Psoriatic arthritis is a chronic systemic inflammatory disease that affects the skin, musculoskeletal structures and other organs and systems compromising functionality, quality of life and reducing the life expectancy of patients. It is a complex disease that requires specialist and timely care and management. The alternatives for treating the manifestations of psoriatic arthritis have increased and the effect of the different agents on specific manifestations has been clarified in recent studies. Therefore, we should incorporate the available evidence to build a strategy for the treatment of these patients. The Mexican College of Rheumatology selected a committee to evaluate these different alternatives and make recommendations.
MethodsThe study group included 16 rheumatologists and 3 certified dermatologists, selected from different health institutions and regions of the country. An executive committee was formed to coordinate the meetings and a committee of experts selected the literature search criteria, prepared the research questions, rated the quality of the evidence, and produced the recommendations in the different disease domains based on the GRADE methodology.
Results24 updated recommendations were generated for the treatment of patients with psoriatic arthritis. The recommendations establish the role of the drugs currently available in our country. The importance of adequate disease control is emphasized, individualizing the level of involvement of each patient in each of the six domains potentially affected by the disease. In addition, the sequence in the choice of treatments available for each domain is established, based on their efficacy, safety profile and accessibility.
ConclusionsWith this consensus document, it will be possible to improve the care of patients with psoriatic arthritis. The recommendations were generated based on the best available information and in consideration of the Mexican health system.
La artritis psoriásica es una enfermedad inflamatoria sistémica crónica que afecta a la piel, las estructuras musculoesqueléticas y afecta otros órganos y sistemas comprometiendo la funcionalidad, la calidad de vida y reduciendo la expectativa de vida de los pacientes. Es una enfermedad compleja que requiere atención y manejo especializado y oportuno. Las alternativas para el tratamiento de las manifestaciones de la artritis psoriásica se han incrementado y, adicionalmente, el efecto de los distintos agentes sobre manifestaciones específicas ha sido aclarado en estudios recientes, por lo tanto, es conveniente incorporar la evidencia disponible para construir una estrategia en el tratamiento de estos pacientes. El Colegio Mexicano de Reumatología seleccionó una comisión para evaluar estas distintas alternativas y generar recomendaciones.
MétodosEl grupo de estudio incluyó a 16 reumatólogos y 3 dermatólogos certificados, que fueron seleccionados de diferentes instituciones de salud y distintas regiones del país. Se conformó un comité ejecutivo que coordinó las reuniones y un comité de expertos que seleccionó los criterios de búsqueda en la literatura, elaboró las preguntas de investigación, calificó la calidad de la evidencia y generó las recomendaciones en los distintos dominios de la enfermedad con base en la metodología GRADE.
ResultadosSe generaron 24 recomendaciones actualizadas para el tratamiento de pacientes con artritis psoriásica. Las recomendaciones establecen el papel de los medicamentos disponibles actualmente en nuestro país. Se enfatiza la importancia del control adecuado de la enfermedad, individualizando el perfil de involucramiento de cada paciente en cada uno de los seis dominios potencialmente afectados por la enfermedad. Además, se establece la secuencia en la elección de los tratamientos disponibles para cada dominio, basada en su eficacia, perfil de seguridad y accesibilidad.
ConclusionesCon este documento de consenso se podrá mejorar la atención de los pacientes con artritis psoriásica. Las recomendaciones se generaron con base en la mejor información disponible y en consideración del sistema de salud de México.
Psoriatic arthritis (PsA) is a severe, heterogeneous, systemic rheumatic disease characterised by inflammation of the joints, periarticular structures and skin and its appendages, manifested as psoriasis. Musculoskeletal involvement may involve different domains including peripheral arthritis, axial involvement, enthesitis and dactylitis1. Dermatological involvement includes the skin and nails.
The heterogeneity of PsA means treatment must be personalised considering each patient’s involvement in each domain: peripheral arthritis, axial involvement, enthesitis, dactylitis, skin and nails. This individualisation is now more important because the efficacy of the different treatments is heterogeneous between domains, therefore, treatment may be suboptimal if it does not consider the profile of the patient's condition.
PsA affects other systems in addition to the skin and joints. The disease, especially in the active stages, induces a state of systemic imbalance, characterised by metabolic syndrome and a significant increase in cardiovascular morbidity. Therefore, appropriate treatment of the inflammatory process becomes a strategy to minimise the impact of the disease.
Treatment of PsA usually starts with non-steroidal anti-inflammatory drugs (NSAIDs) and also includes conventional synthetic disease-modifying drugs (csDMARDs), target-specific disease-modifying drugs (tsDMARDs) including Janus kinase (tofacitinib) and phosphodiesterase-4 (apremilast) inhibitors, as well as biological disease-modifying drugs (bDMARDs), including tumour necrosis factor antagonist agents (TNF-I), interleukin (IL) 12/23 antagonist (ustekinumab), IL-17 (secukinumab and ixekizumab), and IL-23 (guselkumab), and abatacept (CTLA-4 fusion protein), which is a T-cell co-stimulation inhibitor.
