To present recommendations based on the available evidence and the consensus of experts, for risk management of biological treatment and JAK inhibitors in patients with rheumatoid arthritis.
MethodsClinical research questions relevant to the purpose of the document were identified. These questions were reformulated in PICO format (patient, intervention, comparison, outcome or outcome) by a panel of experts, selected based on their experience in the area. A systematic review of the evidence was carried out, grading according to the GRADE criteria (Grading of Recommendations Assessment, Development, and Evaluation). Specific recommendations were then formulated.
Results6 PICO questions were proposed by the panel of experts based on their clinical relevance and the existence of recent information regarding the risk of occurrence of serious infections, the risk of reactivation of the hepatitis B virus, the risk of reactivation of the virus varicella-zoster, the risk of appearance of skin (melanoma and non-melanoma) or haematological cancer, the risk of appearance of thromboembolic disease and the risk of progression of the human papilloma virus.
A total of 28 recommendations were formulated, structured by question, based on the evidence found and the consensus of the experts.
ConclusionsThe SER recommendations on risk management of treatment with biologic therapies and JAK inhibitors in rheumatoid arthritis are presented.
Elaborar recomendaciones basadas en la evidencia disponible y el consenso de expertos, para la gestión del riesgo del tratamiento biológico y los inhibidores de las JAK en pacientes con artritis reumatoide.
MétodosSe identificaron preguntas clínicas de investigación relevantes para el objetivo del documento. Estas preguntas fueron reformuladas en formato PICO (paciente, intervención, comparación, outcome o desenlace) por un panel de expertos, seleccionados en base a su experiencia en el área. Se realizó una revisión sistemática de la evidencia, graduándose de acuerdo a los criterios GRADE (Grading of Recommendations Assessment, Development, and Evaluation). A continuación, se formularon las recomendaciones específicas.
ResultadosSe propusieron por el panel de expertos 6 preguntas PICO en base a su relevancia clínica y a la existencia de información reciente referentes al riesgo de aparición de infecciones graves, el riesgo de reactivación del virus de la hepatitis B, el riesgo de reactivación del virus varicela-zoster, el riesgo de aparición de cáncer de piel (melanoma y no melanoma) o hematológico, el riesgo de aparición de enfermedad tromboembólica y el riesgo de progresión del virus del papiloma humano.
Se formularon un total de 28 recomendaciones, estructuradas por pregunta, basadas en la evidencia encontrada y el consenso de los expertos.
ConclusionesSe presentan las recomendaciones SER sobre la gestión del riesgo del tratamiento con terapias biológicas e inhibidores de las JAK en la artritis reumatoide.
The goals of treatment for patients with rheumatoid arthritis (RA) are the disappearance of signs and symptoms of the disease, the prevention of joint damage, the normalisation of physical function and quality of life, and the prevention of onset of comorbidities and associated consequences. Achieving these goals is becoming more common, thanks to, among other things, the availability of a significant number of treatment options and treatment strategies, which include early initiation of treatment, rational use of available drugs, and treatment-to-target.1
Approved treatments for RA include conventional synthetic disease-modifying antirheumatic drugs (DMARDs); synthetic conventional DMARDs: csDMARDs; biological DMARDs (bDMARDs); and targeted synthetic DMARDs (tsDMARDs).2 Each of these groups includes drugs with different modes of action. bDMARDs include drugs that target TNF (TNF inhibitors) such as monoclonal antibodies, infliximab, adalimumab, certolizumab, and golimumab, the fusion protein etanercept, IL-6 receptor (IL-6Ri) inhibitors such as tocilizumab and sarilumab, abatacept, which is a co-stimulation inhibitor, and rituximab, which is an anti-cell drug B. JAK inhibitors (JAKi) are, so far, the only tsDMARDs approved to treat RA, and in Europe there are four: tofacitinib, baricitinib, upadacitinib and filgotinib. Although their mechanism of action is not exactly the same, these drugs have shown remarkable overlap in efficacy and, in many respects, safety. Although each pharmacological group has a characteristic efficacy and safety profile, some aspects of safety may differ and therefore influence treatment decisions in clinical practice.3
Safety is a key component of the new drug development programme. However, clinical trials are primarily designed to evaluate efficacy. Their follow-up for a limited time and the inclusion of a small number of patients, in general with a disease with few risk factors and comorbidities, limit the ability of clinical trials to study safety precisely. These problems, among others, led to the development of treatment registries that have provided so much information to rheumatology.4 Clinical trials have rarely been designed with the primary objective of assessing safety, and most have been conducted at the request of regulatory agencies in the face of the possibility or indications of the development of potentially serious events, and which provide information on specific safety aspects of certain medicines with the highest level of evidence.5,6 In the absence of clinical trials designed to assess safety, observational studies derived from clinical practice data, where medicines are directly compared in unselected patients over an extended period of time, have been used to study the long-term safety of treatments in a more precise manner.
The purpose of this document is to develop recommendations, useful to rheumatologists and other specialists, on the risk management of treatments used in RA. These recommendations will serve to minimise the risk derived from the prescription of treatments and are based on a systematic review of the literature in order to update the evidence on the safety of csDMARDs, bDMARDs and tsDMARDs.
Clinical research questionsThese recommendations address six clinical issues:
- 1
In patients with RA, what is the risk of serious infections from biological or targeted synthetic DMARD treatments?
- 2
In patients with RA, what is the risk of hepatitis virus reactivation B from targeted biological or synthetic DMARD treatments?
- 3
In patients with RA, what is the risk of varicella-zoster virus reactivation from targeted biological or synthetic DMARD treatments?
- 4
In patients with RA, what is the risk of skin cancer (melanoma and non-melanoma) or haematological development from targeted biological or synthetic DMARD treatments?
- 5
In patients with RA, what is the risk of developing thromboembolic disease from targeted biological or synthetic DMARD treatments?
- 6
In patients with RA, what is the risk of human papillomavirus (HPV) progression from targeted biological or synthetic DMARD treatments?
A qualitative synthesis of scientific evidence and consensus techniques have been used to register the agreement of experts based on their clinical experience and scientific evidence. The process was as follows:
Creation of the working group. An interdisciplinary working group was set up consisting of five rheumatologists who are members of the SER, a family doctor, an infectious disease specialist, a preventivist and a patient. The coordination of the clinical and methodological aspects was undertaken by one of the rheumatologists, as principal investigator (PI), and a specialist in methodology from the SER Research Unit, respectively. The group formed the Recommendation Drafting Group (GH).
Identification of key areas. The Panel participated in structuring the document and setting down the content and key aspects, identifying the clinical research questions with the greatest impact on the risk management of treatments. The questions were rephrased in PICO (Patient, Intervention, Comparison, Outcome) format.
Literature search. A literature search was undertaken in the following databases: Pubmed (MEDLINE), EMBASE (Elsevier) and Cochrane Library (Wiley Online). Searches were closed with dates of September 2021. The process was completed with a manual search of references and posters and abstracts of conferences that the reviewers and experts considered to be of interest. In this way, we have included studies that have been published in 2022, a date after the initial search undertaken, and that could provide relevant data that could lead to new recommendations or change the direction of the recommendation.
Analysis and synthesis of scientific evidence. Systematic reviews of the available scientific evidence were conducted. The assessment of the quality of the evidence was undertaken following the methodology of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) international working group.7 Taking into account components such as: study design, risk of bias, inconsistency, directionality, imprecision, and likelihood of publication bias, the quality of the evidence for each critical outcome was classified and defined as high (very unlikely that new studies will change the estimate), moderate (further studies are likely to change our confidence in the outcome), low (it is very likely that further studies will have an impact on our confidence in the result and may change it) and very low (any estimated result is very doubtful).
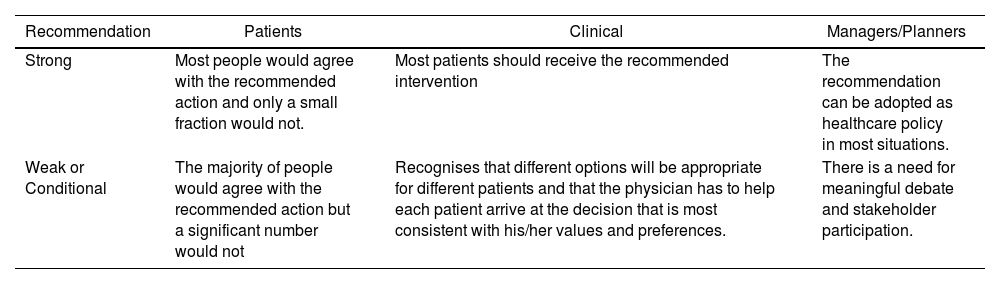
Formulation of recommendations. Once the critical reading was completed, the GoE proceeded to formulate specific recommendations based on scientific evidence. This formulation has been based on "formal assessment" or "reasoned judgment", previously summarising the evidence for each of the clinical questions. The quality or certainty of the scientific evidence identified, the views and preferences of the patients, the balance between desirable and undesirable effects of the interventions, and aspects such as equity, acceptability and feasibility of their implementation have been taken into account following the GRADE methodology. Frameworks that assist in the process of moving from evidence to recommendations (Evidence to Decision (EtD) were used. At the end of this process, the strength (weak or strong) and direction (for or against) of the recommendations were determined, with different implications for the different users of these. (Table 1)
Implications of the strength of recommendation in the GRADE7 system.
| Recommendation | Patients | Clinical | Managers/Planners |
|---|---|---|---|
| Strong | Most people would agree with the recommended action and only a small fraction would not. | Most patients should receive the recommended intervention | The recommendation can be adopted as healthcare policy in most situations. |
| Weak or Conditional | The majority of people would agree with the recommended action but a significant number would not | Recognises that different options will be appropriate for different patients and that the physician has to help each patient arrive at the decision that is most consistent with his/her values and preferences. | There is a need for meaningful debate and stakeholder participation. |
In addition, sometimes the Panel considers that there is some important aspect that it wants to emphasise, for which there is probably no quality scientific evidence to support it. In general, these cases are related to some aspect of the treatment considered good clinical practice and that no one would usually question. These aspects are valued as points or recommendations of good clinical practice (GCP).
