of BMD associated with the prolonged use of glucocorticoids in adult patients at high risk of fracture.
According to the recommendations of the International Groups, the treatment of choice should be maintained for as long as treatment with glucocorticoids lasts and for at least 6 months after suspending the glucocorticoid, unless fractures exist, in which case it would be continued.36
Rheumatic, inflammatory and systemic diseasesRecommendation:It is advisable to assess the risk of fracture and consider performing DXA in patients with rheumatoid arthritis, systemic lupus erythematosus and ankylosing spondylitis especially those over 50 years of age and who are being treated with glucocorticoids or with a severe or evolved disease (RR: √; AL: 100%).
Patients with rheumatoid arthritis, systemic lupus erythematosus and ankylosing spondylitis are at greater risk of OP and/or fractures.188–194 One study reported an increased fracture risk in psoriasis arthritis and in severe psoriasis.195 The conclusions of some studies in patients with rheumatoid arthritis advise screening for fragility fracture and performing rheumatoid DXA, especially in patients >50 years of age in those with severe disease or evolved disease or those treated with glucocorticoids.188,189 Moreover, several documents of consensus on the management of comorbidities in rheumatoid arthritis, psoriasis arthritis and systemic lupus erythematosus suggested making an accurate prophylaxis of OP.196–198 In relation to antifracture treatments, all the drugs approved for OP were used. In the case of denosumab, safety data was limited in patients treated with biologic agents for their baseline disease, although according to the outcome of some studies there does not appear to be a different safety profile from the postmenopausal OP one.199
Osteoporosis in the maleRecommendation:The study of the causes of secondary osteoporosis in all men with low bone mass or fragility fracture is recommended (RR: D; AL: 100%).
It has been observed in different studies that OP in the male is underdiagnosed.200 in fact it has been estimated that a third of hip fractures worldwide occur in males, especially after the age of 70 years. Furthermore, mortality after a hip fracture (over 37% in the first year) is higher than that in women.201 Also, in the male secondary OP is more frequent and studies therefore conclude that appropriate, accurate clinical assessment is needed for systematic exclusion. The most frequent OP causes and risk factors in the man are hypogonadism, alcohol consumption, tobacco and glucocorticoids. Fracture risk assessment in the male would include clinical risk factors, the existence of previous fractures and the measurement of BMD by DXA (if indicated) to better stratify those patients more susceptible for treatment.202 With regard to DXA, the same reference values are used in men as in women.203
With regard to non pharmacological prevention measures, the same may be considered as for OP in women. For hormonal treatment, there are no data on fracture reduction efficacy with testosterone treatment, and the experts therefore only indicate treatment in males with symptomatic hypogonadism and also advising it should be combined with another antiosteoporotic treatment.204 The drugs which have shown improvement of BMD and reduction of vertebral risk fracture both in eugonadal and hypogonadal males are oral BP (alendronic acid and risedronic acid),205,206 zoledronic acid (the only one which was established as the primary aim)207 and teriparatide.208,209 Denosumab was also approved in males with a high fracture risk and to reduce the loss of BMD in the patient with prostate cancer in androgen deprivation treatment.210 All these drugs, except the alendronic acid, have an indication in Spain for OP in the male.
Osteoporosis in premenopausal womenRecommendation:It is recommended that a study of secondary osteoporosis causes be made in all premenopausal women with a low bone mass measured by DXA or with a fragility fracture (RR: √; AL: 100%).