These broadened therapeutic options offer patients new possibilities in the control of their disease, but also force us to rethink strategies in selecting the different treatments, assessing the efficacy of each of the agents for specific manifestations of PsA and, where possible, establishing an order in prescribing them. These recommendations aim to optimise the treatment of patients with PsA based on the available scientific evidence and using expert criteria in their management.
The recommendations consider the PsA disease domains proposed by the GRAPPA2 group as a starting point for defining the usefulness of different therapeutic agents. These recommendations are endorsed by the Mexican College of Rheumatology.
MethodsExecutive committee: An executive committee (EC) was formed that comprised the principal investigators, a project coordinator and two members.
The functions of the EC included the planning and execution of the project, the selection of the members of the expert group, the scheduling and logistics of the meetings and the selection of the personnel in charge of the literature review, and its methodology.
Expert group. A group was formed of 16 rheumatologists and three dermatologists with knowledge and experience in the treatment of patients with PsA and with experience in therapeutic recommendations and guidelines. The functions of the expert group were to select the themes and subthemes in relation to the definition and classification of PsA, to develop the research questions used in the systematic literature review (SLR) considering the six disease domains of PsA, to choose the primary and secondary outcome measures, to prepare the work agendas and, finally, to draft the recommendations.
Systemic literature review. As a prerequisite to the consensus session, two independent investigators conducted an SLR on treatments for PsA. The full methodological description of the SLR is presented in Appendix B, Supplementary data.
The SLR included research questions broken down by disease domain: peripheral arthritis, axial disease, dactylitis, enthesitis, skin psoriasis and nail psoriasis (Ps). For each domain, the following questions were asked: inflammatory activity, structural impairment, improvement, partial remission, inactive disease, moderate activity, high activity, very high activity, significant clinical improvement, very significant clinical improvement, quality of life and health status. It was complemented by the safety and efficacy of pharmacological, non-pharmacological and surgical treatments considered in the SLR. The SLR included articles published in English and Spanish.
The Patient or problem, Intervention, Comparison, Outcomes (PICO) question was used to establish the search criteria, with the following criteria:
Patients. Patients with a diagnosis of PsA with disease onset ≥18 years of age.
Intervention. Pharmacological therapy (bDMARDs, csDMARDs, NSAIDs, glucocorticoids, other), non-pharmacological therapy (exercise and information programmes) and surgical intervention.
Comparison. The different interventions mentioned above were evaluated in various regimens or doses, compared to another intervention, placebo, or none.
Outcomes. The most used outcome measures were used in each of the domains analysed.
In peripheral arthritis, the American College of Rheumatology improvement response criteria (ACR) indices for 20%, 50% and 70% improvement (ACR20, ACR50, ACR70) and the Disease Activity Score for 28 Joints (DAS-28) were used. In axial disease, the Ankylosing Spondylitis Disease Activity Score (ASDAS) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI), the improvement criteria ASAS 20, ASAS 40, ASAS 5/6, ASAS partial remission (ASAS-PR), pain, C-reactive protein (CRP), as well as the radiographic indices Bath Ankylosing Spondylitis Radiology Index (BASRI), modified Stoke Ankylosing Spondylitis Spine Score (mSASS) and Radiographic AS Spinal Score (RASSS) and MRI-based indices (Leeds, Berlin, Spondyloarthritis Research Consortium of Canada [SPARCC Scoring system]) were used.
The outcome measures considered for enthesitis were change from baseline in the enthesitis score, the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), the Leeds Enthesitis Index (LEI), the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC) and the resolution rate of enthesitis cases.
The Leeds Dactylitis Index-Basic (LDI-B), the resolution rate of dactylitis cases, and change from baseline in the Dactylitis Severity Score (DSS) were considered for dactylitis.
The Psoriasis Area Severity Index (PASI) with 50%, 75%, 90% and 100% improvement (PASI50/75/90/90/100), Body Surface Area (BSA) and the Physician's Global Assessment (PGA) response indicator were considered as outcome measures) were used for psoriasis.
Studies reporting the Nail Psoriasis Severity Index (NAPSI) and the modified Nail Psoriasis Severity Index (mNAPSI) were analysed for nail Ps.
The frequency of serious adverse events, infection, serious infection, malignancy, and discontinuation of treatment due to adverse events were evaluated to assess the safety of the treatments (Appendix B, Supplementary data).
The SLR covered the period from January 2016 to June 2020, and pivotal studies of drugs with an indication in PsA were reviewed if they had been previously published. Eligible studies were meta-analyses, systematic reviews, randomised controlled clinical trials, controlled clinical trials and extension clinical trials. The search strategies were replicated in the PubMed, EMBASE, ScienceDirect and CINAHL databases, considering the characteristics and limitations of each information system.
The level of evidence of the articles was rated with the GRADE scale, according to the risk of bias, consistency, direct results, heterogeneity, precision, and publication bias.
Working meetingsThe first working session was planned for the experts to present the main characteristics and definitions in the management of PsA to the full committee and to discuss the characteristics of the health system and the therapeutic options available in Mexico. This discussion guided the search for information for the SLR and specific research questions were considered for each disease domain.
The recommendations were adapted to the context of the national health system and added to with evidence from the SLR and the experts’ clinical experience.
Preliminary meetingDuring the process of drafting this consensus, some factors that potentially limit patients' access to comprehensive care were discussed. In addition, the list of themes included in each of the medical literature searches was selected.
