Salivary gland ultrasound (SGU) provides information about structural gland abnormalities that can be graded and used for primary Sjögren's syndrome (pSS) diagnosis. Its potential role as a prognostic marker for detecting patients at high risk of lymphoma and extra-glandular manifestations is still under evaluation. We aim to assess the usefulness of SGU for SS diagnosis in routine clinical practice and its relationship with extra-glandular involvement and lymphoma risk in pSS patients.
MethodsWe designed a retrospective observational single-center study. Data was collected using the electronic health records of patients referred to an ultrasound outpatient clinic for evaluation over a 4-year period. Data extraction included demographics, comorbidities, clinical data, laboratory tests, SGU results, salivary gland (SG) biopsy, and scintigraphy results. Comparisons were made between patients with and without pathological SGU. The external criterion for comparison was the fulfillment of the 2016 ACR/EULAR pSS criteria.
ResultsA total of 179 SGU assessments were included from this 4-year period. Twenty-four cases (13.4%) were pathological. The most frequently diagnosed conditions prior to SGU-detected pathologies were pSS (9.7%), rheumatoid arthritis (RA) (13.1%), and systemic lupus (4.6%). One hundred and two patients (57%) had no previous diagnosis (sicca syndrome work-up); of these, 47 patients (46.1%) were ANA positive and 25 (24.5%) anti-SSA positive. In this study, the sensitivity and specificity of SGU for SS diagnosis were 48% and 98% respectively, with a positive predictive value of 95%. There were statistically significant relationships between a pathological SGU and the presence of recurrent parotitis (p=.0083), positive anti-SSB antibodies (p=.0083), and a positive sialography (p=.0351).
ConclusionsSGU shows high global specificity but low sensitivity for pSS diagnosis in routine care. Pathological SGU findings are associated with positive autoantibodies (ANA and anti-SSB) and recurrent parotitis.
La ecografía de glándulas salivales (EGS) proporciona información acerca de las anomalías en la estructura glandular, y puede ser utilizado para el diagnóstico del síndrome de Sjögren (SS). Además, su potencial valor pronóstico para detectar pacientes con riesgo de manifestaciones extra-glandulares, así como el riesgo de linfoma se encuentra aún bajo estudio. El objetivo de nuestro estudio es evaluar la utilidad de la EGS para el diagnóstico del SS en la práctica clínica habitual, y su relación con la afectación extra-glandular, así como el riesgo de linfoma en pacientes con síndrome de Sjögren primario (pSS).
MétodosRealizamos un estudio retrospectivo y observacional en un único centro. La información fue recolectada de la historia clínica electrónica del paciente tras un seguimiento de 4 años. Esta información incluye variables demográficas, comorbilidades, datos clínicos, análisis de laboratorio, los resultados de la EGS, biopsia de glándulas salivales y gammagrafía. Se efectuaron comparaciones entre los pacientes que tenían una EGS patológica con aquellos que tenían un resultado normal. El criterio para establecer la comparación fue cumplir los criterios de ACR/ELUAR 2016 para el diagnóstico de pSS.
ResultadosSe realizaron un total de 179 EGS durante el período de 4 años. De estas, 24 (13,4%) resultaron ser patológicas. Las enfermedades más frecuentemente identificadas tras realizar la EGS fueron pSS (9,7%), artritis reumatoide (AR) (4,6%) y lupus eritematoso sistémico (LES) (4,6%). Ciento dos pacientes (57%) no tenían diagnóstico previo (estudio de síndrome seca); de estos, 47 (46,1%) tenían ANA positivo y 25 (24,5%) tenían anti-Ro positivo. La sensibilidad y la especificidad de la EGS para detectar el SS en nuestro estudio fueron del 48 y 98%, respectivamente; con un valor predictivo positivo del 95%. Además, se encontraron diferencias estadísticamente significativas en aquellos pacientes con EGS patológica y parotiditis recurrente (p=0,0083), anti-La positivo (p=0,0083) y gammagrafía positiva (p=0,0351).