External review. The final draft of the document was sent to professionals selected for their knowledge of RA, for an independent external review. The ultimate goal was to increase the external validity of the document and ensure the accuracy of the recommendations. Subsequently, a process of public exhibition of the document was opened to the up members of the SER and to different interest groups (pharmaceutical industry, other scientific societies and patient associations), in order to gather their assessments and their scientific argumentation of the methodology or recommendations.
Preliminary considerationsRisk management in the use of medicines is a very important part of pharmacovigilance. This, in turn, can be defined as the public health activity that aims to identify, quantify, evaluate and prevent the risks of medicines once they have been marketed.8
Despite the undeniable benefits of immunosuppressive treatments, it should not be forgotten that they can inevitably produce side effects that sometimes prevent or hinder their use in some patients. The side effects, related to the treatment that can appear in a patient with RA are very diverse. Some are considered mild and self-limiting, but others are significant, as there is evidence of association with the drug and these side effects are potentially serious.
New drugs are periodically incorporated into RA treatment and new safety descriptions appear derived from their use in increasingly more patients and for a longer period of time, so it is necessary to review and update the safety data that have been reported periodically. In this work, and due to the fact that, for reasons of length, it was not possible to review the overall risk management of all treatments, the panel has chosen those that are considered most relevant and in which there has been recent information that should be considered.
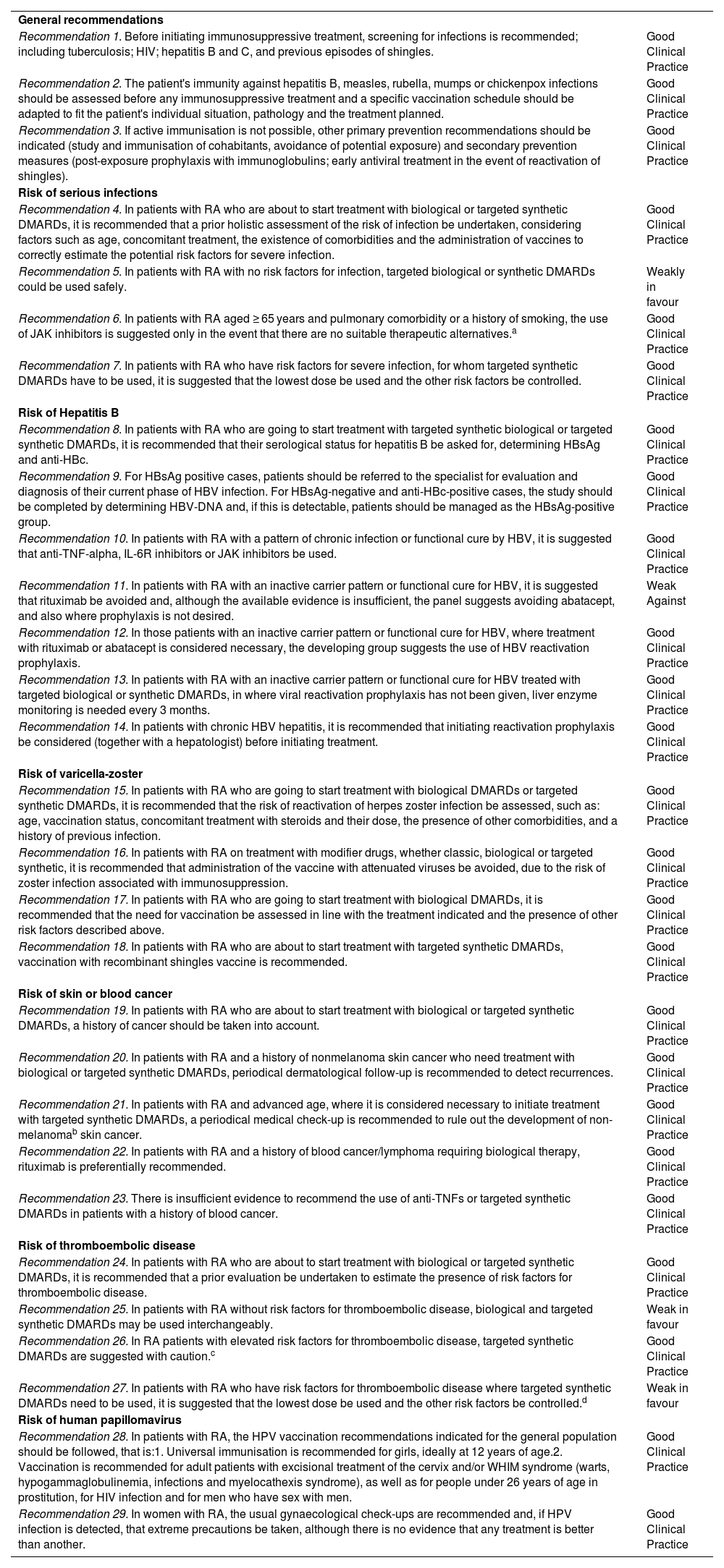
ResultsA total of 29 recommendations have been made for risk management of treatment with bDMARDs and tsDMARDs (Table 2,). Full additional information on the following items can be found in Appendix A. Supplementary data.
SER recommendations on risk management of treatment with bDMARDs and tsDMARDs in rheumatoid arthritis.