Consensus meetingAt the second meeting, the papers reviewed in the SLR were presented, emphasising the efficacy and safety results of each study. In addition, the recommendations, and guidelines for the treatment of PsA (GRAPPA, EULAR and SER) were reviewed in detail to gain an overview of contemporary therapeutic concepts.
The evidence from the SLR was analysed in a structured way and the consensus coordinator presented the associated themes and subthemes to the committee. The proposals for each recommendation were generated from an internal discussion that took each of the disease domains as its structure. The experts gave their opinion on each proposed recommendation, and in cases of disagreement, specific aspects of the SLR were reviewed in more detail.
Prevalence of PsAThe prevalence of PsA or Ps in the Mexican population is unknown. The prevalence of psoriasis varies in different ethnicities and geographical areas. A prevalence of 2.8% has been reported in the Faroe Islands, 1.4% in Sweden and it was 1.3% in the U.S. Black population and 3.15% in the general population3. The prevalence of PsA ranges from one case (in Japan) to 420 cases (in Italy) per 100,000 population (with a mean of 180 cases per 100,000 population) and it is estimated that about 30% of patients with psoriasis develop some form of arthritis4.
A study conducted in Argentina, which examined records of 138,288 private health insurance clients from 2000 to 2006, estimated a prevalence of 74 cases per 100,000 population (95% CI, 57–94)5.
Analysis broken down by geographical area of patients with psoriasis indicates that the prevalence of PsA in Europeans was 22.7% (95% CI, 20.6%–25.0%), in South Americans 21.5% (95% CI, 15.4%–28.2%), in North Americans 19.5% (95% CI, 17.1%–22.1%), in Africans 15.5% (95% CI, .009%–51.5%) and in Asians 14.0% (95% CI, 11.7%–16.3%)6. Hence the importance of conducting epidemiological studies in our country’s population to define the real impact of the disease more accurately.
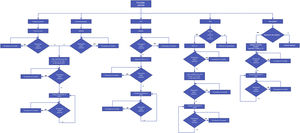
Therapeutic aspects and recommendationsAlgorithms for the treatment of the musculoskeletal manifestations of PsA recommend starting treatment with non-steroidal anti-inflammatory drugs (NSAIDs)7. If there is no response or toxicity, a change to methotrexate (MTX), leflunomide (LEF) or sulfasalazine (SSZ) is recommended (csDMARDs), although efficacy varies and patients with axial disease have poor response rates. Fig. 1 shows the treatment algorithm proposed in this consensus.
Treatment algorithm for psoriatic arthritis.
Anti-TNF; bDMARDs: biological disease-modifying drugs; IL-12/23, IL-17: tumour necrosis factor inhibitors; interleukins 12,23, or 17; LEF: leflunomide; MTX: methotrexate; NSAIDs: non-steroidal anti-inflammatory drugs; PDE4: apremilast; Tofa: tofacitinib.
Each patient’s pattern of involvement in each disease domain must be considered in designing the therapeutic strategy.
In resistant cases of PsA bDMARDs are necessary8–10. The GRAPPA guidelines do not clearly indicate a preference for the use of bDMARDs as first-line therapy, while the EULAR guidelines do consider TNF-I agents as an initial line of therapy for patients who fail with csDMARDs and with predominantly peripheral or early-stage axial disease8,9,11. Not all bDMARD therapy is successful; some patients fail to achieve or maintain a satisfactory status for a variety of reasons. We discuss the different treatments available for PsA below.
csDMARDsMethotrexateMTX is one of the first options for use in patients who have not responded to NSAID therapy. There is low quality evidence that MTX at a weekly dose of 15 mg is superior to placebo and its use is indicated for skin and peripheral arthritis. Furthermore, the effect of MTX on radiographic progression, axial involvement, enthesitis, dactylitis, nails, fatigue or quality of life has not been defined12.
LeflunomideThe drug is administered in daily doses of 20 mg. Efficacy studies of LEF in PsA are not of good quality, either because they have been conducted in a small population13 or because they are observational14,15, although the results obtained are similar to those with MTX. Regarding joints, a slightly higher ACR70 rate is reported with LEF but with less efficacy in the control of psoriasis14. The safety profile is similar to that of MTX and there are no studies that evaluate the combination of the two. LEF has not been demonstrated as useful in the other domains.
SulphasalazineUsed in daily doses of up to 2−3 g. The drug’s use is limited because its only affects peripheral arthritis and does not improve skin condition, and it has not been shown to be useful in the axial or other domains. Like MTX and LEF, the evidence of efficacy of SSZ in the treatment of PsA is of low quality and it is considered inferior to MTX16–21.
TacrolimusTacrolimus is an immunosuppressive drug of the calcineurin inhibitor family, which has been used in the treatment of relapses or resistance in various rheumatic conditions. The available evidence is of low quality, and therefore we cannot make a recommendation based on its efficacy results. The drug can be used after bDMARDs have failed22.
Biological disease-modifying drugsbDMARDsBiological therapy in PsA has recently increased with a variety of therapeutic targets. The number of new agents with these targets has also diversified treatment possibilities, improving the outlook for patients with refractory disease.