According to the different scientific societies and expert groups, in the premenopausal woman a DXA should be carried out when there are fragility fractures and/or risk factors associated with the loss of bone mass.42,211 In this patient group the Z-score would have to be used (which compares with individuals of the same age and sex) instead of the T-score.42,211,212 Thus, “low bone mass” is considered when the Z-score is lower than −2 SD.211 The presence of fragility fractures and particularly when associated with low bone mass, means that OP diagnosis may be established in the premenopausal woman.212
Considering that over 50% of premenopausal women with OP present with a secondary OP212,213 the experts recommend making an exhaustive study to rule out an underlying cause,212,213 with the most frequent being the following: treatment with glucocorticoids, oestrogen deficit, malabsorption diseases and OP associated with pregnancy or breast feeding.212 Idiopathic forms are frequent in premenopausal women and may be associated with hypercalciuria and with a family history of OP.213
The therapeutic approach in the premenopausal woman includes optimum calcium and vitamin D intake, regular physical exercise, suspending tobacco consumption, limiting alcohol consumption and treating the underlying cause.212
For premenopausal OP experts, the specific antiosteoporotic drugs (BP, teriparatide, denosumab) will be considered in patients with fragility fracture or for certain secondary causes such as treatment with glucocorticoids.213 The drugs which they have studied in premenopausal OP are BP (alendronic acid and risedronic acid) and teriparatide.212 According to experts, in young women certain caution should be used in the administration of BP given its long retention in the bone tissue, particularly in women who wish to get pregnant in the future. They also conclude that none of these drugs should be used in pregnancy (BP and teriparatide are in the C category according to the FDA).214,215 With regard to the SERM, it seems reasonable to avoid its use in premenopausal women, whilst with denosumab there is only an indication in postmenopausal OP (according to the FDA: category in pregnancy).212
Some experts suggests that in fertile women anti conceptive measures should be prescribed if any antiosteoporotic treatment is initiated.213
Other special situations: kidney disease, liver disease and digestive problemsChronic renal diseaseIn patients with chronic renal disease (CRD) certain differential aspects should be taken into consideration with postmenopausal OP: (1) DXA does not discriminate the type of underlying bone disease and (2) renal osteodystrophy should be excluded, particularly dynamic bone disease (very low osteodystrophy with remodelling) in chronic dialysis patients, since this contraindicates the use of antiresorptives. PTH levels <120pg/ml with low alkaline phosphatase suggest the presence of dynamic bone disease.216
With regard to a deficiency of vitamin D in the CRD, in states 3–5D, the experts consider the use of therapeutic strategies similar to those accepted for the general population to be appropriate.
Regarding antifracture treatments for stage 1–2 patients and filtered >35ml/min, its use is mostly similar to that of patients without CRD.217,218
The technical specifications of the drugs indicates that BP, teriparatide and raloxifene are not recommended when filtration is <30ml/min (<35ml/min for alendronic acid and zoledronic acid). However, some post hoc studies conducted with risedronic acid219and raloxifene220,221 in patients with glomerular filtration between 15 and 30ml/min (stage 4) have shown these drugs demonstrate most efficacy and safety. Notwithstanding, the limited evidence of this type of post hoc study must be borne in mind. Bazedoxifene has not been sufficiently documented in patients with filtration <30ml/min. Denosumab does not require dose adjustment in kidney failure, but the risk of hypocalcaemia is higher, and it is therefore advised to optimise calcium and vitamin D supplements. Evidence of their safety in patients with stage 4 CRD and in haemodialysis is also limited due to the post hoc studies having a limited number of patients.222
Chronic liver diseaseAccording to the experts, patient management with chronic liver disease includes evaluation of factors which lead to bone loss and the increase in fracture risk such as malnutrition, vitamin D deficit, intensity of cholestasis, glucocorticoids and hypogonadism. The pharmacological studies of reduction in patients with liver disease are limited, with no contraindications existing for the use of BP, which increase BMD.223 There is not enough experience with other treatments.
Digestive diseasesStudies conclude that management of patients with celiac disease, inflammatory bowel disease, and bariatric surgery should consider malabsorption and malnutrition (evaluate calcium and vitamin D supplements), as well as the use of glucocorticoids. In inflammatory bowel disease the BP reduce the risk of vertebral fracture.224
According to the data sheet, oral BP are contraindicated in patients with esophageal anomalies and other factors which may delay emptying, such as stenos is and acalasia. The panel of experts consider that when oral antiresorptive therapy is contraindicated, it is regarded as ineffective due to serious malabsorption is not tolerated, and parenteral route treatment may be considered.47
New treatmentsTwo new drugs for OP treatment: romosozumab and abaloparatide are currently in an advanced phase of clinical development.