| General recommendations | |
| Recommendation 1. Before initiating immunosuppressive treatment, screening for infections is recommended; including tuberculosis; HIV; hepatitis B and C, and previous episodes of shingles. | Good Clinical Practice |
| Recommendation 2. The patient's immunity against hepatitis B, measles, rubella, mumps or chickenpox infections should be assessed before any immunosuppressive treatment and a specific vaccination schedule should be adapted to fit the patient's individual situation, pathology and the treatment planned. | Good Clinical Practice |
| Recommendation 3. If active immunisation is not possible, other primary prevention recommendations should be indicated (study and immunisation of cohabitants, avoidance of potential exposure) and secondary prevention measures (post-exposure prophylaxis with immunoglobulins; early antiviral treatment in the event of reactivation of shingles). | Good Clinical Practice |
| Risk of serious infections | |
| Recommendation 4. In patients with RA who are about to start treatment with biological or targeted synthetic DMARDs, it is recommended that a prior holistic assessment of the risk of infection be undertaken, considering factors such as age, concomitant treatment, the existence of comorbidities and the administration of vaccines to correctly estimate the potential risk factors for severe infection. | Good Clinical Practice |
| Recommendation 5. In patients with RA with no risk factors for infection, targeted biological or synthetic DMARDs could be used safely. | Weakly in favour |
| Recommendation 6. In patients with RA aged ≥ 65 years and pulmonary comorbidity or a history of smoking, the use of JAK inhibitors is suggested only in the event that there are no suitable therapeutic alternatives.a | Good Clinical Practice |
| Recommendation 7. In patients with RA who have risk factors for severe infection, for whom targeted synthetic DMARDs have to be used, it is suggested that the lowest dose be used and the other risk factors be controlled. | Good Clinical Practice |
| Risk of Hepatitis B | |
| Recommendation 8. In patients with RA who are going to start treatment with targeted synthetic biological or targeted synthetic DMARDs, it is recommended that their serological status for hepatitis B be asked for, determining HBsAg and anti-HBc. | Good Clinical Practice |
| Recommendation 9. For HBsAg positive cases, patients should be referred to the specialist for evaluation and diagnosis of their current phase of HBV infection. For HBsAg-negative and anti-HBc-positive cases, the study should be completed by determining HBV-DNA and, if this is detectable, patients should be managed as the HBsAg-positive group. | Good Clinical Practice |
| Recommendation 10. In patients with RA with a pattern of chronic infection or functional cure by HBV, it is suggested that anti-TNF-alpha, IL-6R inhibitors or JAK inhibitors be used. | Good Clinical Practice |
| Recommendation 11. In patients with RA with an inactive carrier pattern or functional cure for HBV, it is suggested that rituximab be avoided and, although the available evidence is insufficient, the panel suggests avoiding abatacept, and also where prophylaxis is not desired. | Weak Against |
| Recommendation 12. In those patients with an inactive carrier pattern or functional cure for HBV, where treatment with rituximab or abatacept is considered necessary, the developing group suggests the use of HBV reactivation prophylaxis. | Good Clinical Practice |
| Recommendation 13. In patients with RA with an inactive carrier pattern or functional cure for HBV treated with targeted biological or synthetic DMARDs, in where viral reactivation prophylaxis has not been given, liver enzyme monitoring is needed every 3 months. | Good Clinical Practice |
| Recommendation 14. In patients with chronic HBV hepatitis, it is recommended that initiating reactivation prophylaxis be considered (together with a hepatologist) before initiating treatment. | Good Clinical Practice |
| Risk of varicella-zoster | |
| Recommendation 15. In patients with RA who are going to start treatment with biological DMARDs or targeted synthetic DMARDs, it is recommended that the risk of reactivation of herpes zoster infection be assessed, such as: age, vaccination status, concomitant treatment with steroids and their dose, the presence of other comorbidities, and a history of previous infection. | Good Clinical Practice |
| Recommendation 16. In patients with RA on treatment with modifier drugs, whether classic, biological or targeted synthetic, it is recommended that administration of the vaccine with attenuated viruses be avoided, due to the risk of zoster infection associated with immunosuppression. | Good Clinical Practice |
| Recommendation 17. In patients with RA who are going to start treatment with biological DMARDs, it is recommended that the need for vaccination be assessed in line with the treatment indicated and the presence of other risk factors described above. | Good Clinical Practice |
| Recommendation 18. In patients with RA who are about to start treatment with targeted synthetic DMARDs, vaccination with recombinant shingles vaccine is recommended. | Good Clinical Practice |
| Risk of skin or blood cancer | |
| Recommendation 19. In patients with RA who are about to start treatment with biological or targeted synthetic DMARDs, a history of cancer should be taken into account. | Good Clinical Practice |
| Recommendation 20. In patients with RA and a history of nonmelanoma skin cancer who need treatment with biological or targeted synthetic DMARDs, periodical dermatological follow-up is recommended to detect recurrences. | Good Clinical Practice |
| Recommendation 21. In patients with RA and advanced age, where it is considered necessary to initiate treatment with targeted synthetic DMARDs, a periodical medical check-up is recommended to rule out the development of non-melanomab skin cancer. | Good Clinical Practice |
| Recommendation 22. In patients with RA and a history of blood cancer/lymphoma requiring biological therapy, rituximab is preferentially recommended. | Good Clinical Practice |
| Recommendation 23. There is insufficient evidence to recommend the use of anti-TNFs or targeted synthetic DMARDs in patients with a history of blood cancer. | Good Clinical Practice |
| Risk of thromboembolic disease | |
| Recommendation 24. In patients with RA who are about to start treatment with biological or targeted synthetic DMARDs, it is recommended that a prior evaluation be undertaken to estimate the presence of risk factors for thromboembolic disease. | Good Clinical Practice |
| Recommendation 25. In patients with RA without risk factors for thromboembolic disease, biological and targeted synthetic DMARDs may be used interchangeably. | Weak in favour |
| Recommendation 26. In RA patients with elevated risk factors for thromboembolic disease, targeted synthetic DMARDs are suggested with caution.c | Good Clinical Practice |
| Recommendation 27. In patients with RA who have risk factors for thromboembolic disease where targeted synthetic DMARDs need to be used, it is suggested that the lowest dose be used and the other risk factors be controlled.d | Weak in favour |
| Risk of human papillomavirus | |
| Recommendation 28. In patients with RA, the HPV vaccination recommendations indicated for the general population should be followed, that is:1. Universal immunisation is recommended for girls, ideally at 12 years of age.2. Vaccination is recommended for adult patients with excisional treatment of the cervix and/or WHIM syndrome (warts, hypogammaglobulinemia, infections and myelocathexis syndrome), as well as for people under 26 years of age in prostitution, for HIV infection and for men who have sex with men. | Good Clinical Practice |
| Recommendation 29. In women with RA, the usual gynaecological check-ups are recommended and, if HPV infection is detected, that extreme precautions be taken, although there is no evidence that any treatment is better than another. | Good Clinical Practice |
The Pharmacovigilance Risk Assessment Committee (PRAC) within the European Medicines Agency, based on the results of the Oral Surveillance study and the data presented by the other three JAK inhibitors on the European market, has issued recommendations to minimise the risk of significant side effects associated with the use of this group of drugs in the treatment of several chronic diseases. Side effects include cardiovascular events, thrombosis, neoplasms, and infections.
This recommendation is based on the statement of the Spanish Medicines Agency issued after the recommendations of the PRAC. Although the statement expressly refers to dermatologists, the Panel believes that this is not operational and that it would overload dermatology impossibly. Since this lesion is easily identifiable, both primary care physicians and specialists will be able to refer all lesions that they consider suggestive to the dermatologist.
This recommendation is derived from the results of the Oral Surveillance study. Although this study is limited to tofacitinib, until more information is available on the other molecules with a similar mechanism of action in patients at risk of TED, they should not be used if there are other alternatives, according to the PRAC resolution.
Recommendation 1. Before initiating immunosuppressive treatment, screening for infections, including tuberculosis, HIV, hepatitis B and C, and previous episodes of shingles, is recommended. (BPC recommendation.)
Recommendation 2. The patient's immunity against hepatitis B, measles, rubella, mumps or chickenpox infections should be assessed before any immunosuppressive treatment and a specific vaccination schedule should be adapted according to the patient's particular situation, pathology and planned treatment. (BPC recommendation.)
Recommendation 3. If active immunisation is not possible, other primary prevention recommendations should be indicated (study and immunisation of cohabitants, avoidance of potential exposure) and secondary prevention measures (post-exposure prophylaxis with immunoglobulins; early antiviral treatment in the event of reactivation of shingles). (BPC recommendation.)
The use of immunosuppressive treatments in rheumatological patients justifies a comprehensive assessment of the immunological status in order to detect undiagnosed infections early and prevent immune-preventable diseases, which present a higher risk of morbidity and mortality in these patients.9,10
First, any infection present that may become unbalanced once immunosuppressive therapy has been initiated, including latent or silent infections, such as latent tuberculosis infection or chronic viral hepatitis, should be ruled out.9,11
Both the autoimmune disease itself and the treatments used to control it increase vulnerability to immune-preventable diseases. Serologically verifying the existing protection, and adapting a specific vaccination schedule before initiating therapy, maximises the benefits and reduces the potential risks associated with this type of treatment.12,13
Finally, patients who have already started immunosuppressive therapy may also benefit from preventive measures, both direct (immunisation with inactivated vaccines, avoidance of exposure to infected patients, passive post-exposure immunoprophylaxis, early antiviral treatment) and indirect (study and immunisation of cohabitants).12–15 The basis for the formulation of these general recommendations has taken into account the point of view of preventive medicine and public health in all patients requiring immunosuppressive therapy.
Risk of developing serious infectionsIn patients with RA, what is the risk of serious infections from targeted biological or targeted synthetic DMARD treatments?Patients with RA are treated with csDMARDs, bDMARDs, or tsDMARDs with the aim of achieving clinical remission or, failing that, a disease with a low inflammatory load. During the course of treatment, efficacy and safety parameters are taken into consideration. There is some evidence that the use of bDMARDs and tsDMARDs is associated with increased infection rates compared to csAMARDs and/or placebo. The data comes from both RCTs and real-life post-marketing data. Sometimes, these real-life studies do not include the existing comorbidities or concomitant drugs (glucocorticoids, among others) of these patients, which undoubtedly influence a higher or lower rate of infections. The increased susceptibility to infections in patients with RA has been attributed to the pathophysiology of the disease, associated comorbidities, lifestyle factors such as smoking and obesity, and the use of drugs such as glucocorticoids and immunomodulators.16
Patients with RA treated with standard-dose bAMARDs (with or without csAMARDs) have a higher incidence of serious infections compared to those treated with cFMARD (OR: 1.31; CI 95%: 1.09–1.58). Therefore, it is advisable to screen prior to the start, an indication that assesses each case individually, and frequent monitoring of clinical and biological parameters.17
For tsDMARDs, the data show that the short- and long-term safety of JAK inhibitors was comparable to that of bDMARDs. As potent immunosuppressive agents, the incidence rates of infections, including opportunistic infections, are comparable to those of bDMARDs, with the exception of the rate of herpes zoster infections, which is slightly higher for JAK inhibitors. Analyses of subsequent RCTs on baricitinib and upadacitinib have suggested a possible dose-dependent infection risk pattern.18 Thus, in the current context, it has been shown that JAKi are clearly superior in efficacy compared to placebo in improving signs, symptoms and health-related quality of life in patients with RA in the short term. However, safety analyses have shown that the risk of adverse effects and infections is slightly higher in the case of the four tsDMARDs: tofacitinib, baricitinib, upadacitinib and filgotinib, especially with regard to the reactivation of herpes zoster.19
The perception of the safety of JAKi has changed following the publication of the results of the ORAL Surveillance post-marketing safety study. Because of potential safety concerns seen with higher doses of tofacitinib, the FDA ordered this study, which compares tofacitinib 5 mg and 10 mg twice daily with anti-TNFs in patients with active RA and insufficient response to methotrexate. Similarly, due to initial data suggesting an increased risk of thromboembolic events with higher doses of baricitinib, the FDA is requesting two post marketing safety studies that are still in development. ORAL Surveillance included patients who were ≥50 years of age and had at least one cardiovascular risk factor. The two primary targets were major adverse cardiac events and malignancies, excluding non-melanoma skin cancer. Other cardiovascular events, including venous thromboembolism, deep vein thrombosis, and pulmonary embolism and infections, were included as secondary endpoints. The study was designed as a non-inferiority comparison with the non-inferiority margin established as the upper limit of CI 95% at <1.8 for the primary comparison between the two combined doses of tofacitinib versus anti-TNF6.