The first group of bDMARDs used in PsA includes tumour necrosis factor (TNF-I) antagonists, as well as IL-12/23 (ustekinumab)23–25, IL-17 (secukinumab and ixekizumab)26,27, IL-23 (guselkumab)28,29, and abatacept, which blocks T-cell co-stimulation.
TNF-IThe TNF-I group includes five drugs: etanercept, infliximab, adalimumab, certolizumab pegol, and golimumab. All are indicated for the treatment of PsA in Mexico. There is evidence from several controlled clinical trials of their efficacy in the treatment of Ps and PsA.
Etanercept showed that 59% of patients with PsA achieved an ACR20 response at 12 weeks and 47% achieved a PASI50 at week 2430. In the IMPACT study infliximab was shown to achieve an ACR20 in 65% of patients at 16 weeks, and a PASI50 in 80% at week 5031. In the ADEPT study, adalimumab achieved an ACR20 in 58% of patients at week 20 and a PASI75 in 59% at week 2432. In the RAPID-PsA study, certolizumab achieved an ACR20 score in 58% of patients at week 12 and a PASI75 of 62.2% at week 2433. Finally, golimumab in its GO-REVEAL study achieved an ACR20 in 51% of patients at week 14 and a PASI75 of 58% at 52 weeks34.
The efficacy of the TNF-I agents has been demonstrated for each of the disease domains identified by GRAPPA (peripheral arthritis, axial involvement, skin, nails, dactylitis and enthesitis). Reduction in radiographic progression in peripheral joints, as measured by the modified Sharp index, was also demonstrated. The drugs’ role in the prevention of structural alterations at the axial level has not been defined. Comparison of the different TNF inhibitors has shown very similar efficacy and safety profiles. In fact, it has been demonstrated that, if any fail, substitution with another agent of the same group can benefit the patient, although the tendency in the event of loss of efficacy is to change the mechanism of action.
IL-12 and IL-23 inhibitorsUstekinumab inhibits the p40 receptor which is shared by IL-12 and IL-23 and therefore antagonises both. It has been widely used in patients with PsA, has been shown to be useful in skin and nails, and is also effective in peripheral arthritis, enthesitis and dactylitis, but its efficacy in the axial component of the disease is uncertain as it has not shown sufficient effect in patients with axial spondyloarthritis. The PSUMMIT trials 1 and 2 demonstrated that ustekinumab at doses of 45 mg and 90 mg at 24 and 52 weeks is effective in the treatment of PsA in patients who fail with DMARDs and TNF-I23–25. In the PSUMMIT-1 trial in patients who failed with DMARDs and NSAIDs, ustekinumab showed an ACR20 response at week 24 in 42.4% (45 mg) and 49.5% (90 mg) of patients vs. 22.8% of patients on placebo (P < .0001), ACR50 response in 24.9% (45 mg) and 27.9% (90 mg) vs. 8.7% on placebo, and ACR70 response in 12.2% (45 mg), and 14.2% (90 mg) vs. 2.4% on placebo (P < .0001). In terms of secondary endpoint, there was improvement in dactylitis (P = .0003) and enthesitis (45 mg and 90 mg) P = 0019 and P < .0001, respectively, vs. placebo. A total of 69.1% achieved a PASI75 at 52 weeks with both doses23.
Specific IL-23 antagonist drugs have been developed more recently. There are published studies of guselkumab, risankizumab and tildrakizumab. Of the three, only guselkumab is indicated for Ps and PsA in Mexico. In the Discover-1 trial, using two dosing regimens of guselkumab, 100 mg every four weeks or 100 mg every eight weeks, after two initial loading doses (weeks 0 and 4), the every-four-week group achieved an ACR 20 at week 24 in 59% and the every-eight-week group in 52 vs. 22% in the placebo group. ACR 50 was achieved in 36% (every four weeks) and 30% (every eight weeks) vs. 9% in the placebo group, and ACR70 in 20% (every four weeks), 12% (every eight weeks) and 6% of the placebo group. PASI100 response was 45% (every four weeks), 26% (every eight weeks) vs. 6% in the placebo group. Guselkumab had a favourable effect on enthesitis reaching statistical significance at the every-four-week dose and although resolution of dactylitis was superior to placebo in both guselkumab groups, the difference was not statistically significant. While the available data on guselkumab show promising results because it has only been included in our country very recently, none of the members of this panel have experience with it and at this stage we do not consider it appropriate to include it as an option in this version of our recommendations.
IL-17 inhibitorsTwo members of this therapeutic class are currently available in Mexico, secukinumab and ixekizumab.
Secukinumab showed a superior effect to placebo at 24 weeks, achieving an ACR20 in 54% of patients at 300 mg SC, and in 51% of patients at 150 mg SC vs. 15% of the placebo group. The PASI75 response was 63% and 48% for skin, respectively, vs. 16% in the group treated with placebo. Initial doses were weekly for the first four applications and monthly from the fifth application onwards. Resolution of dactylitis was 47% vs. 15% in the placebo group and resolution of enthesitis was 40% vs. 22% in the placebo group26. At two-year follow-up, the patients treated with secukinumab had minimal disease activity, as defined by PASDAS, in 36.1% and 35.1% at the doses of 300 mg and 150 mg, respectively35.
