In line with vascular hypothesis of systemic sclerosis (SSc), it is proposed that visceral Raynaud's phenomenon (RP)/endothelial dysfunction causes severe oxygen supply/consumption imbalance in internal organs, leading to visceral ischemic failure in early SSc, especially with cold environmental temperature (Te) and severe RP. There would also be a decrease in body thermogenesis and a decrease in heat loss caused by chronic visceral ischemia and by systemic vasculopathy, respectively. At any given time, these disorders could produce low core temperature (Tc). Hence, SSc is proposed as a candidate cause of secondary hypothermia. It is suggested that SSc could be an adaptive response in cold Te to systemic endothelial damage with secondary chronic visceral ischemia/slow metabolism. This pathophysiological mechanism is proposed in early visceral failure, prognosis, SSc phenotype according to ethnicity, and other manifestations. The impact of Te and Tc on SSc warrants further investigations.
Siguiendo la hipótesis vascular de la esclerosis sistémica (ES), se propone que el fenómeno de Raynaud (FR) visceral y la disfunción endotelial producen un desajuste entre el aporte de oxígeno y su consumo en las vísceras, produciendo isquemia/insuficiencia visceral en la ES precoz con temperaturas ambiente (Ta) frías y FR severo. Habría también una disminución de la termogénesis corporal y de la pérdida de calor causadas respectivamente por la isquemia visceral crónica y por la vasculopatía sistémica. Estas alteraciones podrían producir en un momento dado un descenso de la temperatura central corporal (Tc), postulándose la ES como causa candidata de hipotermia secundaria. Se sugiere que la ES podría ser una respuesta adaptativa en ambientes fríos al daño endotelial sistémico con isquemia visceral crónica y metabolismo lento secundarios. Esta fisiopatología de la ES se propone en la insuficiencia visceral precoz, su pronóstico, fenotipo étnico y en otras manifestaciones. La influencia de la Ta y la Tc sobre la ES requiere más investigaciones.
Currently, systemic sclerosis (SSc) is the classical autoimmune disease with the highest mortality. Genetic and environmental factors influence its clinical manifestations. Among environmental SSc factors, cold environmental temperature (Te) triggers Raynaud's phenomenon (RP), one of its cardinal symptoms. However, the effect of Te on SSc has not been as widely studied as in coronary arterial disease (CAD).1,2 This may be because SSc is a rare and heterogeneous disease and because its large cohorts of published patients come almost exclusively from countries with cold or temperate climates. An integrated view of SSc pathogenesis must include vascular injury, inflammation and immune system and fibroblast activation. However, the relative contribution of these processes to the clinical manifestations varies from one patient to another.3,4 Vascular hypothesis points to systemic endothelial injury as the primary pathophysiological mechanism of SSc. In his pioneer study of temperature in SSc patients, Dr. Le Roi suggested a disorder of thermoregulation caused by cutaneous dysfunction.5 Furthermore, hyper vasoconstriction of RP seems to reflect upregulation of heat conservation.6 But our knowledge of body thermoregulation is based primarily on animal models, and consequently thermoregulation is poorly understood in SSc patients. This paper highlights some features of normal thermoregulation, the SSc vasculopathy and its ischemic tissular microenvironment. Then, in the context of vascular hypothesis, the impaired of physiological thermoregulation and SSc features resulting from interaction between Te, vasculopathy and these thermoregulatory abnormalities are reviewed and analyzed.