The study failed to achieve its primary endpoint, i.e., tofacitinib did not demonstrate non-inferiority to anti-TNFs for MACE and malignancies. The upper limit for both crossed the 1.8 margin. In addition, there was an increased incidence of venous thromboembolism, deep vein thrombosis, and pulmonary embolism with tofacitinib, observed mainly in the 10 mg20 group. The risk of infection also increased with both doses of tofacitinib versus TNF inhibitors, especially herpes zoster.21
More recently, the European Pharmacovigilance Risk Assessment Committed (PRAC) has issued a recommendation based on these results to minimise risks in patients with inflammatory diseases and the use of tsDMARDs. These risks are considered class effects for all tsDMARDs indicated in inflammatory diseases. In the case of treating a patient with any of the aforementioned risk factors because no other therapeutic alternative is available, the dose should be reduced.22
One of the most effective measures to minimise risks while maintaining efficacy has been the optimisation of drug doses, once the therapeutic objective has been achieved and guided by the clinical, serological/biological status, or imaging methods. Optimisation ensures an optimal dose of the drug, with a reduction in possible adverse effects attributed to it. However, the evidence of its benefit/risk balance should be better evaluated. In addition, the observation that frail elderly patients have higher infection rates than younger patients has increased precautionary measures in this population.23–25
Thus, there is a clear need to analyse the available evidence on the risk of serious infections associated with the use of targeted biological and synthetic drugs used in these patients.
Recommendation 4. In patients with RA who are about to start treatment with biological DMARDs or targeted synthetic DMARDs, it is recommended that a prior holistic assessment of the risk of infection be done, considering factors such as age, concomitant treatment and the existence of comorbidities and administration of vaccines to correctly estimate the potential risk factors for severe infection. (BPC recommendation.)
Recommendation 5. In patients with RA without risk factors for infection, targeted biological or synthetic DMARDs could be used safely. (Weak recommendation in favour.)
Recommendation 6. In patients with RA aged ≥65 years and with pulmonary comorbidity or a history of smoking, the use of JAK inhibitors is suggested only in the event that there are no suitable therapeutic alternativess (BPC recommendation.)
Recommendation 7. In patients with RA who have risk factors for severe infection, where targeted synthetic DMARDs have to be used, it is suggested that the lowest dose be used and other risk factors monitored. (BPC recommendation.)
Relevant clinical considerations- •
Subgroup Considerations
▪The subgroup of older patients is a special subgroup because of t heir comorbidity because regardless of their baseline RA, these patients already have higher infection rates. This is where a detailed analysis of their health status is key before starting a bDMARD or tsDMARD. In these patients, treatment optimisation is a key strategy of high value.
- •
Monitoring and evaluation
▪Screening prior to the initiation of tsDMARDs treatment should be accompanied by periodic clinical-biological monitoring during the course of treatment due to changes in susceptibility to infection.
- •
Research Priorities
▪The Panel considers it essential to promote systematic reviews and structured meta-analyses that provide answers to the research question posed. The current data confirming a higher infection rate according to the comparative scenario are not robust enough for a definitive conclusion.
▪It would be necessary to analyse real-life studies and undertake new meta-analyses and systematic reviews adjusted for duration of disease progression, factors associated with treatment, comorbidity and duration of exposure to biological therapy. There is also a need for post-marketing observational studies, which provide the most data on older patients and provide real-life data on treatment optimisation.
The SR by the literature identified 7 RS/MA,26–32 3 RCTs,33–35 one open-label trial36 and 3 observational studies.37–39 The formulation of the recommendations on bDMARDs has taken into account the great variability of the results assessed and the heterogeneity of the quality of the studies. As it is complex to generalise and draw conclusions as a biological therapy group, the emphasis is above all on good screening prior to the onset of BT and a global assessment of the patient. In the case of tsDMARDs, the Panel's justification is based on the fact that the data supporting the differences found between the different comparisons of tsDMARDs are of low or very low quality, with one exception. Thus, it is difficult to draw robust conclusions.
The Panel considers that the studies identified are consistent in suggesting an increase in severe infections in patients treated with anti-TNF bDMARDs compared to placebo or csDMARDs. This is not the case in the comparison of combination treatments of anti-TNF + non-anti-TNF bDMARDs. With regard to tsDMARDs, the Panel considers that the evidence identified is not consistent and, therefore, shows variability in terms of serious infections attributable to this group of drugs.
Finally, the evidence identified, although from low-quality studies, does not confirm the fact that the use of bDMARDS and tsDMARDs is associated with a high rate of severe infection, and the reduction in the risk of severe infection in patients who undergo treatment optimisation versus those who do not, is low.
In addition, additional evidence from the Oral Surveillance study showing an increased incidence rate of all infections and severe infections has been included with tofacitinib 10 mg twice daily (dose not recommended in RA) versus anti-TNF in patients ≥65 years versus patients aged 50 to <65 years. Independent risk factors for infection in patients treated with tofacitinib included age >50 years, pulmonary comorbidity, smoking history, opioid treatment, and cumulative steroid dose.21
The Panel considers that this data suggests that infection is not only drug-dependent or age-dependent but also dependent on the presence of other comorbidities or the use of concomitant drugs such as glucocorticoids. Thus, the recommendations have taken into account the great variability of the results evaluated and the heterogeneity of the quality of the studies. As it is complex to generalise and draw conclusions as a biological therapy group, the emphasis is, above all, on good screening prior to the onset of BT and a global assessment of the patient. Also, on the fact that the risk of severe infection in frail elderly patients treated with tsDMARDs is higher than in young people, but in Oral Surveillance the incidence was higher with tofacitinib than with anti-TNFs.
A detailed description of the evidence considered and the process that has led from evidence to recommendations can be found in Appendix A. Supplementary data.
Risk of hepatitis B virus reactivationIn patients with RA, what is the risk of hepatitis B virus reactivation from targeted biological or synthetic DMARD treatments?Hepatitis B is an immune-mediated disease caused by the hepatitis B virus (HBV). A cytotoxic and humoral response to infected hepatocytes is produced, triggering cytolysis leading to hepatitis. Therefore, the immune status of the patient who suffers from it is extremely important, since the response to the virus and viral replication depends on this being competent.
HBsAg positivity for more than 6 months is the marker of chronic HBV infection. Chronic hepatitis B is a dynamic process, between virus replication and host immune response. Since it is a non-cytopathic virus, liver injury is caused by the immune response, and the increase in the level of transaminases indicates the presence of inflammation (hepatitis).40
To identify hepatitis B infection, the presence of the HBV core antigen (anti-HBc) and HBV surface antigen (HBsAg) antibody must be determined. In all patients with positive anti-HBc Ac, it should also be determined whether HBsAg is negative, Ac anti-HBs (anti-HBs seroconversion). In patients with chronic hepatitis B, it is important to determine HBeAg and its antibody, anti-HBe, since the HBeAg-positive or negative forms have a different evolution and their treatment may be different and quantify viral DNA by PCR (viral replication marker and only mode of detection of occult infections in patients with positive anti-HBc and negative anti-HBs antigen).
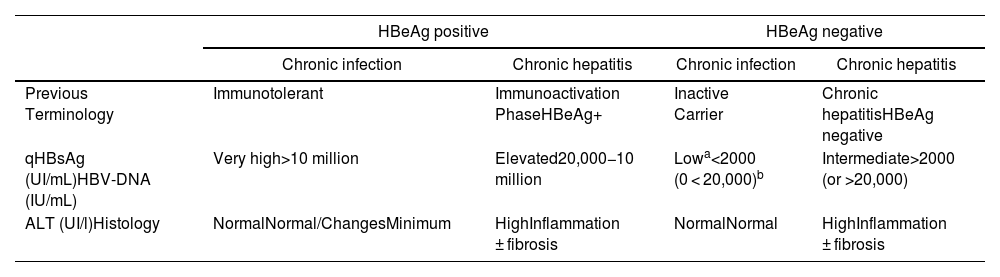
In its natural history, four phases of active infection can be differentiated - not necessarily sequential (Table 3), and a phase of functional healing.
