Septic arthritis due to methylcyllin resistant Staphylococcus aureus (MRSA) is a serious infection that has increased in incidence in the past 10 years.
MethodsWe conducted a retrospective study (1984–2011) in which a description of the clinical and epidemiological characteristics of MRSA arthritis in adults was performed and then compared to native joint infections caused by MRSA vs methylcyllin sensitive S. aureus (MSSA).
ResultsFourteen MRSA infections were included (7 native joint, 5 prosthetic and 2 bursae). No case was polyarticular. There was significant comorbidity, although none was associated to rheumatoid arthritis. Seven patients had bacteremia. Four required surgical treatment. Six died. When comparing the 7 patients with native joint MRSA infection with the 17 cases caused by MSSA, no significant differences in risk factors were seen, except more malignancies in the MRSA group. The infection was polyarticular in 7 cases (41%) of the MSSA group. Bacteremia was more frequent in the MRSA group (71.4 vs 58.8%). Empirical antibiotic was useful in 28.6% of MRSA cases versus 100% of MSSA cases. There was a greater tendency to associated mortality in MRSA arthritis (57.1% vs 17.6%, P=.07).
ConclusionsMRSA septic arthritis is a serious condition that occurs in the elderly and patients with high comorbidity. It is usually monoarticular, with positive blood cultures and higher mortality than MSSA arthritis. In patients at risk, vancomycin empiric antibiotic therapy is indicated.
La artritis séptica por Staphylococcus aureus resistente a la meticilina (SARM) es una infección grave que ha aumentado su incidencia en los últimos 10años.
MétodosEstudio retrospectivo (1984–2011) en el que se realiza una descripción de las características clínicas y epidemiológicas de las artritis por SARM en adultos y se comparan después las infecciones en articulación nativa causadas por SARM vs Staphylococcus aureus sensible a la meticilina (SASM).
ResultadosSe incluyeron 14 infecciones por SARM (7 sobre articulación nativa, 5 protésicas y 2 bursas). Ningún caso fue poliarticular. Tenían importante comorbilidad, aunque ninguno con artritis reumatoide. Siete pacientes presentaron bacteriemia. Cuatro requirieron tratamiento quirúrgico. Seis fallecieron. Se compararon los 7 pacientes con infección de articulación nativa por SARM con los 17 casos causados por SASM. No se encontraron diferencias significativas en los factores de riesgo, excepto más neoplasias en el grupo SARM. La infección fue poliarticular en 7 casos (41%) del grupo SASM. La bacteriemia fue más frecuente en el grupo SARM (71,4 vs 58,8%). El antibiótico empírico resultó apropiado en el 28,6% de los casos SARM, frente al 100% de los casos SASM. Existió mayor tendencia a la mortalidad en las artritis por SARM (57,1% vs 17,6%, p=0,07).
ConclusiónLa artritis séptica por SARM es una entidad grave que acontece en pacientes ancianos y con gran comorbilidad. Es generalmente monoarticular, con hemocultivos positivos y mayor mortalidad que la artritis por SASM. En los pacientes de riesgo el tratamiento antibiótico empírico indicado es la vancomicina.
Septic arthritis is an infectious arthritis caused by the colonization of a joint cavity by a pyogenic microorganism. The rapid joint destruction that it produces leads to the deterioration of joint function. Moreover, it is associated with high morbidity and mortality and, thus, is considered a true medical emergency.
The incidence in Europe is estimated to be 4–10 cases per 100000 patients/year.1–3 It occurs in patients with the classical risk factors for infection4: longevity (age >80 years; odds ratio [OR]=3.5), diabetes mellitus (OR=3.3), hemodialysis, intravenous drug use, and treatment with glucocorticoids and immunosuppressive agents. In addition, specific risk factors have been reported: the presence of skin ulcers (OR=27.2) and joint replacement (OR=15), as well as previous intervention of the joint using other invasive techniques. It has been calculated that 4 of every 10000 local glucocorticoid injections and 14 of every 10000 arthroscopies result in infections. The existence of previous joint disease, particularly rheumatoid arthritis (RA) predisposes to infection, but the same occurs with other inflammatory joint diseases and, to a lesser extent, osteoarthritis.2,5,6 Recent data6 suggest a higher risk of septic arthritis in patients treated with anti-tumor necrosis factor α therapy when compared with those treated with disease-modifying drugs (DMD).