Highlights on physiologic thermoregulationTransient receptor potential (TRP) family of cation channels detect Te and core body temperature (Tc).7 Reflecting the characteristics of an organism's ecological niche, TRP display dramatically different thermal activation ranges consistent with their respective Te and Tc (e.g. frogs versus mammals), thus supporting the notion that properties of these TRP channels are under strong evolutionary pressure.8,9 During exposure to cold, the body attempts to maintain normal Tc by reducing heat loss and later by increasing heat production. The rate at which heat is lost is determined almost entirely by the speed at which it is transferred from the core to the skin, and from there to the surroundings.10–12 Blood circulates slowly through the subcutaneous veins of the four limbs, which covers 50% of skin surface.10 The rate of blood flow in the skin can vary from barely above zero to as high as 30% of cardiac output.10 To increase the insulating capacity of the skin, heat is conserved by constriction of cutaneous arterioles, arteriovenous anastomoses (AVAs) and superficial veins.11,12 Thus, these constrictions direct blood flow to the deep veins. AVAs have a thick muscular vessel wall and they are more densely innervated than arterioles. They are capable of making large adjustments in blood flow through synchronous activation and their capacity to supply the subcutaneous venous plexus of the extremities. AVAs are mainly located in hairless acral areas of the skin; fingers, palms, soles, toes, externa! ears and tip of the nose, dense vascularized areas where the skin surface/volume is highest and most exposed to Te.6,10–12 Within the thermoneutral zone (TNZ) Tc is controlled solely by adjusting skin blood flow. AVAs probably have a greater capacity to adjust blood flow in TNZ than arterioles in non-acral skin.12 This is controlled almost entirely by the sympathetic nervous system in response to changes in Tc and Te.6,11,12 As higher vertebrates, the heat produced in the body is generated in the deep organs. As warm-blooded animals, we can maintain a relatively constant Tc quite independently of Te because we have a high metabolic rate that can drastically increase in response to cold (tachimetabolism).10 Even without sweating insensible evaporation of water from skin results in continued heat loss. If Te is greater than that of the skin the body gains heat, there is vasodilatation of cutaneous blood vessels in healthy people and sweating is the only means of cooling it down.10,11
Highlights on SSc vasculopathyIn accordance with its seriousness, RP of SSc (RP-SSc) develops digital ulcers and necrosis and visceral vasospasm, called visceral RP, which has been proven in the heart13 and kidney.14 During the early stages of SSc, RP-SSc/microvascular dysfunction (MVD) perfusion impairment caused by cold temperatures can be completely, or almost completely, reversed. This can be followed by structural arteriolar lesions leading to irreversible lesions with secondary chronic ischemia and visceral fibrosis.3,4 Systemic widespread proliferative/obliterative vasculopathy of small and medium-sized arteries are pathologic hallmarks of SSc, primarily affecting microcirculation.3,4,15 Peripheral macrovascular involvement in SSc16 is predominantly distal and upper limbs (digital and ulnar arteries).17 It remains unclear why vasculopathy is expressed mainly in different anatomical areas in different patients.4 The interactions between microvasculature and microvasculature have rarely been studied18 and they are at the crossroads between SSc and atherosclerosis. These interactions can increase vascular damage with consequent visceral ischemia/dysfunction, as occurs in the digital ischemic ulcers in SSc19 and in coronary arterial disease (CAD).20 Furthermore, likely similar to association of diabetes and hypertension,18 in SSc, especially in association with hypertension, large elastic arteries lose their buffering capacity as they increase in stiffness,21 which can increase pulsatile load transmission on the microvasculature in high flow low impedance organs such as the brain and kidney, because this high pulsatile load can penetrate deeply in to their microcirculation,17 although this does not seem to influence the prognosis.22 Atherosclerosis and SSc share endothelial dysfunction, arterial stiffness, inflammation, age risk and cold Te triggering clinical manifestations, but both have different stenosing arteriopathies. Prevalence of atherosclerosis in SSc is controversial4,15 (see atherosclerosis – SSc section), and the association between SSc and CAD has only been recently, but worldwide, demonstrated.23–30 Coronary atherosclerosis can begin before age 20 and is present among asymptomatic adults. Prevalence of many pathogenic mechanisms for increased atherosclerosis are possible in patients with SSc and likely overlap with the pathophysiology of SSc.27 The chronic, clinically silent mechanisms underlying both atherosclerosis and connective tissue diseases (CTD) can persist for prolonged periods. Therefore, CTD associated with atherosclerotic CAD events do not necessarily appear only after establishment of CTD diagnosis.27