ConclusionesLa EGS parece tener una muy baja sensibilidad y una alta especificidad para el diagnóstico del pSS en la práctica clínica. Los hallazgos patológicos en la EGS se asocian con positividad de los auto-anticuerpos (ANA y anti-La) y la presencia de parotiditis recurrente.
Salivary gland ultrasound (SGU) is already widely available in clinical practice and is a simple, fast and useful examination, providing information about structural gland abnormalities that can be graded and used for the diagnosis of Sjögren's Syndrome (SS).1
The specific SGU findings for SS are multiple hypoechoic areas within the gland and heterogeneity of the gland parenchyma.2
SS not only generates a wide variety of extra-glandular manifestations, from interstitial lung disease to kidney and brain involvement, but also carries a higher risk of lymphomas.3 SGU's potential role as a prognostic marker for detecting patients at high risk of lymphomas and extra-glandular manifestations is still under evaluation.4
Classification criteria for SS have twice been changed in recent years.5,6 One study showed that the original 2016 criteria had a sensitivity of 95.9% and a specificity of 92.2%. Inclusion of SGU in the 2016 ACR/EULAR criteria would likely improve its sensitivity to 97.3%.7
These properties have led to SGU being increasingly performed not only for research, but also for clinical purposes, especially in centers boasting experience in diagnosing patients with sicca symptoms, although not exclusively.
The present study aimed to evaluate the diagnostic value of SGU for SS diagnosis in clinical practice by calculating the positive predictive value, negative predictive value, sensitivity and specificity of the test. Our secondary objective was to determine the relationship between SGU findings with extra-glandular manifestations and the risk of lymphoma in SS patients.
Materials and methodsStudy design and participants. We conducted a retrospective observational single-center study. We included all patients over 18 years of age who visited the Ultrasound Unit of the Rheumatology Department and coded as SGU during the last 4 years.
ProceduresDemographics (age, sex), reasons for ordering the SGU, prior and final diagnoses, clinical data (including extraglandular involvement, recurrent parotid swelling and lymphoma), SGU results and grading (when available), as well as other diagnosis procedures such as the Schirmer test, an SG biopsy and scintigraphy were collected from the electronic clinical history. The laboratory data collected included the presence of autoantibodies (ANA, Anti-SSA, Anti-SSB and RF), complement levels (C3 and C4) and cryoglobulinemia (Table 1).
Demographic data of all patients, divided by pathological SGU findings and clinical diagnoses of Sjögren's syndrome.
| Total (173)N (%) | SGU pathological (23)N (%) | Clinical pSS or sSS diagnosis (45)N (%) | pSS or sSS syndrome fulfilling any criteria (34)N (%) | |
|---|---|---|---|---|
| Female | 162 (92.6) | 20 (87) | 42 (93) | 32 (94.1) |
| Pathological SGU | 23 (13.1) | 23 (100) | 22 (48) | 20 (58) |
| Diagnosis prior to SGUn (%) | ||||
| None | 83 (47.4) | 8 (34.8) | 15 (33.3) | 11 (32.3) |
| Primary Sjögren's syndrome | 17 (9.7) | 9 (39.1) | 17 (37) | 12 (35.2) |
| Systemic lupus erythematosus | 8 (4.6) | 1 (4.3) | 4 (8) | 4 (11.7) |
| Mixed connective tissue disease | 6 (3.4) | 0 | 0 | 0 |
| Osteoarthritis | 15 (8.6) | 0 | 3 (6) | 1 (2) |
| Rheumatoid arthritis | 23 (13.1) | 5 (21.7) | 6 (13) | 4 (11.7) |
| Other diagnosis | 19 (10.9) | 0 | 0 | 2 (5) |
SGU: salivary gland ultrasound; IR: interquartile range; N: number; sSS: secondary Sjögren's syndrome; pSS: primary Sjögren's syndrome.