Romosozumab is a humanised IgG2 monoclonal antibody which links and blocks sclerostin. It is administered monthly subcutaneously for a year, followed by denosumab or alendronic acid for a further year and has shown a significant reduction in the incidence of new vertebral and non vertebral fractures.225,226 in one of the studies an increase in the rate of serious adverse cardiovascular events was observed compared with alendronic acid,226 which motivated a requirement by the US Food and Drug Administration (FDA) to provide the results of additional studies.
Abaloparatide is a synthetic peptide which activate the type 1 receptor of the PTH, approved by the FDA in 2017 and rejected by the EMA in 2018. In daily subcutaneous administration it obtained a significant reaction in the rate of vertebral and non vertebral fractures compared with placebo, with no differences to teriparatide.227 Its safety and tolerability profile was similar to teriparatide, but with a lower rate of hypercalcaemia.
ConclusionAs mentioned in the introduction, the aim of this document was to provide an update on the new advances in the different aspects of OP relating to clinical practice: diagnosis, evaluation, treatment and follow-up.
The document is based on a critical review of the previous consenus,5 the best available scientific evidence and expert clinical experience. This was a joint task between panel members and the SER Unit of Research which, together with SER reviewers, carried out an extensive systematic review on the different areas of interest and provided the necessary scientific rigour to produce the recommendations with their level of evidence and also their level of consensus, to provide the reader with the most objective evaluation of these recommendations.
The new definition of OP is incorporated into the diagnostic assessment of this document, and which encompasses patients with fragility fractures and low bone mass, the concept of imminent risk of fracture and the utility of TBS.
With regard to treatment, the effectiveness of each drug for the prevention of fractures together with several selection criteria such as cost-effectiveness, the existence of multiple fractures or the limitations for oral use are presented. The panel members assessed the possibility of including an extensive treatment decision algorithm no agreement was reached on this.
The SER reviewers for their part have updated osteonecrosis of the jawbone and atypical femur fracture guidelines. Special situations were maintained in keeping with the 2011 recommendations, adding OP of inflammatory rheumatisms and a few precautions which should be taken into account in patients with kidney disease and gastrointestinal and hepatic problems. Finally, a section on the new treatments (romosozumab and abaloparatide) was produced.
We would like to highlight from these recommendations that fracture risk and treatment indication were the issues most debated among panel members. The use of the FRAX® was unanimously accepted for hip fracture with the threshold of 3% whilst the level of agreement for primary fracture was somewhat lower. In the document the reason why of this discrepancy was exposed, which was basically derived from the absence of sufficient studies on the calibration of the FRAX® for the Spanish population.
To conclude, we may say that the recommendations of this document are a draft consultation framework for OP management. They are general regulations which require individualisation in each case and this is the role we must accept as professionals.
Conflict of interestsAntonio Naranjo Hernández received financing from Amgen, Lilly and FAES for attendance to courses/congresses, fees from Amgen for congress presentations and grants from FHOEMO-Amgen and Lilly to contract staff and provide IT teams to the service.
María Pilar Aguado Acín received financing from Lilly for attendance to congresses; fees from Alexion, Faes, Lilly and de Rubió for presentations and financing for educational programmes in the Rheumatology Unit.
Luis Arboleya Rodríguez received financing from Janssen-Cilag, Lácer and Lilly for attendance at congresses and received fees from Pfizer and Lilly for congress presentations.
Enrique Casado Burgos received financing from Amgen, Lilly, FAES, Gebro, ItalDrug and Rubió for attendance to courses/congresses and fees for presentations.
Santos Castañeda Sanz received financing from Amgen for attendance to courses/congresses; fees from Lilly for congress presentations; and financial aid from Amgen for consultation for pharmaceutical companies or other technologies and for educational programmes or courses.