Tissue ischemic microenvironment in SSc patientsMetabolic reprogramming of glycolysis and glutamine metabolism are key events in myofibroblast transition in SSc.31,32 Acidic microenvironment, resultant of increased lactates production as end metabolic product of glycolysis, may impairs angiogenesis and drives endothelial-to-mesenchymal transition.32,33 SSc dermal fibroblasts32 and endothelial cells,33 are characterized by increased glycolytic metabolism. Mesenchymal stem like cells (MST like cells) are present in SSc sections (but not in control sections) in dermis and adjacent to damaged microvasculature.33 Analyzing dermal blister fluid from patients with diffuse SSc (dSSc), lactates and other microenvironmental factors in early skin SSc promote MSC-like cells differentiation to myofibroblast signature.33 Compared to oxidative phosphorylation, glycolysis is much less efficient energy producing ATP. But glycolysis provides more rapid access to ATP and it generates biosynthetic intermediates (biomass).34 Glutamine metabolism produces these intermediates as well, thus supporting proliferation and protein synthesis.31 SSc is a disease in which myofibroblast proliferation and exuberant production of extracellular matrix deposition are key. Being particularly important in this microenvironment the production of the key collagen I precursor α-ketoglutarate and building blocks for non-essential amino acid synthesis.31,34 This metabolic reprogramming introduces the possibility of applying metabolically targeted interventions for SSc therapy.32,34 Tissular ischemia with scarce local supplies, would facilitate this metabolic reprogramming towards glycolysis by yielding less ATP energy, thereby would favor cell survival in this microenvironment. α-Ketogluturate is a cofactor for hypoxia inducible transcription factors (HIF}-α prolyl hydroxylases which could alter HIF function and play a role in myofibroblasts generation.34 HIF control cellular responses to oxygen availability by activating a myriad of genes involved in angiogenesis, PH regulation, glycolysis and cell grow.35 HIF are present in all tissues. Taken together, this HIF-mediated reprogramming of metabolism supports a shift toward anaerobic production.36 Aside this, HIF are involved in diverse process including cell development, inflammation and immune regulation.35 The protein stability of the two main HIF subunits-lα is regulated by human oxygen sensors (3 oxygen-and-iron-dependent prolil-4-hydroxylase-domain (PHD)) enzymes, which are druggable targets.37 Roxadustat and daprodustat are first-in-class PHD inhibitors with regulatory approval in China and Japan for renal anemia treatment. In a mouse model of AMI, selective inhibition of PHD preserves heart function. Repurposing PHT inhibitors for tissue protection37 could be a line of research for future therapy in SSc. α-Ketogluturate can alter HIF-la that can then indirectly affect epigenomics through HIF-α mediated demethylases. HIF is important in SSc.34
Thermoregulation in SSc patientsSystemic vasculopathy and vascular rarefaction lead to chronic tissue ischemia. This, together with the malnutrition described in SSc38 may produce slow metabolism, thus suppressing the human physiological visceral tachimetabolism to generate much more heat after exposure to cold Te.10 In SSc, fingertips AVAs are transformed into a passive vascular bed with no spontaneous activity.39 This, together with stenosing microangiopathy and cutaneous vascular rarefaction, causes the skin to lose its thermoregulatory function.3 In this way, the rate of heat loss decreases in patients with SSc because heat conduction from muscles and viscera to the skin, and possibly from the skin to the air, is slower. At the macrovascular level stenosis/obliteration of the ulnar artery, characteristic of evolved SSc, leaves out of circulation the cutaneous surface most exposed to the ambient temperature (the hands). Fibrous thickening of the dermis obliterates sweat glands, thus eliminating this heat evaporation mechanism. Probably, this fibrous thickening decreases thermal conduction by increasing the insulating capacity of the skin,40 what might be relevant in dSSc.