Diagnosis of SS (primary (pSS) or secondary (sSS)), was carried out by the responsible rheumatologist. We confirmed whether or not those patients diagnosed with pSS fulfilled the 2002 or the 2016 American-European classification criteria by reviewing their electronic clinical histories.8,9
Extra glandular involvement was considered present based on the following parameters: articular (arthralgia, arthritis), renal (nephritic syndrome or renal tubular acidosis), peripheral nervous system (inflammatory demyelinating polyneuropathy, vasculitis, or pure sensory axonal polyneuropathy), neurological (cerebrovascular stroke, optic neuritis, seizures, cerebral vasculitis, or multiple sclerosis-like syndrome) and pulmonary (interstitial lymphocytic pneumonitis).
UltrasonographyThree expert rheumatologists in SGU performed a standardized SGU assessment by utilizing a real-time, high-resolution ultrasound machine (EsaoteMyLab™X8 Platform Ultrasound System). The assessment was performed on B mode with a linear transducer (frequency from 7.5 to 15MHz and gain from 35 to 50dB) and Doppler mode was applied when needed.
The patients were assessed lying on the bed in a supine position with the neck slightly extended and turned away from the assessed side. Both the parotid and submandibular glands were assessed; the submandibular glands were assessed only in the longitudinal scan, while the parotid glands were assessed in both the longitudinal and transverse scans.
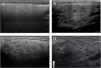
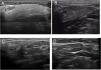
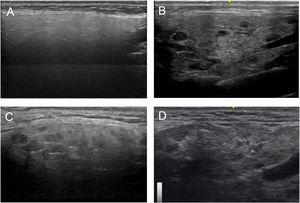
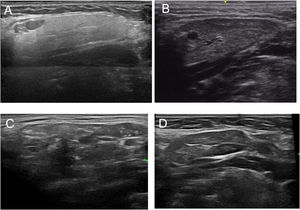
Based on all of the possible glandular aspects assessed with US (hyperechoic lines, number and size of hypoechoic images, etc.), we evaluated only parenchymal homogeneity for diagnostic purposes in accordance with the available literature.10 These findings were graded based in the experience of the authors with a 4 grades semiquantitative score, quite similar to the proposed by OMERACT group in 20192 that considers parenchymal homogeneity ranging from 0 to 3 and can be assessed in both parotid (Fig. 1) and submandibular (Fig. 2) glands. The definition of each grade is specified below: Grade 0: homogeneous normal glandular parenchyma; grade I: minimal glandular heterogeneity without hypoechoic images; grade II: moderate parenchymal heterogeneity with hypoechoic images; grade III: severe heterogeneity with hypoechoic lesions in the entire glandular parenchyma.
We determined the autoimmunity status from the electronic clinical history. ANA were considered positive when >1:80, as measured by indirect immunofluorescence of cryostat sections from rat tissues (kidney, liver, and stomach) and in cultured HEp-2 cells (MarDx Diagnostics, Carlsbad, CA, USA) using a fluorescein-conjugated rabbit antihuman antibody (DAKO, Copenhagen, Denmark) according to standard procedures. RF (positive at >20IU/mL) was measured by laser nephelometry (Beckman Coulter, Brea, CA, USA). Anti-SSA and anti-SSB antibodies were detected using an enzyme-linked immunosorbent assay (Rheuma ELISATM System; Whittaker Bioproducts, Walkersville, MD, USA).
Based on the SGU results, we calculated the positive predictive value, negative predictive value, sensitivity and specificity of the test. We also compared patients who fulfilled any classification criteria (the 2002 American-European Consensus Group (AECG) criteria or the 2016 ACR/EULAR criteria) with those who had been only clinically diagnosed with Sjögren's syndrome.
Finally, for in-patients who fulfilled any of the classification criteria (2002 AECG or the 2016 ACR/EULAR criteria) we compared clinical and laboratory characteristics, comparing those who presented either pathological or normal SGU findings.