Septic arthritis is associated with a mortality rate of 11% when a single joint is affected and up to 40% in patients with RA and polyarticular infection.2,7
Over the last few decades, Staphylococcus aureus has been the pathogen responsible for 40%–50% of the cases of septic arthritis, and, in recent years, between 6% and 22% of them can be attributed to methicillin-resistant S. aureus (MRSA).2,3,8,9 This strain was first isolated in the United Kingdom in 1961.10 Until the 1970s, MRSA infections were limited to occasional outbreaks in hospitals, initially in intensive care units and, later, in regular wards. Since the end of the 1980s, its frequency has gradually increased, until recently, when community-acquired cases of MRSA infection began to be reported, making it the real epidemiological problem that it is at the present time.11
Despite all these implications, information on the epidemiology of septic arthritis due to MRSA is limited. Most of it comes from retrospective cohort studies, which include heterogeneous populations (children, individuals with prostheses and others with native joints, different causative germs) and in which the case definition does not always take into account the isolation of the microorganism in synovial fluid and/or blood samples.
The objective of this study is to describe the clinical characteristics and outcome of septic arthritis due to MRSA in adults, and compare it with that caused by methicillin-sensitive S. aureus (MSSA).
Material and MethodsWe report a retrospective study carried out at Hospital Universitario Germans Trias i Pujol, a tertiary level hospital located in the metropolitan area of Barcelona, Spain, with a referral population of 800000 patients. All the cases of septic arthritis in peripheral joints documented between 1984 and 2011 were reviewed. The inclusion criteria were: age over 18 years and a synovial fluid culture positive for MRSA during that period.
The descriptive part of the study involved MRSA infections in both native and prosthetic peripheral joints. Subsequently, a subanalysis of the patients with MRSA infections of a native joint was carried out to compare them with MSSA infections produced during the same period. The statistical analysis was performed using nonparametric tests, Fisher's test and χ2. Statistical significance was set at P<.05.
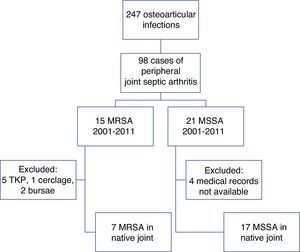
ResultsDuring the period from 1984 to 2011, the rheumatology department recorded 247 osteoarticular infections, 98 of which corresponded to peripheral arthritis in adults (Fig. 1).
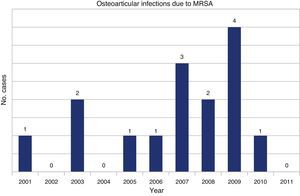
A review of the microbiological records of our center identified 14 cases of MRSA isolated in synovial fluid, the first in 2001 (Fig. 2). There were 7 cases of septic arthritis in a native joint, 5 of septic arthritis in a prosthetic joint and 2 of bursitis. The patients were 8 men and 6 women, with a mean age of 70 years (range 35–88 years).
The demographic and clinical characteristics of the patients can be seen in Table 1. Thirteen of the 14 patients (92.8%) had significant comorbidity: 11 (78.5%) had hypertension (HT), 4 (28.6%) had ischemic heart disease, 3 (21.4%) had diabetes mellitus, 3 (21.4%) had liver cirrhosis, 2 (14.3%) had a solid tumor, 2 (14.3%) had a hematologic disease (myelodysplastic syndrome and myeloma, respectively), 1 (7.1%) had chronic obstructive pulmonary disease (COPD) and 1 (7.1%) was receiving hemodialysis. There was no evidence of RA, arthrocentesis, recent local injections or trauma as predisposing factors. Previous hospital stays (within the preceding 12 months) could be confirmed in only 4 cases.