Vascular hypothesisIt has been recognized for many years in SSc that prolonged vasospasm causes anoxic injury with resultant fibrosis and visceral failure. Endothelial injury triggered by unidentified circulating factor is likely the initiating factor in SSc. At cold Te, this endothelial injury causes an acute and widespread microvascular dysfunction (MVD) – vasospasm (RP) and an imbalance in viscera between the supply and consumption of oxygen causing vascular organ damage. This imbalance could be especially severe in the very early stages of SSc, when visceral RP may be particularly intense with more flexible prearterioles and arterioles, and with viscera still free of structural vasculopathy, vascular rarefaction, ischemia or fibrosis.4 If the visceral damage resulting from this has a poor prognosis, these patients are overlooked in preSSc and SSc cohorts (survival bias). Either way, it is logical to assume that the combination of cold and serious (autoantibodies) endothelial systemic injury at the onset of SSc, with or without atherosclerotic plaque, maybe serious. Acute and systemic vascular RP–MVD can occur, possibly with intense, diffuse and prolonged vasospasm and resultant visceral ischemia/failure. Due to the already mentioned heterogeneity of SSc and its vasculopathy, the significance of this vascular hypothesis can vary widely between patients or in the same patient overtime. In summary, at cold Te patients with established SSc appear to have an impaired ability to increase thermogenesis, and a delay and decrease of heat loss generated in their viscera. Thus, it is possible to adequately preserve Tc in SSc. Alternatively, according to this delicate balance, an equally acceptable hypothesis raises the possibility that patients with SSc, or a subgroup in some circumstances, have low Tc. Secondary hypothermia may occur in sick persons with a wide variety of illnesses, even indoors in a warm environment. Important; conditions such as hypoglycemia, hypoxia and malnutrition may trigger it.41
Low Tc (hypothermia) in SScA low Tc is important in this setting because it increases the effect of cold Te on this model. A review of the literature (PubMed) uncovered only very preliminary reports of Tc in patients with SSc with inconclusive results.5 Several indirect clues favor low Tc in SSc. Compared with healthy controls, in either TNZ or with cold stimulus, patients with primary RP, undefined RP, or RP-SSc had significantly lower skin temperature,5,42,43 and those ones with undefined RP had lower Tc. In addition, Tc dropped significantly more in the undefined RP group following the cold stimulus.42 Thus, authors suggested that a subgroup of subjects with RP are less efficient in maintaining Tc in response to body cooling.42 As in undefined RP,42 low skin temperature in SSc may reflect low Tc temperature; hyper vasoconstriction of RP-SSc though excessive could be the immediate response to maintain Tc by restricting heat loss.11 Hypothermia cools inflammation and adaptive immunity.44 Hypothermia is beneficial as a tissue protection mechanism when energy supply is low by decreasing the metabolic needs of ischemic tissues. This happens in cold-blooded animals, and in some human diseases such as acute myocardial infarction (AMI), where restoration of blood flow in the ischemic myocardium also induces inflammation and additional injury/necrosis (myocardial ischemic reperfusion injury (MIRI)). Selective therapeutic hypothermia is a treatment designed to MIRI and it decreases infarct myocardial size.45 This beneficial situation could also occur in SSc in patients with normal/minimally low Tc. These patients would adapt to cold by increasing their insulation, as previously discussed, and with hypothermia. But this delicate balance would be broken with colder temperatures or with a rapid drop in Tc; not being able to generate heat quickly these patients, low Tc would increase or appear with resultant visceral failure. It is well known that mild hypothermia is often subtle and the mortality rate is high in patients who develop secondary hypothermia as a complication of generalized endothelial injury.
Clinical implicationsAtherosclerosis and sclerodermaAtherosclerosis is the main cause of CV disease. Although there is more CV involvement in patients with SSc,23–30 compared with the general population there are fewer traditional (atherosclerotic) CV risk factors in these patients.23,30 Initial autopsy studies found either no excessive15 or strikingly little atherosclerosis.46,47 Decades later a higher incidence of atheroma plaques relative to controls has been found in cross-sectional cohort studies of hospitals and in meta-analyses of SSc patients48–52 (see the section “Metabolic syndrome”). Controversy about the prevalence of atherosclerosis in SSc4,53,54 underlies confluence of vasculopathies of SSc and atherosclerosis. Although digital necrosis is considered a primary manifestation of SSc, ischemic heart disease is not considered a primary cardiac involvement of SSc.55 There is a known additive synergistic effect between the macroangiopathy and microangiopathy of atherosclerosis and SSc. In addition, SSc inhibits angiogenesis and vasculogenesis necessary for the development of collateral circulation induced by the chronic tissue ischemia of atherosclerosis.56 Surrogate markers of subclinical atheromatosis have varied, and SSc can influence these markers. Carotid intima-media thickening (CIMT) has been found in meta-analyses and hospital-based cohorts of SSc patients,49,57,58 but not in all studies.48,53,59 CIMT is a surrogate marker of atherosclerosis in the general population, but CIMT may reflect an intrinsic vascular reaction to hypertension rather than incipient plaque.48 CIMT has also been considered to be part of the subclinical macroangiopathy of SSc.17 Dilatation-mediated arterial brachial flow and the ankle-arm index are altered in both diseases.53,54,57,58,60,61 Macrovascular structural damage in SSc patients is proportional to age and time of evolution.58 Survival rate of SSc patients is on the rise (see the section “Improved prognosis for SSc patients”) and aging is an independent risk factor for atherosclerosis. When collagen vascular disease (CVD) populations age, the accumulation of atherosclerosis and its risk factors (hypertension, diabetes, hyperlipidemia, and years of smoking) partially overshadow the effect of pre-existing CVD vasculopathy, but young people with CVD are at the forefront.27 Young patients with early/very early SSc, especially those genetically predisposed to systemic vasospasm,62 are postulated to die of severe vasospasm (RP/DMV) (see the sections on patien's age and vascular hypothesis).