This study was approved by the ethics committee for drug research at the Hospital General Universitario Gregorio Marañon.
Statistical analysisWe used IBM® SPSS® Statistics 25 for the statistical analysis. Quantitative measures are expressed as the mean, range, and standard deviation. Categorical measures are expressed as the absolute frequency and percentage. We also calculated the sensitivity and specificity of the SGU in our cohort. Finally, we compared groups by using the Student's T-test and the Chi-squared test and Fisher test when applicable.
ResultsA total of 173 patients were assessed with SGU during this 4-year period, 179 ultrasound scans were performed in total. Of these, 162 (92.6%) were women, the mean age being 59.5 (± 14.55).
Forty-seven percent of the patients did not present any diagnosis prior to SGU assessment. The most frequent previous diagnoses were rheumatoid arthritis (13.1%), osteoarthritis (8.6%), and primary Sjögren's syndrome (9.7%).
Among the most frequent reasons for requesting SGU, the sicca syndrome was the most common (76% of cases), followed by follow-up control (8.6% of cases), and suspicion of SS (6.9% of cases).
Pathological ultrasoundUltrasound findings were pathological in 23 patients (13.1%), 20 of them (87%) women. In addition, 34% of cases had no previous diagnosis, while the most common previous diagnoses were pSS (39.1%), rheumatoid arthritis (21.7%) and SLE (4%).
Only one patient with sicca syndrome but with antibodies and sialography that were not compatible, had a pathological SGU but did not fulfilled any diagnosis criteria; also no biopsy was made.
After the diagnostic exercise, of the 23 cases with pathological SGU, 17 (73.9%) were diagnosed with pSS and 5 (21%) with sSS, with only one patient presenting abnormalities in the SGU assessment.
Sjögren's syndromeThere were 45 patients clinically diagnosed with SS by the treating rheumatologist and 34 of them also fulfilled the SS classification criteria, being 27 pSS (12 cases with previous diagnosis and 15 with new diagnosis) and 7 sSS (all new diagnoses, 4 of them with RA and 3 with SLE) (Table 2).
Comparison between Sjögren's patients (pSS and sSS) who fulfilled any classification criteria divided according to salivary gland ultrasound pattern.
| Sjögren's syndrome with normal SGU (14)N (%) | Sjögren's syndrome with pathological SGU (20)N (%) | ‘p’ | |
|---|---|---|---|
| Female n (%) | 14 (100) | 18 (90) | 0.501 |
| Lymphoma n (%) | 0 | 0 | |
| Recurrent parotitis n (%) | 0 | 7 (35) | 0.014 |
| Extra-glandular involvement n (%) | 9 (64) | 10(50) | 0.403 |
| None n (%) | 5 (35) | 10(50) | 0.2568 |
| Arthralgia/arthritis n (%) | 9 (64) | 7 (35) | 0.2023 |
| Interstitial lung disease n (%) | 0 | 1 (5) | 0.8708 |
| Peripheric neuropathy n (%) | 0 | 2 (10) | 0.6858 |
| Other EGI | 0 | 0 | |
| ANA n (%) | 8 (57.1) | 16 (80) | 0.290 |
| Anti-SSB n (%) | 2 (14.3) | 11 (55) | 0.054 |
| Anti-SSA n (%) | 8 (57.1) | 16 (80) | 0.337 |
| Positive biopsy | 1/1 (100) | 2/7 (28.6) | 0.731 |
| Positive syalography | 7/11 (63) | 12/12 (100) | 0.094 |
| Positive Schirmer test | 8/8 (100) | 16/17 (94) | 0.246 |
EGI: extra-glandular involvement; SGU: salivary gland ultrasound; ANA: antinuclear antibodies; N: number; sSS: secondary Sjögren's syndrome; pSS: primary Sjögren's syndrome.
Of the 27 cases of pSS, the ultrasound was pathological in 59.3%, all meeting the 2002 classification criteria, while 20 (76%) met the 2016 criteria, and 6 of them (22%) presented recurrent parotitis.