Characteristics of the Patients With MRSA Septic Arthritis.
| Patient | Age (years) | Sex | Infection site | Route of infection | Comorbidity | Outcome |
|---|---|---|---|---|---|---|
| 1 | 63 | M | Knee | TKP | HT, neobladder | Septic shock, ARF |
| 2 | 85 | W | Knee | Patellar cerclage | HT, asthma, tachyarrhythmia | Good |
| 3 | 73 | W | Knee | TKP | Liver cirrhosis | Septic shock, EAD, death |
| 4 | 76 | M | Elbow | Hematogenous | HT, DM2, liver cirrhosis, neoplasm of the pancreas | Septic shock, EAD, death |
| 5 | 82 | M | Knee | Unknown | HT, ischemic heart disease, myelodysplastic syndrome, bladder neoplasm, liver cirrhosis | Death |
| 6 | 88 | W | Knee | TKP | HT, DM2, dyslipidemia, venous insufficiency | Good |
| 7 | 47 | M | Ankle | Diabetic foot ulcer | HT, DM2, dyslipidemia, obesity, dilated cardiomyopathy, | Septic shock, gout, anemia, leukocytoclastic vasculitis, nephrotic syndrome |
| 8 | 80 | W | Knee | Unknown | HT, dyslipidemia, Colles’ fracture | AMI, gout |
| 9 | 77 | M | 1st MTCP | Hematogenous | HT, ischemic and hypertensive heart disease, mitral-aortic valve disease, COPD, CNS vascular disease, epilepsy, dementia, amaurosis of right eye | Death |
| 10 | 74 | M | Knee | TKP | HT | Good |
| 11 | 35 | M | Prepatellar bursa | Cellulitis | Good | |
| 12 | 54 | M | Olecranon bursa | Cellulitis | HT, DM2, PHT, venous insufficiency +venous ulcers | Death |
| 13 | 85 | W | Knee | TKP | Venous insufficiency +venous ulcers | Good |
| 14 | 74 | W | Knee | Hematogenous (endocarditis) | HT, ischemic heart disease, multiple myeloma, CKD in hemodialysis | Death |
AMI: acute myocardial infarction; ARF: acute renal failure; CKD: chronic kidney disease; CNS: central nervous system; COPD: chronic obstructive pulmonary disease; DM2: type 2 diabetes mellitus; EAD: edematous ascitic decompensation; HT: hypertension; M: man; MRSA: methicillin-resistance Staphylococcus aureus; MTCP: metacarpophalangeal; PHT: pulmonary hypertension; TKP: total knee prosthesis; W: woman.
Septic arthritis was localized to knee in 9 cases (64.3%). In 5 of them (35.7%), the affected joint was a prosthesis, whereas 1 patient had undergone cerclage for fixation of a traumatic patella fracture that she had had years before. There was one case of infectious arthritis of the elbow, one of the ankle and another involving a metacarpophalangeal joint. Two patients had bursitis (olecranon and prepatellar, respectively). There were no cases of multifocal septic arthritis due to MRSA.
In the 5 cases in which the infection involved a prosthetic knee, this was considered to be the route of infection; in 3, the route was hematogenous; in another 3, the infection had spread from a skin infection; and in the remaining 3, the route was unknown.
Seven of the 14 patients (50%) had MRSA bacteremia: 4 of the 7 (57.1%) had native joint infections and 3 had prosthetic infections (60% of the 5 known prosthetic infections). Only 8 (57.4%) had fever on presentation. The mean erythrocyte sedimentation rate was 85mm after 1h (range 28–107) and the C-reactive protein level, 193mg/l (range 28–480mg/l). The mean blood leukocyte count was 12.9×109mm−3 (5.50–27.20×109/mm3). The cellularity of the synovial fluid was 40,132 leukocytes/mm3 (250–133330 leukocytes/mm3), with a mean glucose level of 40.2mg/dl (5.4–203mg/dl).