Raynaud's phenomenonMost studies SSc patients with ischemic digital ulcers (serious RP) have worse outcomes compared with SSc patients without ulcers.63,64 Serious visceral RP/DMV at SSc onset could explain the inverse relationship between severity of SSc and time elapsed between RP and the next SSc clinical symptom.65,66 RP prevalence and severity (RP-SSc) is related to cold geographical areas.67,68 Usually RP is the first symptom of SSc, but it may not be the first one, especially in dSSc,69 or it may go unnoticed. In this regard, there was a striking discrepancy in the presence of RP between two hospitals in Madrid (Spain) studying very similar cohorts of patients with dSSc induced by toxic oil syndrome during their first six months of illness.70,71 A posteriori, this discrepancy can be explained by the severe visceral involvement that immediately followed it, its onset in spring and by heating differences between hospitals.
Pulmonary hypertension (PH)Natives and residents of extremely cold environments in northeastern Russia have increased pulmonary artery systolic pulmonary artery pressure (PASP), and the percentage of residents with PASP is related to years of stay in the region.72 In a study of pulmonary arterial blood pressure (Dopler echocardiography) in two populations of Kyrgyzstan, one at 700m altitude (lowlanders) and the other at 3400m altitude (highlanders). Pulmonary arterial blood pressure increased at the end of winter in both populations, more so in highlanders.73 Chronic exposure to hypoxia underlies at high altitude. Cold is also an important environmental factor present in high-altitude regions.73 In a study performed in Israel with cardiac ultrasounds of 11,348 patients, it was found that ultrasounds performed during the months of June to November were 19% less likely to have a PASP >40mmHg compared to those performed in other months, demonstrating that environmental heat stress can affect hemodynamics.74 The lungs are the only viscera directly exposed to hypoxia and the one most exposed to cold Te, being susceptible to cold stimulus.75 It has been suggested that RP is a predisposing factor for pulmonary vasospasm. Early and repeated vasospasm would be one of the predisposing factors for pulmonary vascular remodeling, which may lead to PH, but results from studies of cold-induced pulmonary vasospasm have been inconsistent.76,77One study with negative results showed evolved SSc in patient's cohort; mean evolution 8 years, 14/21 with pulmonary fibrosis, mean SPAP of 46mmHg,77 suggesting semi-rigid/stiff pulmonary arterioles. SScPH is associated with subclinical atheromatosis49,52 and it is classically established after many years of RP onset, but it also occurs in the early phase of SSc, so, SScPH is also a major cause of mortality in that phase.78,79 Survival of patients with SScPH and interstitial lung disease (ILD) is lower than that of patients with ILD or SScPH alone.78 In tropical climate of Thailand PH incidence in patients with early SSc was low, but survival was poor, with ILD or left heart involvement in all cases. RP and higher oxygen saturation protected the development of PH in that study.79 Authors suggest that SSc patients with RP tend to be treated with vasodilators that may have prevented the development of PH at this early stage of SSc.79
Metabolic syndrome (MetS)In 2014 a Mexican study found that the prevalence of MetS among patients with SSc was 36.4%, higher than that among the general Mexican population.80 Insulin resistance, the main pathophysiological mechanism of MetS, would increase tissue ischemia as insulin stimulates nitric oxide production.80 These results have subsequently been confirmed in other cross-sectional studies of patients from hospital settings in Italy and Turkey,49,52,81,82 and in a large multicenter study.51 The prevalence of MetS in the Italian studies was lower than in Mexico, which they attributed to the use of different MetS criteria, ethnic differences, and environmental factors.81,82 It should be noted that of the clinical components of the MetS (hypertension, central obesity, and type 2 diabetes), the last two are lower or not increased in the general SSc population.23,30 Patients with central obesity have less sweating and are less exposed to cold Te as they have the smallest body surface area. In the general population MetS is a predictor marker for the development of atherosclerotic CV events, thus tripling the risk of AMI and stroke. According to this, in a cohort of 116 SSc patients, 31% had MetS compared to 52% MetS in the subgroup of patients with subclinical atherosclerosis.49 Considering the global epidemic of obesity, the increasing prevalence of MetS with age, and the current increased survival of SSc, MetS contributes to the increased mortality of older age SSc patients.83,84
Early serious visceral damagePatients with dSSc have worse prognosis. Early dSSc with progressive cutaneous fibrosis within one year is associated with overall disease progression and reduced survival.64,69 Functionally predominant systemic vasculopathy causing visceral ischemia could contribute to observed mortality from visceral failure in early SSc patients,63,66,83,84 especially in cold Te.