About extra-glandular manifestations, the most common was arthritis (7.4%) and there were no lymphoma cases reported.
Regarding the other diagnostic tests, Schirmer test was pathological in about 94% of the patients in whom it was performed (18/19), scintigraphy was positive in about 83% (15/18), and finally, of the 6 biopsies performed, 50% were pathological.
Of the 102 assessments as part of the diagnostic work-out for SS, 74% were ANA positive, 77.8% anti-Ro positive, 44% anti-LA positive, 48% RF positive, 3.7% positive for cryoglobulins. 36% also presented hypocomplementemia for C3 and 28% for C4.
Sensitivity and specificity were 48% and 98% respectively, with a positive predictive value of 95% and a negative predictive value of 70%.
DiscussionSGU is an easy-to-perform and well-tolerated imaging technique that is useful in routine clinical practice for diagnostic work-ups of SS. SGU has been used to assess sicca syndrome in both primary and secondary SS.11 In our center, SGU has been performed for several years and is frequently ordered to assess multiple types of patients.
As reported in the literature, the sensitivity of SGU in SS is wide ranging, from less than 50% to more than 90%.11 As an example of this, Theander et al.12 investigated the sensitivity and related factors of SGU in pSS. These authors observed hypoechoic lesions in 52% of pSS patients versus 1.8% of controls, with a very high specificity and a positive predictive value of 98.0% in both groups, while sensitivity and negative predictive values were 52.0% and 53.0%, respectively. However, in juvenile SS sensitivity seems to be much higher, as shown by a recently published study involving a pediatric cohort diagnosed with SS, in which the findings for 62 of the 64 patients were pathological (94%).13
Our study showed that SGU had a sensitivity of 50% for clinical SS diagnosis, with a very high specificity (about 96%), a positive predictive value of 74% and a negative predictive value of 89%, which was even higher for those who meet any of the SS criteria.
These results are similar to those previously reported in the literature, in particular regarding the very high specificity of SGU.12 On the other hand, in daily clinical settings a pathological SGU was uncommon (13.1%), which might be explained by the fact that SGUs can be ordered for many different reasons, from sicca syndrome to autoimmune disease with specific auto-antibodies such as anti-SSA, including with SS patients. These results suggest that a clinical indication of SGU in routine clinical practice is quite important and should not be overused.
In our clinical practice, as in previously published studies, associations between pathological SGU and diagnosis of pSS have been confirmed; e.g., in terms of its relationship with recurrent parotitis. Unexpectedly, there was a tendency but we did not find an association with anti-SSA or anti-SSB, as has been described before, probably due to a lack of statistical power because we had only 45 patients diagnosed with SS.
We would like to emphasize that 26% of our patients did not undergo a salivary gland biopsy (SGB) due to positive anti-SSB antibodies plus a pathological SGU. This is similar to the EULAR study group on SGU recommendations.5
In addition to its strengths, our study has some limitations that should be acknowledged. One of the objectives of our study was to identify any relation between a pathological SGU finding in SS patients and extra-glandular manifestations or lymphoma. We did not find any difference, however, the small sample size of our SS cohort lacks sufficient statistical power to confirm these assumption.
In conclusion, SGU is a very specific imaging test for the diagnosis of both pSS and sSS. However, one should keep in mind that a clinician's judgment remains the most important factor in the decision to order an imaging technique such as an SGU.
Consent for publicationNot applicable.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Financial supportThis research was supported by internal funding and not by any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no competing interests. Patient and public involvement: patients and/or the public were not involved in the design, conduct, reporting, or disseminating of this research. Patient consent for publication: waived by the local IRB.
We are grateful to all of the rheumatologists at H. G. U. Gregorio Marañón who assisted in the patient work-up process and made this study possible.
The authors thank the Spanish Foundation of Rheumatology for providing medical writing/editorial assistance during the preparation of the manuscript (FERBT2021).