- 1
Phase of chronic HBeAg positive infection (before, immunotolerance). This is characterised by the presence of HBeAg, very elevated HBV-DNA levels, normal ALT values, mild necroinflammatory activity or normal liver on liver biopsy, and slow or no progression of fibrosis. This phase is typical of young people (<40 years) who have acquired the virus by vertical transmission, when the immune system is still immature.
- 2
Chronic hepatitis phase or HBeAg positive immune activity. This is characterised by the presence of HBeAg, a decrease in the level of HBV-DNA compared to those observed in the previous phase, high or fluctuating ALT values, and an increase in histological activity with progression of fibrosis.
- 3
Chronic hepatitis phase HBeAg negative. In some patients with chronic HBV infection, inflammatory activity persists in the absence of HBeAg positivity. This phenomenon is mostly due to infection by viruses that have certain mutations in the genome that prevent infected hepatocytes from producing HBeAg. Chronic HBeAg-negative hepatitis B is very common in the Mediterranean area and the East and is currently the most common form of chronic hepatitis B in our environment.
- 4
Chronic infection phase HBeAg negative (formerly, inactive carrier). It is defined by having HBeAg negative and anti-HBe positive, persistently normal ALT values with low HBV-DNA levels (generally less than 2000 IU/mL). Quantification of HBsAg (qHBsAg) level may be useful to differentiate this phase from patients in the grey area (normal ALT or <1.5 times the upper limit of normal with oscillating HBV-DNA >20,000 IU/mL). qHBsAg levels below 1000 IU/mL are highly suggestive of chronic HBeAg-negative infection. The long-term prognosis of patients in this phase is generally good, although sometimes reactivations of the infection occur with elevations in the level of HBV-DNA and ALT, so it is advisable to monitor these parameters at least once a year.
- 5
Functional healing phase after loss of HBsAg. It is defined by HBsAg-negative with or without anti-HBs, anti-HBc-positive, and is characterised by normal ALT values and usually undetectable serum HBV-DNA levels. Loss of HBsAg in patients without advanced fibrosis is associated with minimal risk of cirrhosis, decompensation, and hepatocellular carcinoma (HCC), and improved survival.
Summary of the main characteristics of the phases of chronic hepatitis virus infection B.40
| HBeAg positive | HBeAg negative | |||
|---|---|---|---|---|
| Chronic infection | Chronic hepatitis | Chronic infection | Chronic hepatitis | |
| Previous Terminology | Immunotolerant | Immunoactivation PhaseHBeAg+ | Inactive Carrier | Chronic hepatitisHBeAg negative |
| qHBsAg (UI/mL)HBV-DNA (IU/mL) | Very high>10 million | Elevated20,000−10 million | Lowa<2000 (0 < 20,000)b | Intermediate>2000 (or >20,000) |
| ALT (UI/l)Histology | NormalNormal/ChangesMinimum | HighInflammation ± fibrosis | NormalNormal | HighInflammation ± fibrosis |
People with chronic infection (HBsAg positive) or previous contact with HBV (HBsAg negative and anti-HBc positive regardless of anti-HBs) who receive immunosuppressive treatments or biological drugs are at risk of reactivation of the infection, the severity of which can range from asymptomatic and transient elevation of transaminases to fulminant hepatitis.
HBV reactivation is defined as an increase in viral replication (usually >1 log of HBV-DNA) in people with detectable viral load or as HBV-DNA positivity in people with undetectable viral load or resolved infection. Host, virus, and type of immunosuppressive therapy factors associated with an increased risk of HBV reactivation have been described. The level of immunosuppression required to produce reactivation in HBsAg-positive patients is lower than in those with HBsAg-negative and anti-HBc-positive.
In situations of immunosuppression, patients with a serological pattern of chronic infection or functional cure may have an elevated viral load in the blood (known as reactivation), with reappearance of surface antigens and elevation of transaminases, which can lead to acute or even acute liver failure.41
Treatment of RA includes immunomodulatory and immunosuppressive drugs (DMARDs) that have changed the prognosis of the disease. However, it is a priority to know what the risk of these treatments is, since the inhibition of certain inflammatory pathways can also lead to reactivation of infections that had previously been stopped by a competent immune system. Cases of HBV reactivation have been observed in patients with RA in relation to the administration of csDMARD,42 or with bDMARD43 or DMARDs. But it is necessary to know what the real risk of activation is with all the drugs used.
Recommendation 8. In patients with RA who are about to start treatment with biological DMARDs or targeted synthetic DMARDs, it is recommended that their serological status of hepatitis B be asked for by determining HBsAg and anti-HBc. (BPC recommendation.)
Recommendation 9. In HBsAg positive cases, patients should be referred to the specialist for evaluation and diagnosis to establish which phase of HBV infection they are in. In HBsAg-negative and anti-HBc-positive cases, the study should be completed by determining HBV-DNA and, if this is detectable, patients should be managed as for the HBsAg-positive group. (BPC recommendation.)
Recommendation 10. In patients with RA with an inactive carrier pattern or functional cure due to HBV, what is suggested is the use of anti-TNF-alpha, IL-6R inhibitors, or JAK inhibitors. (BPC recommendation.)
Recommendation 11. In patients with RA with a pattern of chronic infection or functional cure for HBV, it is suggested that rituximab be avoided and, although the available evidence is insufficient, the development group suggests also avoiding abatacept, if prophylaxis is not desired. (Weak recommendation against.)
Recommendation 12. In those patients with a pattern of chronic infection or functional cure of HBV, in whom treatment with rituximab or abatacept is considered necessary, the developing group suggests the use of HBV reactivation prophylaxis. (BPC recommendation.)
Recommendation 13. In patients with RA with a pattern of chronic infection or functional cure of HBV, treated with targeted biological or targeted synthetic DMARDs, where viral reactivation prophylaxis has not been given, liver enzyme monitoring is necessary every 3 months. (BPC recommendation.)
Recommendation 14. In patients with chronic HBV hepatitis, it is recommended that initiating reactivation prophylaxis be considered (together with the hepatologist) before initiating treatment. (BPC recommendation.)
The SR has identified only observational studies,44–63 many of them retrospective, where most are along the same lines, showing that the risk of HBV reactivation in patients with past infection is very low when treated with anti-TNF, anti-IL-6R and tsDMARDs. The use of this type of drug in the management of RA has amply shown its benefits for the joints, as well as mortality and cardiovascular risk. The recommendation is weak due to the small number of studies and the quality of the available evidence, however it is in favour of treatment with these drugs, as the benefits outweigh the risks of HBV reactivation.
Regarding the use of abatacept and rituximab, although the data are inconclusive due to the types of studies identified (with probable patient selection bias), the mechanism of action of these drugs and the recommendations of the expert groups, together with the availability of other drugs that appear to be safer, makes them unadvisable for use in patients with a history of past HBV infection, if prophylaxis cannot be given. In the case of rituximab, based on the evidence identified, consensus documents and its own criteria, the Panel considers that the risk-benefit ratio advises against its use in these patients unless prophylaxis is previously initiated to avoid HBV reactivation, and that prophylaxis should be extended at least one year after the end of treatment with rituximab.
Finally, the group considers that there is not enough evidence to issue treatment recommendations in patients with chronic HBV infection, although it is specified that the assessment of prophylaxis together with the hepatologist may be beneficial. This could improve patients' chances of treatment.
A detailed description of the evidence considered and the process that has led from evidence to recommendations can be found in Appendix B's Supplementary material.
Risk of herpes zoster reactivationIn patients with RA, what is the risk of varicella-zoster virus reactivation from biological or targeted synthetic DMARD treatments?Herpes zoster (HZ) is caused by the chickenpox-zoster virus, the same virus that causes chickenpox. After having chickenpox, the virus lies dormant in the dorsal ganglia near the spinal cord. The virus is kept under control, however when it undergoes change, the virus can reactivate as HZ or “shingles”, which is characterised by a painful, unilateral vesicular rash, usually in a single dermatome that can spread in cases of profound immunosuppression. HZ causes a great deal of morbidity, including pain, depression, and long-term disability in the form of post-therapeutic neuralgia, pain that continues after the rash has subsided or disappeared.