Scleroderma renal crisis (SRC)SRC was significantly more prevalent in winter and fall months in New York from 1952 to 197214 and Pittsburgh (USA) from 1955 to 1981.85 But in the same database of the university of Pittsburgh there was no such seasonal pattern from 1972 to 1982.86 Blood flow to the kidney is related to its excretory function, and it is by far in excess of its metabolic requirements. In SRC, vascular damage with ischemia predominates over immune system,87 and this can lead to grossly visible renal infarcts. SRC appears in the early years of SSc,86,87 possibly with especially serious renal RP. In established SSc, without or moreover with hypertension, structural vasculopathy of interlobular and afferent arterioles can damp the increased pulsatile load, resulting from stiffness of aorta, on the glomerulus distal to them.
Coronary arterial disease (CAD). The high mortality in the early years of SSc is caused mainly by CV disease.69,83,88–91 Possibly due to the difficulty of distinguishing primary SSc myocardial disease from CAD,92 the association between CAD and SSc has only recently been demonstrated.23–30 But it has been known for decades that AMI occurs in SSc patients with normal coronary arteries.47,93 Myocardial cells are strictly aerobic and the rate of blood flow through the heart is controlled mainly in response to its needs for oxygen. AMI induced by cold spells may result in deaths from acute coronary events rather chronic ischemic heart failure disease.1 AMl-mortality-cold association is mainly explained by coronary vasoconstriction due to cold-enhanced sympathetic activity.1,2 Thus, in the absence of significant atherosclerotic epicardial obstructions, intramyocardial prearterioles and arterioles determine the resistance to blood flow due to their great capacity for dilatation. These vessels are also the ones that are characteristically involved cardiac vasculopathy of SSc.15 Taken into account cardiac RP and considering the whole, myocardium appears especially vulnerable to acute ischemia in SSc. In SSc patients the risk of acute myocardial infarction (AMI) is highest within the first year following SSc diagnosis.26 Furthermore, patients with RP have higher CV risk,94 according to this, a minority of patients with AMI presented RP prior to admission to ICU (personal observation, unpublished data). In this setting, it makes sense to speculate that in some patients, severe cardiac ischemia (AMI, sudden cardiac death, congestive heart failure), may be the first manifestation of SSc.
Improved prognosis for SSc patientsThe phenotype of vasculopathy95 and of SSc itself is changing due to earlier diagnosis and broader treatments with vasoactive and immunosuppressive drugs.96 Thus, SRC prevalence has declined,94 and its seasonal pattern seems to have disappeared.86 Survival rate of SSc patients is on the raise,90,96,97 and in Spain in recent decades we have observed fewer SSc patients suffering from digital necrosis in emergency departments in winter. However, the increase in Te in recent decades and the improvement of heating systems may have contributed to these changes by decreasing the vascular pathogenesis mechanism. In this sense, benignity of RP and SSc in Thailand has been attributed to genetic factors and tropical climate.98
EthnicityIt seems that humans in general are less proficient in adapting to cold in the long term than to heat, possibly because we come from Africa, where there have been no major changes since man first appeared.99 Despite this, natives in cold terrestrial areas seem to have developed behavioral and physiological adaptations to cold.1 Multiple factors linked to race, including genetic, environmental factors and epigenetic influences, may modulate SSc disease manifestations.100 Black patients appear to have more severe SSc clinical phenotype than white ones. Accordingly, compared to whites, in recent studies of large multiethnic cohorts with SSc in France91 and United States,100,101 subgroups of African origin had worse prognosis. By other side, these associations do not count for cultural and social determinants of health, such as poverty and access to care.102 Poverty with poor heating is closely related to cold Te and to hypothermia in temperate and cold climate countries. In this sense, when socioeconomic status was taken into account differences between races disappeared.101 Furthermore, in the only study carried out in a publicly funded universal healthcare system (Canada), despite ethnic variation in SSc manifestations, there were no differences in short-term survival across ethnicities.102 It may be that health inequities/socioeconomic status,1,100,101 acclimatization (1)/generational status (foreign-born versus native-born) and acculturation100 are potential confounding variables on these racial data. Te and body thermoregulation influence these variables.