Jodi Fitter Arrested received financing from Novartis, Roche, MSD, Amgen, Lilly, Maharani, UCB and Pfizer for attendance to courses/congresses; fees from Amgen-Ferrier, Roche, Maharani and Amgen for congress presentations and received financing from UCB to participate in a research study.
Labia Gofer Sale received financing from Lilly for attendance to congresses; fees from Lilly, Amgen and FAES Farman e for congress presentations; and financing from Alexion for research projects.
Carmen Gomez Vaquero received financing from Abby, Amgen, Bristol, Lilly, Maharani, MSD, Pfizer, Procter Gamble, Roche, Sandoz, Servicer, UCB for attendance to courses/congresses; fees from Abbie, Amgen, Lilly, MSD, Pierre Fabré, Roche and UCB for congress presentations; financing from Lilly for educational programmes or courses; financing from Novartis, Amgen and Lilly for participating in a research study; financial aid from Nycomed Pharma for consultation for pharmaceutical companies or other technologies; and received financing from Lilly for educational programmes or courses for the unit.
Núria Guañabens Gay received financing from Amgen y Lilly for attendance to courses/congresses, for congress presentations and for educational programmes or courses; financing from Amgen to participate in a research study and financial aid from Lilly, Amgen, UCB y Alexion as consultation for pharmaceutical companies.
The group of experts of this study wish to express their gratitude to Mercedes Guerra Rodríguez, the SER documentalist for her collaboration in search strategies and to Dr. Hye Sang Park for her collaboration in the RS report reviews. They also wish to thank Dr. Federico Diaz González, director of the SER Research Unit, for his participation in the final manuscript review and for contributing to maintaining the independence of this document.
| Levels of evidence | |
|---|---|
| 1++ | High quality meta-analysis, systematic reviews of clinical trials or high quality clinical trials with very low risk of bias. |
| 1+ | Well executed meta-analysis, systematic reviews of clinical trials or well executed clinical trials with low risk of bias. |
| 1− | Meta-analysis, systematic reviews of clinical trials or clinical trials with high risk of bias. |
| 2++ | Systematic reviews of high quality cohort studies or cases and controls. Cohort studies or case and control studies with a very low risk of bias and high probability of establishing a causal relationship. |
| 2+ | Well executed cohort studies or case and control studies with low risk of bias and a moderate probability of establishing a causal relationship. |
| 2− | Cohort studies or case and control studies with high risk of bias and significant risk that there will be no causal relationship. |
| 3 | Non analytical studies, such as case reports and case series. |
| 4 | Expert opinions. |
| Recommendation rates | |
|---|---|
| A | At least one meta-analysis, systematic review or clinical trial classified as 1++ and directly applicable to the target population of the guide; or a volume of scientific evidence composed by studies classified as 1+ and with major agreement between them. |
| B | A volume of scientific evidence composed by studies classified as 2++, directly applicable to the target population of the guide and which show major concordance between them; or scientific evidence extrapolated from studies classified as 1++ or 1+. |
| C | A volume of scientific evidence composed by studies classified as 2+, directly applicable to the target population of the guide and which shows major consistency between them; or scientific evidence extrapolated from studies classified as 2++. |
| D | Scientific evidence level 3 or 4; or scientific evidence extrapolated from studies classified as 2+. |
Studies classified as 1− and 2− should not be used in the production of recommendations due to their high possibility of bias.
| Good clinical practice | |
|---|---|
| √ | Recommended practice based on clinical experience and consensus of the drafting team |
aOn occasions the drafting group notices an important practical aspect which has to be highlighted and for which there is probably no supporting evidence. In general, these cases concern some aspect of treatment which is considered good clinical practice and which nobody would usually question. These aspects are valued as points of good clinical practice. These messages are not an alternative to the recommendations based on evidence but should only be considered when there is no other way of highlight this aspect.
Please cite this article as: Naranjo Hernández A, Díaz del Campo Fontecha P, Aguado Acín MP, Arboleya Rodríguez L, Casado Burgos E, Castañeda S, et al. Recomendaciones de la Sociedad Española de Reumatología sobre osteoporosis. Reumatol Clin. 2019;15:188–210.