The incidence rates of HZ in the healthy general population aged 50–59 years are 6.7 cases per 1000 person-years,64 and in the 60−90 year age group, it is approximately 10 cases per 1000 person-years in the unvaccinated group.65 The risk and severity of HZ increases with age and in situations where cellular immunity, responsible for keeping the chickenpox virus under control, such as systemic lupus erythematosus (SLE) and RA, is impaired. In SLE and RA, the incidence rates per 1000 patient-years are about 20 and 13 cases, respectively.66,67
Antiviral medications approved for the treatment of HZ include acyclovir, valacyclovir or famciclovir, which reduce the severity and duration of HZ but may not prevent pain caused by HZ or the development of postherpetic neuralgia, which can persist for years and may be refractory to treatment.68
The increased risk of HZ infection from immune-mediated diseases may be caused by the inflammatory disease itself, as well as by the use of immunosuppressive treatments. This increase has been described in association with the use of corticosteroids, especially in relation to dose and DMARDs, which are related to their mechanism of action. bDMARDs increase the risk of HZ compared to csDMARDs, and tsDMARDs can increase this up to 3 fold.
Recommendation 15. In patients with RA who are going to start treatment with biological DMARDs or targeted synthetic DMARDs, it is recommended that the risk of reactivation of herpes zoster infection be assessed, considering: age, vaccination status, concomitant treatment with steroids and their dose, the presence of other comorbidities, and a history of previous infection. (BPC recommendation.)
Recommendation 16. In patients with RA on treatment with modifier drugs, whether classical, biological or targeted synthetic drugs, it is recommended that administration of the vaccine with attenuated viruses be avoided due to the risk of zoster infection associated with immunosuppression. (BPC recommendation.)
Recommendation 17. In patients with RA who are going to start treatment with biological DMARDs, it is recommended that the need for vaccination be assessed according to the treatment indicated and the presence of other risk factors described above. (BPC recommendation.)
Recommendation 18. In patients with RA who are about to start treatment with targeted synthetic DMARDs, vaccination with recombinant herpes zoster vaccine is recommended. (BPC recommendation.)
The SR has identified a total of 15 studies, including RS29,69,70 RCTs71–75 and observational studies9,76–81 that have focussed on evaluating the reactivation of herpes zoster infection, the spread of the infection to other areas, and the occurrence of serious events such as postherpetic neuralgia. None of the identified studies recorded any cases of postherpetic neuralgia. Additional considerations derived from two other studies have been included.21,82
The evidence is conclusive regarding the frequency of HZ infection in patients treated with tsDMARDs in comparison with the csDMARDs or between them. Additional considerations from the ORAL Surveillance study showed an increased risk of HZ reactivation in patients over 65 years compared to those between 50 and 65 years, in all treatment groups. However, the development group has considered the favourable benefit-risk balance in favour of the use of b/tsDMARDs in patients with RA, since these drugs have great benefits for the patient, without this meaning a high risk of reactivation of the HZ, in most cases and especially now that there is a recombinant vaccine.
The Panel has taken into account safety studies and registries that have identified other factors influencing the risk of HZ reactivation. Age, sex, use of glucocorticoids (SLN), place of origin (Asia vs. Europe) or previous infections are factors that appear to be associated with an increased risk of HZ.66,67,83,84
In general, live attenuated vaccines should be avoided in patients on immunosuppressive therapy, as these vaccines contain live attenuated microorganisms that could theoretically cause infections in the immunocompromised population. Regarding vaccination against HZ, a study in patients with immune-mediated diseases has evaluated the safety of live attenuated vaccine. In patients aged 60 years or older treated with immunosuppressants, including bDMARDs, no increase in the incidence of vaccine-related HZ was demonstrated in the first few days after vaccination.85 Finally, the 2019 update of the EULAR recommendations on vaccination in patients with immune-mediated diseases also includes similar recommendations on HZ vaccination.10
Thus, the development group, based on the evidence, their clinical experience and their own criteria, has issued a recommendation that is in favour of evaluating the possible risks of reactivation of HZ infection and another against the administration of the vaccine with attenuated viruses due to immunosuppression and the fact that a recombinant vaccine already exists. In addition, a PCB recommendation has been issued in favour of recombinant vaccine prior to treatment with bDMARDs, depending on risk factors, and another in favour of vaccine before tsDMARDs in all cases. As there is a recombinant vaccine suitable for everyone, the Panel has ruled out the possibility of not recommending certain treatments in patients at high risk of HZ reactivation.
No data have been found on the effect of recombinant vaccine in reducing the incidence of HZ in RA patients treated with bDMARDs or tsDMARDs.
A detailed description of the evidence considered and the process that has led from evidence to recommendations can be found in Appendix B's Supplementary material.
Risk of developing skin or blood cancerIn patients with RA, what is the risk of skin cancer (melanoma and non-melanoma) or blood cancer from targeted biological or targeted synthetic DMARD treatments?The association between cancer and RA was first described in 1978 when it was identified that RA patients had an elevated risk of lymphoma.86 Since then, multiple studies have been published on the incidence of cancer in RA patients, sometimes with mixed results. A 2015 meta-analysis, which included 23 observational studies, comparing cancer risk in RA patients versus the general population, calculated an overall standardised incidence rate (SIR) for all cancer types of 1.09 (95%CI 1.06–1.13) for patients with RA.87 The overall SIR for lymphoma was 2.46 (95% CI 2.05–2.96), 3.21 (95% CI 2.42–4.27) for Hodgkin lymphoma, and 2.26 (95% CI 1.82–2.81) for non-Hodgkin lymphoma. It was also suggested that there is an increased risk of melanoma in patients with RA (SIR: 1.23; CI 95%: 1.01–1.49), although a more recent analysis of several European registries has not confirmed this association.88
Following the large increase in immunosuppressive drugs available for the treatment of RA, interest has been aroused in the effect that new therapies may have on the development of cancer. There are numerous registries and observational cohorts analysing the relationship between biological therapies and the development of cancer in patients with RA.89–93 The evidence from these observational studies does not show that there is an increased risk of cancer in general, however it is possible that there is a greater risk of specific neoplasms.94 Therefore, a specific assessment of the risk of certain cancers is required in RA patients treated with advanced therapies, including skin cancer and haematological malignancies.
Recommendation 19. In patients with RA who are about to start treatment with biological or targeted synthetic DMARDs, a history of cancer should be taken into account. (BPC recommendation.)
Recommendation 20. In patients with RA and a history of non-melanoma skin cancer, who need treatment with targeted biological or synthetic DMARD, periodic dermatological follow-up is recommended to detect recurrences. (BPC recommendation.)
Recommendation 21. In patients with RA and advanced age, in whom it is considered necessary to initiate treatment with targeted synthetic DMARDs, a periodical medical review is recommended to rule out the development of non-melanoma skin cancer.t (BPC recommendation.)
Recommendation 22. In patients with RA and a history of blood cancer/lymphoma requiring biological therapy, rituximab is preferentially recommended. (BPC recommendation.)
Recommendation 23. There is insufficient evidence to recommend the use of targeted synthetic anti-TNFs or targeted synthetic DMARDs in patients with a history of blood cancer. (BPC recommendation.)
The SR has identified 15 studies including 3 RS82,95,96 one RCT and one extension study6,97 and 12 observational studies37,98–108 with data on the development of melanoma, non-melanoma skin cancer and different types of blood cancer.
Studies with bDMARDs do not show an increased risk of skin or blood cancer in patients receiving biologicals compared to csDMARDs, except for abatacept, which has shown a slightly higher risk of non-melanoma skin cancer in several studies.82,104
Clinical trials with tsDMARDs have not shown an increased risk of developing non-melanoma skin cancer,6,95 although there does seem to be a trend. In the SR by Sepriano et al.82 we did not specifically analyse this outcome, however we did review the safety aspects of the studies included in general, noting that there were a higher number of cases of non-melanoma skin cancer in patients treated with JAKi compared to those treated with placebo or active comparator. The SR by Solipuram et al.95 did not find a significant increase in non-melanoma skin cancer in patients treated with JAKi. In the ORAL Surveillance6 study, tofacitinib did not meet non-inferiority criteria for cancer development compared to anti-TNFs. Subsequent sub-analysis detected a non-significant increase in non-melanoma skin cancer in patients receiving tofacitinib compared with anti-TNFs, when both doses of tofacitinib were compared together and also separately. In addition, this sub-analysis found that 10 lymphomas occurred in the group of 2911 patients who received tofacitinib (both doses) compared to a single case of lymphoma in the 1451 patients who received anti-TNF97. In the real-world STAR-RA study,108 the HR for non-melanoma skin cancer development in patients treated with tofacitinib compared with patients receiving anti-TNF was 1.15 (95% CI 0.96–1.39). This study also evaluated the occurrence of lymphatic and hematopoietic cancers, with the HR for tofacitinib compared to anti-TNF being 0.91 (CI 95%: 0.53–1.58); however, the disadvantage of this study is that the duration of treatment was much shorter than that of the ORAL Surveillance study, so the results were inconclusive.
Regarding the recommendation made for elderly patients, it is true that all DMARDs used in the treatment of RA (including methotrexate) are associated with a small incremental risk of nonmelanoma skin cancer. This recommendation is made only by the Spanish Agency for Medicines communiqué, which the Panel does not agree with, and it has been proposed to the SER that they suggest this be deleted.22
The recommendations issued are weak because the quality of the available evidence was low or moderate. In any case, the evidence supports the use of biological drugs and JAKi for the treatment of RA, as the benefits for disease control and prognosis far outweigh the risk of cancer.