Once the diagnosis was confirmed microbiologically, all the patients received intravenous vancomycin (1g every 12h). The mean duration of antibiotic therapy was 30 days (range 3–56 days). Four patients (28.7%) required surgical intervention: articular debridement in 2, replacement of a knee prosthesis in 1 and removal of the cerclage of the patella in the fourth. The mean hospital stay was 26 days (range 3–55 days). There were no significant differences between the patients who died during the hospital stay and those who did not.
There were 6 in-hospital deaths (42.8%). Four patients developed septic shock and 2, hemodynamic shock, secondary to their concomitant disease. One patient had an acute myocardial infarction and another, acute renal failure.
In a second subanalysis, the 7 patients with septic arthritis caused by MRSA in a native peripheral joint were compared with the 17 patients with synovial fluid cultures positive for MSSA occurring during the same time period (2001–2011) (Fig. 1 and Table 2).
Comparison Between the Clinical and Epidemiological Characteristics of MRSA and MSSA Arthritis.
| MRSA | MSSA | P | |
|---|---|---|---|
| n | 7 | 17 | |
| M/W | 4M/3W | 13M/4W | .374 |
| Mean age (years) | 77 (47–85) | 66 (30–82) | .023 |
| Site | |||
| Knee | 4 (57.14%) | 10 (58.82%) | .767 |
| Shoulder | 0 | 4 (23.53%) | .494 |
| Ankle | 1 (14.28%) | 4 (23.53%) | 1 |
| Carpus | 0 | 2 (11.76%) | .53 |
| Elbow | 1 (14.28%) | 3 (17.65%) | .659 |
| ACV | 0 | 2 (11.76%) | 1 |
| SCV | 0 | 1 | 1 |
| Discitis | 0 | 1 | 1 |
| Others | 1 | 1 | 1 |
| Bursae | 0 | 2 | |
| Polyarticular | 0 | 7 (41.18%) | .13 |
| DM | 2 (28.57%) | 5 (29.41%) | 1 |
| CKD | 1 (14.28%) | 4 (23.53%) | 1 |
| Hemodialysis | 1 (14.28%) | 2 (11.76%) | 1 |
| Alcoholism | 0 | 5 (29.41%) | .272 |
| Cirrhosis | 2 (28.57%) | 3 (17.65%) | .608 |
| Neoplasm | 3 (42.86%) | 2 (11.76%) | .013 |
| Neutropenia | 0 | 0 | |
| PDU | 0 | 0 | |
| AIDS | 0 | 0 | |
| Systemic corticosteroids | 0 | 2 (11.76%) | .515 |
| Immunosuppression | 0 | 0 | |
| Rheumatoid arthritis | 0 | 0 | |
| Other arthritides | 2 | 8 | .825 |
| Arthrocentesis+local injection | 0 | 2 (11.76%) | 1 |
| Arthrocentesis without local injection | 0 | 2 (11.76%) | 1 |
| Arthroscopy | 0 | 1 (5.88%) | 1 |
| Route of injection | .247 | ||
| Unknown | 3 (42.86%) | 4 (23.53%) | |
| Hematogenous | 3 (42.86%) | 3 (17.65%) | |
| Cutaneous | 1 (14.28%) | 8 (47.06%) | |
| Intra-articular procedure | 0 | 2 (11.76%) | |
| Fever | 4 (57.14%) | 9 (52.54%) | 1 |
| ESR (mm 1st hour) | 97 (29–102) | 95 (20–109) | 1 |
| CRP (mg/l) | 174 (28–480) | 259 (8–537) | .608 |
| Plasma leukocytes (×109/mm3) | 11.6 (7–27.5) | 14.09 (4.1–34) | 1 |
| Synovial fluid | |||
| Leukocytes (×109/mm3) | 22.5 (4.7–62.5) | 48.54 (14.5–120.00) | .266 |
| Glucose (mg/dl) | 12 (5–203) | 59 (44–99) | |
| Crystals | 3 (42.86%) | 3 (17.65%) | .51 |
| Blood cultures | 5 (71.43%) | 10 (58.82%) | .001 |
| No. of days since initiation of clinical-diagnostic management | 12 (1–21) | 7 (1–52) | .341 |
| Length of hospital stay (days) | 33 (8–49) | 20 (7–82) | .361 |
| Appropriate empirical antibiotic therapy | 2 (28.57%) | 17 (100%) | .003 |
| Surgical treatment | 3 (42.86%) | 7 (41.18%) | .788 |
| Death | 4 (57.14%) | 3 (17.65%) | .071 |
ACV: acromioclavicular joint; AIDS: acquired immunodeficiency syndrome; CKD: chronic kidney disease; CRP: C-reactive protein; DM: diabetes mellitus; ESR: erythrocyte sedimentation rate; M: man; MRSA: methicillin-resistant Staphylococcus aureus; MSSA: methicillin-sensitive Staphylococcus aureus; PDU: parenteral drug use; SCV: sternoclavicular joint; W: woman.