Patient's agePatients with digital ulcers (severe RP) are younger at SSc diagnosis.64,66 The higher mortality rates in younger-age groups,88 may be related, via visceral RP/MVD, to the intact reflect vasoconstrictor response with cold exposure at that age.11 At the other end, aging is associated with an attenuated cold-induced vasoconstriction and subsequent higher skin blood flow, which in turn leads to greater heat loss.11 Aging is also associated with an impaired ability to increase thermogenesis with cold. AII together leads to increased susceptibility to hypothermia,11 which together with age-related atherosclerosis may contribute to the worse prognosis of late onset SSc.88,89,91
Heat strokesDespite inability to increase skin flow and impaired sweating during heat Te in SSc patients, there is only one published case (PubMed) of heat stroke in SSc.40 Authors believe that there are more cases, but they go unnoticed because dSSc is a rare disease and because large cohorts of patients with SSc come almost exclusively from countries with temperate or cold climates.40 Possibly the already mentioned decrease in visceral thermogenesis (low Tc), protects these patients in hot weather. With heat waves currently on the rise through the world, including geographic zones traditionally classified as temperate or cold climates, therefore, more heat strokes and more AMI1 can be expected.
This work has several limitations. The paper focus on vascular hypothesis as pathophysiological mechanism of SSc and on many, but not all, of its clinical manifestations. There is no integrated view of its pathogenesis (autoimmunity, genetic, etc.). But the aim of this article is to discuss a little studied aspect of SSc, and SSc heterogeneity is well known. The assumption that RP is more severe at the onset of SSc, although very logical, remains unproven. The discussion of the effects of Te on thermoregulation in these patients has been over-simplified; these effects can vary depending on intensity or duration of exposure to cold, or during transition to cooler temperatures in autumn, as found in RP103 and CAD.2
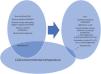
Conclusionlt is postulated that in early SSc, especially with cold ambient temperature, systemic endothelial damage causes a severe oxygen supply/consumption imbalance in internal organs, leading to early mortality in SSc. Due to profound changes in physiological mechanisms of thermoregulation in SSc, it is feasible to hypothesize that patients with SSc, or a subgroup in some circumstances, may have lost or reduced their physiological adaptation to cold characteristic of warm-blooded animals. SSc is a candidate for triggering secondary hypothermia. In any case. It is suggested that SSc could be a human adaptive response to cold Te and systemic endothelial injury/vasculopathy with resultant visceral ischemia leading to low metabolism (Fig. 1). The impact of Te and Tc on SSc warrants further investigations, especially in patients with recent onset SSc and those with acute-subacute visceral failure.
Scheme of the proposed model of cold Te adaptation in SSc patients. MVD: microvascular dysfunction; Tc: body core temperature. Cold ambient temperature has less effect on † non-onset (evolved) SSc patients. ‡ Especially on skin surfaces most exposed to cold Te and most thermoregulatory (rich in arteriovenous anastomosis); cheeks, fingers, nose.
Table 1 shows a summary of the different sections of this article.
Summary of the different sections of this article.