Ixekizumab (IXE) at a dose of 80 mg every two weeks or every four weeks in bDMARD-naïve patients with PsA (SPIRIT-P1) showed superiority to placebo in response with ACR20 (62.1% biweekly IXE, 57.9% monthly IXE and 30.2% in the placebo group [P ≤ .001], and a PASI75 in 79.7% with biweekly IXE, 71.2% with IXE every four weeks and 10.4% in the placebo group) (P ≤ .001) at 24 weeks36, this improvement was maintained until week 5237. Significant improvements in skin symptoms, health-related quality of life and productivity at work were also identified38. In the SPIRIT-P2 trial in patients with TNF-I-resistant PsA, a difference in ACR20 (48% IXE fortnightly, 53% IXE every four weeks, compared with 20% in the placebo group) (P < .0001) was achieved at week 2439, as well as a significantly higher response than placebo in minimal disease activity (MDA), Disease Activity Index for PsA (DAPSA) and DAS28-PCR in patients treated with IXE40. Combining the data from the SPIRIT-P1 and P2 trials, treatment with IXE resulted in significant improvement in patients with pre-existing enthesitis and dactylitis41,42.
AbataceptAbatacept has proved useful in the management of peripheral arthritis. Its usefulness in cutaneous manifestations, nail manifestations or axial component, dactylitis or enthesitis is inferior to the other bDMARD alternatives, therefore it is recommended primarily for patients whose predominant musculoskeletal manifestation is peripheral arthritis and in those whose Ps is under control43,44.
Targeted synthetic disease-modifying antirheumatic drugsTofacitinibIn addition to biological therapy, inhibition of Janus kinases by agents such as tofacitinib (JAK-i) has also been shown to be effective in several disease domains. Tofacitinib is currently indicated for the treatment of PsA and has proved effective in skin and musculoskeletal disorders. In the OPAL study45, tofacitinib achieved an ACR20 of 68% and a PASI75 of 56% at 12 months. The same study showed improvement in dactylitis and enthesitis as well as other domains such as fatigue and physical function. The twice daily dose of 10 mg was superior to the twice daily dose of 5 mg. Tofacitinib is also useful in patients with PsA who have failed with TNF-I, the approved dose is 5 mg every 12 h46.
There are currently active clinical trials with other Janus kinase inhibitors (upadacitinib and filgotinib). However, these drugs are not yet available in Spain.
ApremilastApremilast is a tsDMARD, phosphodiesterase-4 inhibitor, used in doses of up to 60 mg per day. It has an acceptable safety profile, hence its use in PsA patients with several comorbidities. The results of apremilast therapy can be seen after four months. Combination with biological drugs is not recommended47–49. The PALACE studies consist of three phases, with reports at week 16, 28, and 52, and a four-year extension phase (open phase), in a series of three publications they reported the results of studies with the same design: randomised, placebo-controlled clinical trials to demonstrate the efficacy of apremilast at doses of 20 and 30 mg orally twice daily in 1493 patients with active PsA and a history of failure with tsDMARDs.
At week 260, 67.2% of patients had achieved ACR 20 response, 44.4% and 27.4% ACR 50 and ACR 70 response, respectively. For enthesitis and dactylitis, 62.4% had a MASES index of 0 and 80.9% had a dactylitis count of 0. Skin response in patients with more than 3% involvement of body surface area recorded a PASI75 response in 43.6% of cases.
Restrictions in the use of bDMARDs and JAK-iA screening process is a prerequisite before initiating biological or JAK-i therapy. This process includes screening for active or latent tuberculosiss, either by Quantiferon or purified protein derivative (PPD). Each of these two tests must be complemented by chest teleradiography. Screening also includes a complete blood count, liver function tests, hepatitis serology and human immunodeficiency virus (HIV) testing.
As a rule, biological or JAK-i therapy is contraindicated in patients with active infections, about to undergo elective surgery in the very short term, or with serious systemic diseases that are out of control (metabolic, cancer, chronic infections). These drugs have relative contraindications in patients with increased risk factors for infections (advanced age, cytopenia, history of recurrent infections) and have specific contraindications depending on their mechanism of action.
TNF-I should be used with caution in patients with risk factors for developing TB and their use in patients with latent TB requires a period of prophylactic treatment. Their use is also restricted in patients with congestive heart failure or demyelinating diseases. The association of TNF-I with skin cancer has been of interest, as their use is contraindicated in patients with psoriasis undergoing ultraviolet light treatment.
A history of inflammatory bowel disease or recurrent uveitis is a relative contraindication for the use of I-IL-17 agents. Contraindications for JAK-i include a history of disseminated herpes zoster or multidermatoma, and patients at increased risk of thrombosis50,51. Ustekinumab and abatacept have no specific contraindications.
Results of the systematic literature reviewThrough the SLR we found information on PsA published from January 2016 to June 2020. Its level of evidence was graded, and the questions posed were answered and recommendations were generated from this analysis.
RecommendationsGeneral aspects of treatment:
In the opinion of the expert group, the following were defined as general principles for the treatment of patients with PsA:
- 1
Treatment should be defined by mutual agreement between the doctor and the patient, after providing the patient with the information for them to make their own judgement.