We found no differences between the two groups in terms of age or sex distribution. Likewise, the most common infection site, the knee, was the same in both groups. There were 7 patients (41.1%) with more than 1 affected joint in the MSSA group and none in the group with septic arthritis due to MRSA (P=.13).
There were no differences in the classical risk factors associated with septic arthritis. However, there was a significantly higher incidence of neoplasms among the patients in the MRSA group (42.8%) as compared to the MSSA group (11.8%) (P=.013). There were no cases of RA in either of the two cohorts during the study period. No patient in the MRSA group had undergone recent intra-articular procedures, whereas 2 patients in the MSSA group had received a local corticosteroid injection, another 2 had undergone arthrocentesis, and arthroscopy had been performed in a fifth. The comparison of the routes of infection in the two groups revealed no significant differences (P=.247).
The two groups did not differ with respect to the presence of fever, increase in acute phase reactants, peripheral blood leukocyte count, or synovial fluid cell count or glucose concentration.
The patients with MRSA septic arthritis showed a greater tendency to develop bacteremia than those with MSSA infection (positive blood cultures in 71.4% vs 58.8%, P=.001).
Initial empirical antibiotic therapy was appropriate in 28.5% of the MRSA group versus 100% of the MSSA group (P=.003). The mean duration of antibiotic therapy was 30 days and 45 days, respectively (P=.608), and the mean length of the hospital stay, 33 and 20 days (P=.361). The percentage of patients who required surgical intervention was similar (42.9% vs 41.2%).
The patients with MRSA septic arthritis had a greater tendency to develop complications and a higher mortality rate (57.6% vs 17.6%) during the hospital stay than the MSSA group, although the differences were not statistically significant (P=.07).
DiscussionThis study describes the clinical and epidemiological characteristics of the cases of septic arthritis due to MRSA documented over the last 20 years in a university hospital in Barcelona, Spain, and compares them with the cases produced by MSSA.
We should point out that the first case of septic arthritis due to MRSA isolated in our center occurred in 2001, 40 years after MRSA infections were first reported in Europe10 and some 20years after the first cases in Spain.11
Although many cases have been reported,12–18 only 2 studies published to date directly compare a cohort of patients with septic arthritis due to MRSA in a native peripheral joint with another with septic arthritis produced by MSSA.19,20 We find it striking that, in both articles, the overall incidence of septic arthritis, as well as the percentage of that caused by MRSA, is notably higher than in our center. Between 2000 and 2005, the group from the United States recorded 59 cases of septic arthritis (25% involved MRSA)19 and the British group, 58 cases (25.8% due to MRSA).20 In our hospital, over a time period nearly twice as long (2001–2011), there were 37 cases of septic arthritis, 24 caused by S. aureus, 7 of which were due to MRSA, that is, 18.9% of the overall group.