| Physiological thermoregulation | Heat generated in viscera and muscles (thermogenesis) and rate of its loss to the outside largely determine Tc. Heat loss is regulated by the subcutaneous blood circulation. Rate heat loss and thermogenesis depend to a large extent on Te. |
| Vasculopathy of SSc | In very early/early SSc there is severe cutaneous and visceral RP/MVD, followed by irreversible arteriolar lesions causing tissue ischemia/fibrosis. Structural stenosing macroangiopathy of SSc (with arterial stiffness) is distal and upper limb predominance. |
| Tissue ischemic microenvironment | Tissue ischemia would favor metabolic reprogramming towards glycolysis. Glycolysis generates intermediates that increase protein synthesis and produce less ATP energy, which would favor cell survival in this situation. Lactic acid microenvironment (final metabolite of glycolysis), drives the endothelial–mesenchymal transition and promotes differentiation of MSC-like cells to myofibroblast signature. |
| Thermoregulation in SSc patients | SSc patients have a slow metabolism due to chronic tissue ischemia and malnutrition. This prevents them from generating sufficient heat with cold Te. In addition, heat conduction from the viscera to the skin is slower. |
| Vascular hypothesis | At cold Te, endothelial injury causes an acute and widespread RP–MVD and visceral imbalance between oxygen supply and consumption causing vascular organ damage, especially severe in VEDOS/early SSc patients with viscera intact. At cold Te SSc patients have an impaired ability to increase thermogenesis, and a delay and decrease of heat loss generated in their viscera. This delicate balance raises the possibility that some SSc patients in some circumstances have low Tc (hypothermia). |
| Hypothermia in SSc patients | This delicate balance would be broken with colder Te or with a rapid drop in Tc; being unable to generate heat quickly these patients, low Tc would increase or appear with resultant visceral damage/failure. |
| Atherosclerosis and SSc | The boundaries macrovascular structural angiopathy between SSc and atherosclerosis are not well defined. There is an additive/synergistic effect between the two. As the survival of SSc patients is increasing, their aging allows atherosclerosis and its risk factors to partially overshadow the pre-existing SSc vasculopathy, but young SSc patients, especially those genetically predisposed, are leading candidates for exitus/severe visceral involvement due to severe RP/MVD. |
| Raynaud's phenomenon | Serious visceral RP/MVD at SSc onset could explain the inverse relationship between severity of SSc and time elapsed between RP and next SSc clinical symptom. |
| Pulmonary hypertension | Although the lung is the only viscera directly exposed to cold Te, studies of cold-induced pulmonary RP are inconsistent to date. Pulmonary arterial pressure is elevated with cold Te, and it is even greater when hypoxia is present (high altitude). Survival of SSc patients with both pulmonary hypertension (PH) and ILD is worse than those with isolated SScPH or SScILD. |
| Metabolic syndrome | MetS is an important predictor of CV disease in the general population. MetS has recently been identified in patients with SSc, being associated with subclinical atherosclerosis. Prevalence of MetS increases with age. Thus, MetS may contribute to increased mortality of elderly SSc patients |
| Severe early visceral damage | Scleroderma renal crisis (SRC). Vascular damage due to ischemia predominates over the immune system in SRC. SRC appears in the first years of SSc, possibly with severe renal RP. Prevalence in autumn and winter of SRC initially described has now disappeared. Coronary arterial disease. Early mortality in SSc is mainly due to CV disease. Acute myocardial infarction (AMI) occurs in patients with SSc with normal coronary arteries. The association AMI-cold mortality is mainly due to coronary vasoconstriction. Considering also cardiac RP and other factors, it can be speculated that AMI could be the first visceral manifestation of SSc. |
| Improved prognosis of SSc patients | The increase in Te and improved heating systems in recent decades may have contributed to the disappearance of SRC seasonal rhythm and increased survival of SSc patients by decreasing the pathophysiological vascular mechanism. |
| Ethnicity | Worse prognosis of SSc black population is clearly influenced by socioeconomic status. Poverty with poor heating is associated with cold Te and hypothermia in countries with cold or temperate climates. |
| Patient's age | Patient's age. Mortality rates in younger-age SSc patients may be related, via visceral RP/MVD, to intact reflect vasoconstrictor response with cold exposure at that age. At the other end, aging is associated with attenuated cold-induced vasoconstriction and subsequent higher skin blood flow, which in turn leads to greater heat loss. Aging is also associated with an impaired ability to increase cold thermogenesis. |
| Conclusion | In early SSc, especially with cold Te, systemic endothelial damage causes a severe oxygen supply/consumption visceral imbalance, leading to early mortality. SSc is a candidate for secondary hypothermia It is suggested that SSc disease could be a human adaptive response to cold Te and systemic endothelial injury/vasculopathy with resultant visceral ischemia leading to low metabolism. Impact of Te and Tc on SSc warrants further investigations. |
I have used IA (DeepL) to help translation.
Conflict of interestThe author declares no conflict of interest.