- 2
Treatment should be started from the earliest stages of the disease.
- 3
Treatment should be provided by the specialist physician, the rheumatologist, for musculoskeletal manifestations and it is advisable for the dermatologist to be involved in the management of skin disorders in severe cases from the initial diagnosis, or in those refractory to treatment at any stage.
- 4
Treatment is aimed at reducing the symptoms of the disease in the affected domains, improving the patient's functionality, preventing structural damage and loss of function. The objective should be clinical remission or, if this is not possible, a low degree of disease activity. There are different validated instruments for each disease domain, however, the concept of the therapeutic goal achieved did not reach unanimous consensus. The definition of improvement in each domain should preferably be based on the use of validated instruments.
- 5
Treatment should be tailored to the patient, the domains that the disease affects in each patient need to be identified, the domain that most impacts the patient's wellbeing should be selected, and the drugs that achieve control should be selected based on this.
- 6
The patient should be re-assessed periodically to achieve closer control of their disease activity.
- 7
Treatment includes the detection and appropriate management of comorbidities.
Recommendation 1. NSAIDs are recommended to improve the symptoms of peripheral arthritis, using full doses, without combination, intermittently and with due precaution for possible adverse events.
Quality of evidence: Low. Strength of Recommendation: Weak.
Recommendation 2. In patients with no previous use of csDMARDs, it is highly recommended to initiate treatment with drugs in this group, such as MTX12, LEF14, or SSZ16–21. There is no evidence of efficacy to support the use of cyclosporine (CyA) for the treatment of PsA and caution is recommended due to its toxicity profile.
Quality of evidence: High. Strength of recommendation: Strong.
Recommendation 3. In the event of failure with csDMARDs, the use of bDMARDs should be considered. During the review period, studies were identified that reinforce this recommendation for TNF-I33,52–57, IL-12/2323–25, I-IL-1726,27. If the response to the drugs in this first group is not adequate, it is recommended to start treatment with tofacitinib46 or abatacept58, and in mild cases with apremilast.
Quality of evidence: High. Strength of Recommendation: Strong.
Recommendation 4. If a bDMARD fails due to inefficacy or adverse events, it can be exchanged for another bDMARD or JAK-i preferably with a different mechanism of action.
Quality of evidence: High. Strength of recommendation: Strong.
Recommendation 5. Oral half-life glucocorticoids (prednisone or deflazacort) are recommended in periods of reactivation of PsA, at doses lower than 7.5 mg/day of prednisone or its equivalent if used orally, for short periods, to avoid adverse events that include the exacerbation of psoriasis after discontinuing treatment. Systemic use of fluorinated glucocorticoids is not recommended to treat musculoskeletal manifestations. Intra-articular application of glucocorticoids may be considered in refractory cases in mono- or oligo-articular presentations.
Quality of evidence: Low: Strength of recommendation: Weak.
Axial diseaseRecommendation 6. NSAIDs are the first-line treatment for the symptoms of axial disease, in therapeutic doses only, without combining them with other NSAIDs, taking due precautions for possible adverse events. (Agreement: 100%).
Quality of evidence: Low, Strength of recommendation: Weak.
Recommendation 7. In the event of failure with NSAIDs, TNF-I agents59–63 are recommended as first-line treatment, and in refractory cases or cases with contraindications to TNF-I, the use of I-IL-17 is recommended26,27. The use of I-IL-17 should be considered in patients with extensive or very active psoriasis, I-IL-17 should be avoided in patients with inflammatory bowel disease. Preferably TNF-I (except etanercept) should be used in patients with a history of uveitis.
Quality of evidence: High. Strength of recommendation: Strong.
Recommendation 8 In the event of an inadequate response to a bDMARD, switching to a bDMARD with a different mechanism of action is recommended, restricting this change to the two previously indicated mechanisms of action (TNF-I, I-IL-17) only. There is insufficient evidence of effectiveness with ustekinumab, apremilast, abatacept or tofacitinib, and therefore they are not recommended for axial disease.
Quality of evidence: High. Strength of Recommendation: Strong.
Recommendation 9. Physiotherapy and physical rehabilitation are recommended to complement pharmacological treatment64.
Quality of evidence: Moderate. Strength of recommendation: Weak.
Recommendation 10. There is no evidence of the effectiveness of DMARDs, including SSZ, in this disease domain, and therefore their use is not recommended.
Quality of evidence: Low. Strength of recommendation: Weak.
EnthesitisEnthesitis is a common manifestation of PsA, it impacts several measures of disease activity, impairs prognosis and quality of life. In most cases enthesitis accompanies peripheral arthritis or axial involvement. A subgroup of isolated enthesitis in PsA has been described but this is less than 5% of all patients65. Therefore, the treatment of enthesitis in general does not determine overall treatment. The following options are considered for cases where control of the other manifestations has been achieved and enthesitis remains resistant to these therapeutic measures.
Recommendation 11. In the opinion of the experts, the first line of treatment for enthesitis includes NSAIDs, although the evidence does not come from specific trials.
Quality of evidence: Low. Strength of recommendation: Weak.