Infectious arthritis usually occurs in elderly patients with considerable comorbidity. In contrast to other reports,19,20 our study found no association with the classical risk factors for septic arthritis. We only observed a higher number neoplasms in the MRSA group.
Other authors affirm that the presence of a rheumatic disease is a predisposing factor of vital importance. One noteworthy finding in the series we studied is that there were no cases of MRSA or MSSA infection in patients with RA. A possible explanation is that, over the past decade, early and intensive treatment of RA has become generalized. It has been demonstrated that, over the long term, this strategy reduces both the need for systemic glucocorticoids and the structural damage, as well as the need for a prosthesis. Moreover, in our center, all the injections were performed under strict asepsis, and it is recommended that biological therapies be discontinued 2–4weeks prior to major elective surgery. In contrast, there were 8 cases of crystal arthritis coexisting with the infection. In the MRSA group, 2 patients presented with monosodium urate crystals and another with calcium pyrophosphate in the same fluid from which S. aureus was isolated. In the MSSA group, urate crystals were observed in 4 cases and pyrophosphate crystals in a fifth. In addition, one patient with MSSA arthritis had a previous psoriatic arthropathy.
Irrespective of the causative pathogen, the knee was the joint most frequently affected. We encountered no patients with polyarticular MRSA infection, a circumstance that contrasts with a recent review that reported an incidence of polyarticular infections of 23%.15 There were more positive blood cultures in the cases of MRSA infection. Bacteremia due to MRSA is known to be associated with a higher mortality than MSSA bacteremia (OR=1.88; 95% confidence interval [CI]: 1.33–2.69; P<.001).7 Its virulence is linked to the presence of staphylococcal chromosomal cassette mec (SCCmec), which contains the mecA gene. This gene is present in the great majority of MRSA strains and encodes the protein PBP2a (penicillin binding protein 2A), a transpeptidase that allows continued bacterial cell wall synthesis in the presence of β-lactam agents.21
We found no differences between the two staphylococcus infection groups in terms of the duration of antibiotic therapy, the number of cases requiring surgical treatment or the length of the hospital stay, findings that are shared with other authors.20 The greater diagnostic delay in the MRSA group (12 vs 7 days, P=.34) was probably due to the initial low index of suspicion. Moreover, the empirical antibiotic therapy proved to be appropriate in only 28.6% of the cases of MRSA arthritis, whereas in the MSSA infections, it was appropriate in 100% of the cases. This fact suggests the need to include vancomycin in the initial antibiotic regimen to treat septic arthritis in patients in whom there is a clinical suspicion. We should point out that over half of the patients with MRSA septic arthritis had been hospitalized during the preceding year, although MRSA colonization was not documented in any of these hospital stays. Nor was it recorded in the series presented by other authors.
The virulence of this pathogen, together with the frailty of the affected patients, results in an elevated mortality rate, although in our series, it did not prove to be statistically significant, probably because of the small sample size; the in-hospital mortality due to MRSA infection was higher than that caused by MSSA (57% and 7%, respectively) (P=.071). Both data are worse than those reported in the earlier series. The in-hospital mortality in the North American series was 20% in cases of MRSA and 7% in cases of MSSA,19 whereas the British group reported a 6-month sepsis-related mortality of 13% vs 5% (MRSA vs MSSA).20
The present report has the characteristic limitations of studies performed in a single hospital, in addition to the retrospective design and relatively small number of patients.
Septic arthritis due to MRSA is a potentially life-threatening infection, the incidence of which has increased over the last decade. It should be suspected in patients who had recently or frequently been exposed to the hospital or social health care setting, as well as in those with documented previous infection or colonization by this microorganism.
The inclusion of vancomycin in the initial empirical antibiotic regimen in these cases could achieve a reduction in the mortality rate associated with infection by this pathogen.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Mínguez S, Molinos S, Mateo L, Gimenez M, Mateu L, Cabello J, et al. Artritis séptica por Staphylococcus aureus resistente a la meticilina en adultos. Reumatol Clin. 2015;11:381–386.