A detailed description of the evidence considered and the process that has led from evidence to recommendations can be found in Appendix B's Supplementary material.
Risk of developing thromboembolic diseaseIn patients with RA, what is the risk of thromboembolic disease from biological or targeted synthetic DMARD treatments?Different epidemiological studies have shown that patients with RA have a 2–3 times higher risk of thromboembolic disease (TED) than the general population, even when statistically adjusted for confounding factors, such as the presence of other cardiovascular risk factors.109 In fact, an interdependence has been established between inflammation and the elevation of certain pro-inflammatory cytokines, such as IL-6 and IL-8, and the activation of coagulation pathways responsible for the thrombotic tendency. In addition, it has been reported that endothelial dysfunction and venous stasis occur in relation to sustained inflammation, which provokes activation of the coagulation cascade.110–112
Given the link with the inflammatory activity of the disease, the use of drugs capable of controlling this reduces the risk of TED but does not completely eliminate it, and TEDs have been described during treatment with different cs/b/tsDMARDs.70,82,113 In RA, in addition to the classic risk factors for TED, such as a sedentary lifestyle, obesity, post-surgical status, the intake of contraceptives or COX2 inhibitors, it should be borne in mind that one of the most important factors is the activity of the disease, so its reduction, regardless of treatment, should be a factor in the prevention of TED.114 It is therefore necessary to analyse the available evidence on the risk of TED associated with the use of targeted biological and synthetic drugs to improve the care of our patients.
Recommendation 24. In patients with RA who are about to start treatment with biological or targeted synthetic DMARDs, it is recommended that a prior evaluation be done to estimate the presence of risk factors for thromboembolic disease. (BPC recommendation.)
Recommendation 25. In patients with RA without risk factors for thromboembolic disease, biological and targeted synthetic DMARDs may be used interchangeably. (Weak recommendation in favour.)
Recommendation 26. In RA patients with elevated risk factors for thromboembolic disease, targeted synthetic DMARDs are suggested with caution.u (BPC recommendation.)
Recommendation 27. In patients with RA who have risk factors for thromboembolic disease in which targeted synthetic DMARDs need to be used, it is suggested that the lowest dose be used and the other risk factors be controlled.v (Weak recommendation in favour.)
The SR has identified 23 studies including 5 SRs70,82,115–117 2 RCTs 118,119 and 16 studies including long-term extension analyses, administrative registries or databases, and other observational,77,79,120–132 that assess the risk of venous thromboembolic events (VTEs), including deep vein thrombosis (DVT) and pulmonary embolism (PE). Studies with bDMARDs are along the same lines, showing a similar risk of thromboembolic event in patients who receive these drugs, versus placebo or csDMARDs. In studies with tsDMARDs, the risk is maintained, although a greater trend is observed, which does not reach statistical significance, in the groups with higher cardiovascular risk and higher doses. However, the Oral Surveillance study in the population over 50 years of age with risk factors for cardiovascular disease has shown an increased incidence of TED from tofacitinib compared to anti-TNF, and especially with high doses of 10 mg twice daily. All TEDs occurred in patients with significant risk factors, especially having had a previous event of these characteristics. Risk factors for PD in the treatment groups included a history of VTE, use of oral contraceptives or hormone replacement therapy, a body mass index ≥ 30 kg/m2, an age ≥ 65 years, and a history of hypertension.129,133 Although this study is limited to tofacitinib until more information is available on the other molecules with a similar mechanism of action, it should not be used in patients at risk of TED, according to the resolution FROM the PRAC, if there are other alternatives.
The recommendations issued are weak or on good clinical practice due to the quality of the available evidence, however they are in favour of the use of these drugs, which can have great benefits for the patient, without posing a high risk of thromboembolic events. Nonetheless, the EG highlights the importance of prior patient evaluations to estimate whether there are risk factors for thromboembolic disease, in which case it is suggested that tsDMARDs should be avoided.
A detailed description of the evidence considered and the process that has led from evidence to recommendations can be found in Appendix B's Supplementary material.
Risk of human papillomavirus progressionIn patients with RA, what is the risk of human papillomavirus progression from biological or targeted synthetic DMARD treatments?There is particular interest in human papillomavirus (HPV) infection, precancerous disease, and cancerous cervical changes in women with RA in relation to the effects of bDMARDs and tsDMARDs on RA and immunosuppressive therapy.
The global prevalence of HPV infection is estimated at 11.7% (95%CI: 11.6–11.7), with considerable regional differences and higher incidence rates in sub-Saharan Africa (24%), Eastern Europe (24%), and Latin America (16%).134 The highest prevalence is in women under 25 years of age. Studies on immune-mediated inflammatory diseases have shown that patients with systemic lupus erythematosus have a higher incidence of HPV compared to RA and scleroderma. In a small cohort of RA patients treated with anti-TNF (for 6 months), the risk of exacerbation and/or progression of HPV135 was not increased.
It is a priority to establish what the risk of bDMARDs and tsDMARDs is, since the inhibition of certain inflammatory pathways can also cause reactivation of infections that had previously been halted by a competent immune system
Recommendation 28. In patients with RA, the HPV vaccination recommendations indicated in the general population should be followed, that is:
- 1
Universal immunisation is recommended for girls, ideally at 12 years of age.
- 2
Vaccination is recommended in adult patients with excisional treatment of the cervix and/or WHIM syndrome (warts, hypogammaglobulinemia, infections and myelocathexis syndrome), as well as in those under 26 years of age in prostitution, those with HIV infection, and men who have sex with men (BPC recommendation).
Recommendation 29. In women with RA, the usual gynaecological check-ups are recommended and, if HPV infection is detected, that extreme precautions be taken, although there is no evidence that any treatment is better than another (BPC recommendation).
The SR has identified 7 observational studies,135–141 most of them prospective based on cohort or registry follow-up, that evaluate the risk of HPV progression in RA patients treated with bDMARDs. No evidence has been identified to assess the impact of treatment with tsDMARDs. The use of these types of drugs in the management of RA has amply demonstrated their benefits.
The Panel considers that there is not enough evidence to issue recommendations for or against some drugs or others, but the recommendations for HPV vaccination indicated for the general population for this type of patient with RA are endorsed.
The HPV vaccine is commonly used in our environment and is currently included in the Spanish vaccination schedule for the general female population,142 in addition to being considered for people of both sexes with certain risk pathologies or some risky sexual practices.142–144 Routine vaccination programmes against HPV in adolescent women, and specifically in those focussed on risk groups of both sexes (men who have sex with men, HIV, prostitution, Whim syndrome and conization) have achieved high effectiveness in reducing the incidence of infections by the genotypes included in the vaccines that cause anogenital warts. precancerous lesions (cervix and other anogenital locations) and cervical cancer, resulting in highly cost-effective treatment.
Systematic HPV vaccination programmes in men have not yet been shown to be sufficiently cost-effective in relation to these results, although they would be possible if we consider other parameters: a lower price for the vaccine and/or broadening the objectives of these programmes (also considering the prevention of other tumours: oropharynx, head and neck), provided that high vaccination coverage (close to 90%) is achieved. Almost half of European countries have incorporated this type of programme since 2020, considering other criteria such as equity, homogeneity or confidence in vaccines, in line with recommendations from the World Health Organisation and the European Union. In Spain, at the time of writing, this type of programme is already in place in three regions (Galicia, Valencia and Catalonia), and, in accordance with these criteria, the Ministry of Health has decided to approve funding in the remainder of the regions from 2023 onwards.145,146
A detailed description of the evidence considered and the process that has led from evidence to recommendations can be found in Appendix B's Supplementary material.
Applicability and usefulnessThis paper on the risk management of treatment with bDMARDs or tsDMARDs in patients with RA has been drafted to provide rheumatologists with recommendations for decisions they frequently face in clinical practice. The authors recognise that the characteristics of the disease and the associated comorbidities will be different in each patient and should have a major influence over clinical decision-making, so that sometimes views different from those proposed in these recommendations may be fully justified.
The last update on the risk management of SER biological treatments was in 2011,8 and included all the biological drugs existing at that time, regardless of their indication. More than 10 years now, a new biological, sarilumab, an inhibitor of the IL-6 receptor, was introduced for the treatment of RA, as was tocilizumab and, above all, four inhibitors of JAKs, small oral molecules included in the new group of DMARDs, have been approved by regulatory agencies which they are the only representatives of for the time being.2
The inclusion of this new family alone would justify updating this document, however in 2021 the FDA issued a safety alert that pointed out a possible increased risk of major cardiovascular events and malignancies in RA patients over 50 years with at least one risk factor for cardiovascular disease, taking part. in an RCT (Oral Surveillance) designed to compare the safety of tofacitinib with anti-TNF147 A year earlier, a similar safety study comparing tocilizumab with etanercept had shown no significant differences between the two drugs,5 so these results had an unprecedented impact on the rheumatology community. The publication of the original study6 and the most important sub-analyses, some of which are still only in abstract form, have made it possible to better identify the real risks and put them in the context of the patient and the disease, and it is imperative that these recommendations are aligned with the recommendations published by the PRAC after analysing all the available evidence.148 Nonetheless, it should not be overlooked that the incidence rates of MACE and cancer with tofacitinib were similar to those observed in controlled trials, registers, and claims databases, but the margin of non-inferiority was exceeded and differences in rates favoured anti-TNFs. This assay has important aspects that have no answers; for example, whether differences in baseline smoking rates between the two groups might be responsible for the differences in cardiovascular events and pulmonary malignancies, or whether both therapies were protective but anti-TNFs were more so; whether or not these effects found in relation to JAK inhibitors is a class effect that can be extended to the whole family, and, finally; whether selectivity or the absence of clear selectivity is important.149,150 It is clear that more comparative studies of JAK inhibitors are needed.