Recommendation 12. There are no studies to support the efficacy of cDMARDs in the treatment of enthesitis, however, in the opinion of the experts, SSZ is the only cDMARD with effect in enthesitis, and it is recommended for use in refractory patients only, with some contraindication, or in patients who, due to the budgetary limitations of health institutions, cannot be given biological therapy or btsDMARDs.
Quality of evidence: Low. Strength of recommendation: Weak.
Recommendation 13. In enthesitis resistant to NSAIDs and cDMARDs, the use of anti-TNF33,52–57, ustekinumab66, I-IL-1767,68 is recommended. If there is a contraindication to biological therapy, tofacitinib or, if the condition is very mild, apremilast should be considered36,39,43,46,69.
Quality of evidence: High. Strength of recommendation: Strong.
Recommendation 14. The local application of glucocorticoids to enthesitis sites is not recommended.
Quality of evidence: Low. Strength of recommendation: Weak.
Recommendation 15. Although there are no studies to evaluate the efficacy of physiotherapy in enthesitis, it is often prescribed as complementary treatment in these patients.
Quality of evidence: Low. Strength of recommendation: Weak.
DactylitisDactylitis is a clinical manifestation with adverse impact on patients with PsA, however, it does not usually occur in the absence of arthritis or enthesitis. Therefore, in most cases the presence of dactylitis is not a determining factor in the choice of treatment. The following recommendations have been established for the cases in which it is.
Recommendation 16. NSAIDs are the first-line treatment for dactylitis, only therapeutic doses are recommended without combining more than one NSAID and monitoring for possible adverse events.
Quality of evidence: Low. Strength of recommendation: Weak.
Recommendation 17. In dactylitis resistant to NSAIDs and cDMARDs, the use of anti-TNF33,52–57, ustekinumab66, IL-1767,68, is recommended. If there is a contraindication to biological therapy, consider tofacitinib or, if the symptoms are very mild, apremilast36,39,43,46,69.
Quality of evidence: High. Strength of recommendation: Strong.
Recommendation 18. The Mexican experts do not consider glucocorticoid infiltration to be advisable. However, in resistant cases, and only when strictly necessary, systemic glucocorticoids can be used, provided that a dose not exceeding 7.5 mg/day of prednisone or equivalent is used for short periods of time, to avoid adverse events, including the exacerbation of psoriasis after the treatment is discontinued.
Quality of evidence: Low. Strength of recommendation: Weak.
Recommendation 19. csDMARDs should be used in the second line of treatment, experience is limited to SSZ.
Quality of evidence: Low. Strength of recommendation: Weak.
Cutaneous psoriasisIn most patients, the treatment of psoriasis is a challenge, and therefore in resistant, moderate, or severe cases where body surface area (BSA), PASI or Dermatology Life Quality Index (DLQI) scores are greater than 10, it is recommended that the patient be referred to dermatology or treated in conjunction with a dermatologist. Provided the patient has not been assessed by a dermatologist, treatment can be initiated to alleviate symptoms with antihistamines and barrier function maintained by applying topical agents such as emollients (e.g., sweet almond oil) or lubricants (e.g., petroleum jelly or vegetable minerals) two to three times daily.
Recommendation 20. In mild cases (ASC ≤ 10 or PASI ≤ 10 or DLQI ≤ 10), the first line of treatment includes topical agents, followed by csDMARDs and phototherapy. In mild cases with DLQI > 10, treatment with dermatology-specific cDMARDs or bDMARDs should be considered. (Agreement: 100%).
Quality of evidence: Low. Strength of recommendation: Weak.
Recommendation 21. In moderate or severe cases (ASC > 10 or PASI > 10 or DLQI > 10), treatment should be decided by a dermatologist. It may start with topical agents combined with (dermatology-specific) cDMARDs or phototherapy. It is important to note that a mild case can be considered moderate or severe if quality of life is severely affected. (Agreement: 100%).
Quality of evidence: Low. Strength of recommendation: Weak.
Recommendation 22. Patients with moderate or severe disease in whom DMARDs have failed can be treated with I-TNF36,45,70,71, ustekinumab58,72, I-IL-1736,58,69,72, and while tofacitinib has not yet been approved for plaque psoriasis, apremilast can be used in patients with mild psoriasis or risk factors for developing infections. Certain patients without prior treatment with csDMARDs can also be treated with bDMARDs. It is recommended to switch between csDMARDs, from csDMARDs to bDMARDs or between bDMARDs with different mechanisms of action.
Quality of evidence: High. Strength of recommendation: Strong.
Nail psoriasisIn most cases, nail Ps is part of the profile of the Ps patient, therefore, the choice of treatment of Ps in its cutaneous component usually provides a response to most patients’ nail condition. For those in whom nail Ps persists as a significant disorder despite a good response in other domains, we make the following recommendations:
Recommendation 23. Few studies of nail Ps have been conducted in patients with PsA, therefore, treatment is primarily based on data from skin psoriasis or nail Ps studies with subgroups of patients with PsA. The efficacy of TNF-I36,73, I-IL-1736,39, ustekinumab74, and tofacitinib75 on nail disorders has been demonstrated and their use is recommended.
Quality of evidence: High. Strength of recommendation: Strong.
Recommendation 24. The local application of glucocorticoids to the nail matrix is not recommended.