A meta-analysis of three registers and 11 claims databases in the United States, Europe, and Japan was published in 2022 with the aim of evaluating the safety of baricitinib compared to anti-TNFs by comparing patients who started baricitinib (2 mg or 4 mg) versus an anti-TNF. The objectives were to compare the occurrence of venous thromboembolism, major cardiovascular events, and severe infections in patients with RA. The mean duration of treatment was 9 months. The results showed that incidence rates with baricitinib were significantly higher for VTE and numerically higher for MACE and serious infectious episodes, compared to anti-TNFs.151
Although the potential adverse effects associated with treatments used in RA are varied and some are not well understood, in these recommendations the authors have focused on some of the least evidence-based safety events and those most closely related to recently published studies, such as infections, herpes zoster, skin tumours and lymphomas, thromboembolic events, reactivation of hepatitis B virus and human papillomavirus.
Considerations on the desirability of future evidenceAfter the SR on the existing scientific evidence for the risk management of treatment with b/tsDMARDs undertaken for the drafting of these recommendations, the Panel considers that there are aspects such as the following that should be taken into account when included in future research:
- •
Observational studies already mentioned above, and others that are underway and published with very short follow-up periods,108,152 will provide very relevant information when these follow-ups are large enough to be able to evaluate the occurrence of side effects that require follow-up time, such as cardiovascular events and neoplasms.
- •
The two clinical trials, RA-BRANCH (NCT04086745) and RA-BRIDGE (NCT03915964), which aim to compare baricitinib and anti-TNF in patients with at least one risk factor for VTE and with insufficient response to bDMARDs or tsDMARDs, will provide very relevant information to confirm or not the safety events described.
Since the introduction of the first biological drug to treat RA, there has been a rapid expansion of advanced therapies licensed for its treatment, culminating in the arrival of a new family of drugs which are JAK inhibitors. While this is clearly beneficial for people with RA, it presents additional challenges for clinicians in selecting the best therapy for these individuals. These recommendations aim to provide practical support to rheumatology physicians to support prescribing bDMARDs and tsDMARDs in RA.
Ethical responsibilitiesProtection of people and animals. The authors state that no experiments have been conducted on humans or animals for this research.
Data Confidentiality. The authors state that they have followed their workplace protocols regarding the publication of patient data.
Right to privacy and informed consent. The authors state that no patient data appear in this article.
FundingSpanish Rheumatology Foundation.
AuthorshipThe authors have made substantial contributions based on: 1) the conception and design of the study and the analysis of the data; the draft of the article or the critical review of the intellectual content, as well as the final approval of the version that is presented, and 2) the review of the evidence and the preparation of the report on the systematic review.
Conflict of interestAlejandro Balsa received funding from AbbVie, Nordic, Novartis and Pfizer to attend courses/conferences; fees from AbbVie, BMS, Galapagos, Lilly, Nordic, Novartis, Pfizer, Sandoz, Sanofi and UCB for presentations; funding from AbbVie, BMS, Lilly, Nordic, Novartis, and Pfizer for educational programmes or courses; funding from AbbVie, BMS, Nordic, Novartis, Pfizer and UCB for participating in research; fees from AbbVie, BMS, Galapagos, Gilead, Nordic, Novartis, Pfizer, Roche, Sandoz and Sanofi for consulting services for pharmaceutical companies or other technologies; financial support from AbbVie, BMS and Pfizer for research funding and funding from BMS, Novartis, Pfizer, Sandoz, Sanofi and UCB for educational programmes or courses for the unit.
Petra Díaz del Campo Fontecha has declared that she has no interests in this.
José María Aguado García has declared that he has no interests in this recommendations document.
Rafael Cáliz Cáliz has received funding from AbbVie, BMJ, MSD and Novartis for attendance at courses/conferences and for presentations; significant provision of equipment to the AbbVie service unit, and financial support from AbbVie, BMJ and MSD for the funding of research.
Héctor Corominas has received funding from AbbVie, Lilly and Pfizer to attend courses/conferences; fees from AbbVie, Lilly, Gebro and Pfizer for presentations; funding from Amgen, Jansen, Lilly, and Sandoz for educational programmes or courses; funding from Gebro, MSD and Sanofi for participating in research; fees from AbbVie, Amgen, MSD and Sanofi for consultancies for pharmaceutical companies or other technologies, and funding or financial support from AbbVie, Amgen, BMS, Gebro, GSK, Jansen, Kern, Lilly, MSD, Nordic, Pfizer, Roche and UCB for the creation of the unit or service.
M. Vanesa Hernández Hernández has received funding from AbbVie, Gebro, Jansen, Lilly, Novartis, Roche, Pfizer and UCB for attendance at courses/conferences; fees from AbbVie, BMS, Gebro, Grunenthal, Jansen, Lilly, Novartis, Roche, Sanofi and UCB for presentations, and funding from Novartis for research studies.
Fernando León Vázquez has received funding from Astra Zeneca and MSD for attendance at courses/conferences and fees from Astra Zeneca, MSD, Novartis and Roche for presentations.
Virginia Nistal Martínez has declared that she has no interests in relation to this recommendations document.
José Valencia Martín has declared that he has no interests in relation to this recommendations document.
Lucía Silva Fernández has received funding from BMS, Galapagos, Lilly, Novartis and Pfizer to attend courses/conferences; fees from AbbVie, Amgen, Janssen, Lilly and Novartis for presentations; fees from MSD, Novartis, and Sanofi for consulting services for pharmaceutical companies or other technologies, and funding from BMS and Novartis to participate in research.
Gloria Candelas Rodríguez has declared that she has no interests in relation to this recommendations document.
Nora Ibargoyen Roteta has declared that she has no interests in relation to this recommendations document.
Arturo Martí Carbajal has declared that he has no interests in this recommendations document.
M. Nieves Plana Farras has stated that he has no interests in relation to this recommendations document.
Janet Puñal Riobóo has stated that she has no interests in relation to this recommendations document.
Hye Sang Park has received funding from Galapagos, Lilly, Novartis and Pfizer for attendance at courses/conferences and fees from AbbVie, Amgen, Galapagos, Janssen, Lilly, Novartis and Pfizer for presentations.
Yolanda Triñanes Pego has declared that she has no interests in these recommendations document.
Virginia Villaverde García has received funding from Lilly, Novartis, Pfizer and UCB to attend courses/conferences and fees from AbbVie, Janssen, Lilly and Novartis for presentations.
The group of experts in this study would like to express their gratitude to Mercedes Guerra Rodríguez, documentalist of the Spanish Society of Rheumatology (SER), for her collaboration in the strategies for evidence searches. In addition, they expressly thank Dr Marta Abadía Barnó (hepatologist), Dr José Luis Andreu Sánchez (rheumatologist) and Dr Julián Torres Cisneros (infectious disease specialist) as expert reviewers of the document, for their critical review and contributions to it.
We would also like to thank Dr Federico Diaz González, director of the SER Research Unit, for his participation in the review of the final manuscript and for helping to preserve the independence of this document.
Recommendations group.
Evidence reviewers group.
The European Medicines Agency PRAC, based on the results of the Oral Surveillance study and the data presented by the other 3 JAK inhibitors on the European market, has issued recommendations to minimize the risk of significant side effects associated with the use of this group of drugs used in the treatment of several chronic diseases. Side effects include cardiovascular events, thrombosis, neoplasms, and infections.
This recommendation is based on the statement from the Spanish Medicines Agency issued after the recommendations of the PRAC. Although the statement expressly refers to dermatologists, the development group believes that this is not operational and that it would overload dermatology impossibly. Since this lesion is easily identifiable, both primary care physicians and specialists will be able to refer to the dermatologist all lesions that they consider should be suggested.
This recommendation is derived from the results of the Oral Surveillance study. Although this study is limited to tofacitinib, until more information is available on the other molecules with a similar mechanism of action in patients at risk of TED, according to the resolution of the PRAC, they should not be used if there are other alternatives.
There is insufficient evidence to add prophylactic anticoagulation in these cases, unless indicated by the existence of other cardiovascular risk factors.
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Léalo en español
- Download PDF
- Bibliography
- Additional material