Quality of evidence: Low. Strength of Recommendation: Weak.
DiscussionThe Mexican College of Rheumatology generated these recommendations through the consensus effort of a group of experts in the clinical aspects, assessment, and treatment of PsA.
The group included dermatologists as well as rheumatologists to refine treatment recommendations for PsA and nail disease and to jointly define treatment for the different manifestations, and to address general aspects of care and share perspectives on delayed diagnosis and the barriers to access to different aspects of their medical care.
Where possible, the recommendations were generated based on evidence from an SLR updated as of 30 June 2020, but considering the clinical experience of experts and the characteristics of the Mexican health system. The evidence was rated using the GRADE system. In several recommendations, the evidence is strong, and the recommendations are well-supported. This is even clearer for the use of new therapies that underwent rigorous trials in their approval process; these trials clearly define the scope of usefulness of each of the treatments.
We considered the six main domains of PsA and sought evidence to update the recommendations. However, and in line with more recent recommendations, we consider that enthesitis, dactylitis and nail involvement are unlikely to guide the choice of treatment in the earliest stages and their importance is highlighted in cases where these manifestations are refractory, to consider a secondary adjustment.
NSAIDs were included in these recommendations as a starting point in all four musculoskeletal domains. In the case of peripheral arthritis and enthesitis, the use of cDMARDs and in the case of resistance, starting bDMARDs or tsDMARDs. The use of the latter two treatment groups requires special consideration as access to them is restricted by the medical coverage for a significant proportion of our population. This requires strategies to be optimised prior to starting biological therapy. Fortunately, peripheral arthritis is the most common presentation seen in Mexico and this means several cDMARDs can be used as initial treatment.
Axial involvement in NSAID-resistant cases limits treatment with bDMARDs to only two cytokines, TNF-I, and I-IL-17. Although there is evidence of a favourable effect of tofacitinib and filgotinib in axial spondyloarthritis, we cannot recommend them from the currently available information. Ustekinumab is not considered an effective treatment in axial disease because it has not demonstrated an effect in axial spondyloarthritis trials other than in patients with PsA and axial disease. Recently, however, differences have been established between the axial involvement of axial spondyloarthritis and that of PsA76. Therefore, further evaluation of the effect on axial involvement in patients with PsA treated primarily for peripheral involvement would be desirable, as there is a possibility that a drug that does not prove useful in axial spondyloarthritis not associated with psoriasis may be useful in the axial involvement of PsA.
In contrast to axial involvement, new treatment options have emerged for the remaining PsA domains. New bDMARDs have been licensed and there is evidence to support the high efficacy of ustekinumab, I-IL-17, I-IL-23, tsDMARDs in peripheral arthritis, enthesitis and dactylitis, as well as in skin manifestations and nail disease. The experience of Mexican rheumatologists with the use of local glucorticoids to infiltrate the entheses, in dactylitis or nail beds is poor and is not recommended in our population.
In our population access to the Mexican health system is heterogeneous, which is an element to be considered. There is diverse coverage, and this restricts access to some drugs for certain populations; several public health systems provide access to some bDMARDs and tsDMARDs but in differing regimens that do not necessarily include all the drugs in each group. However, all the drugs listed in these recommendations are indicated in PsA by our regulatory agency (COFEPRIS) and are available in private care. The absence of some of the drugs mentioned here in a specific list of essential drugs provided by state insurance will require treating physicians to select viable alternatives to meet the needs of patients in their specific setting. To this end, the relative redundancy in the effect of various groups of drugs is fortunate as it opens a diversity of strategies for the initial treatment and management of refractory cases.
FundingNovartis Mexico covered the costs of the meetings, without intervening in the discussions or the proposals made by the participating physicians.
Conflict of interestsJ. Casasola-Vargas: Abbvie, Janssen, UCB, Novartis; D. Flores-Alvarado: Janssen, UCB, Novartis, Abbvie, Roche; Luis H. Silveira, Jannsen; G. Reyes-Cordero: Novartis, Eli-Lilly, Abbvie, Pfizer, UCB, Gilead; G. Villanueva Quintero, Mario Amaya Guerra: Abbvie, Celgene, Janssen, Isdin, Leo, Lilly, Novartis. Pfizer, Sanofi; B.E. Zazueta Montiel: Novartis, Eli Lilly, Roche, Amgen, UCB; A. Mendoza-Fuentes: Novartis, Abbvie; A. López Rodriguez: Novartis, Janssen, Eli Lilly; R. Burgos-Vargas: Novartis, UCB, Abbvie; C. Pacheco-Tena: Pfizer, Abbvie, R-Pharm, AstraZeneca, UCB, Janssen, Merck-Serono, Sanofi, Eli-Lilly, Novartis, Gilead.
The Committee for the development of recommendations for the management of PsA is grateful for the support of the Mexican College of Rheumatology and the company Novartis for their unreserved support of our committee's activities.
Please cite this article as: Casasola-Vargas J, Flores-Alvarado D, Silveira LH, Sicsik-Ayala S, Reyes-Cordero G, Villanueva Quintero G, et al. Recomendaciones del Colegio Mexicano de Reumatología para el manejo de la artritis psoriásica. Reumatol Clin. 2021;17:611–621.