To review the available evidence on the impact of rheumatoid arthritis (RA) treatments in associated diffuse interstitial lung disease (ILD).
MethodsSystematic review of studies evaluating the impact of pharmacological treatment in patients with RA and ILD. A bibliographic search in MEDLINE, EMBASE and Cochrane, a selection of articles and the methodological quality assessment (FLC 3.0 OSTEBA) and grading of the level of evidence (SING) of the selected articles were performed.
Results1,720 references were identified in primary search and 7 in manual or indirect. Forty-three articles were included: 7 systematic reviews, 2 randomized clinical trials, 5 cohort studies, 8 case-control studies and 21 case series. Methotrexate (MTX) and leflunomide (LEF) do not increase incidence, complications or mortality due to ILD. Although the results are not uniform, anti-TNF have often had worse outcomes in incidence, progression and mortality due to ILD than MTX, LEF, abatacept (ABA) and rituximab (RTX). The evidence found is scarce for JAK kinase and antifibrotic inhibitors, and controversial for IL-6 inhibitors.
ConclusionsThere is no evidence that MTX or LEF worsens the prognosis of patients with AR-EPID. RTX and ABA seem to have better results than other biologicals, such us TNFi, often achieving stabilization and, in some cases, the improvement of ILD in patients with RA.
Revisar la evidencia disponible sobre la repercusión de los tratamientos de la artritis reumatoide (AR) en la enfermedad pulmonar intersticial difusa (EPID) asociada.
MétodosRevisión sistemática de estudios que evalúan el impacto del tratamiento farmacológico en pacientes con AR y EPID. Se realizó una búsqueda bibliográfica en MEDLINE, EMBASE y Cochrane, selección de artículos y evaluación de la calidad metodológica (FLC 3.0 OSTEBA) y graduación del nivel de evidencia (SING).
ResultadosSe identificaron 1.720 referencias en búsqueda primaria y 7 en manual o indirecta. Se incluyeron 43 artículos: 7 revisiones sistemáticas, 2 ensayos clínicos aleatorizados, 5 estudios de cohortes, 8 estudios de casos-controles y 21 series de casos. Metotrexato (MTX) y leflunomida (LEF) no aumentan la incidencia, complicaciones ni mortalidad por EPID. Aunque los resultados no son uniformes, los anti-TNF han tenido con frecuencia peores resultados en incidencia, progresión y mortalidad por EPID que MTX, LEF, abatacept (ABA) y rituximab (RTX). La evidencia encontrada es escasa para los inhibidores de JAK quinasas y antifibróticos, y controvertida para los inhibidores de la IL-6.
ConclusionesNo existe evidencia de que MTX o LEF empeoren el pronóstico de los pacientes con AR-EPID. RTX y ABA parecen tener mejores resultados que otros biológicos, como anti-TNF, consiguiendo con frecuencia la estabilización y, en algunos casos, la mejoría de la EPID en pacientes con AR.
Rheumatoid arthritis (RA) is a chronic immune-mediated inflammatory disease of unknown aetiology. It primarily affects the joints, but extra-articular clinical manifestations are common. The lung is one of the most frequently affected organs and it causes significant morbidity and mortality.
Diffuse interstitial lung disease (ILD) is the most common pulmonary manifestation in RA, with an estimated incidence of 4–4.5 cases/1,000 patients/year.1,2 Its prevalence ranges from 10%–30% of cases in early RA (<2 years’ duration), and from 3.6%–42% in established RA.2,3 This large variation is because studies are very heterogeneous with different diagnostic methods, populations are not very homogeneous, and there is a lack of terminology and validated classification criteria.
ILD is included in the international classification of idiopathic interstitial pneumonia of the American and European Pneumology Societies, which take histopathological findings into account. The most common forms are usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP) (44%–46% and 33%–44%, respectively), although cases have been reported with all the anatomopathological forms described in the context of the disease.4
ILD in RA is associated with certain risk factors such as male gender, older age, smoking and RF and ACPA-positivity.5 The clinical course of ILD varies from asymptomatic to rapidly progressive disease in a minority of cases. It has been estimated that patients with RA and ILD have up to three times the risk of death than those without ILD, constituting the second cause of mortality after cardiovascular disease.3,6 This high mortality has been attributed to uncontrolled systemic inflammatory burden, infections and complications of therapies. Treatment of RA has improved substantially in recent years with the introduction of the biological therapies; however, use of these agents has been restricted in patients with ILD due to concerns about their safety.
Despite the frequency and potential severity, there is currently no consensus on the most appropriate treatment for patients with RA and ILD. We conducted a systematic review (SR) of the literature with the aim of answering the research question with the best available evidence: In patients with RA and associated ILD, what is the impact of treatments used in RA on ILD in terms of efficacy and safety?
Material and methodsWe undertook a SR of treatments of patients with RA and associated ILD.
Search strategy. We conducted a sensitive search on MEDLINE (from 1966), EMBASE (from 1974) and COCHRANE (from 1992) up to 31 September 2019, using MESH terms and free text. The strategy included synonyms for 'rheumatoid arthritis', 'diffuse interstitial lung disease', 'disease-modifying anti-rheumatic drugs (DMARDs)' and the different DMARDs and immunosuppressants (IS), and for the terms 'response' and 'mortality'. The search was restricted to human studies published in English, French or Spanish. The process of selecting studies for review was conducted in parallel and independently by two reviewers (CCC and ECC) in two steps: 1) selection by title and abstract of the studies located through the literature search and 2) selection by full text of those previously selected, eliminating articles that did not meet the inclusion criteria. Any doubts or discrepancies were resolved after discussion and when there was no consensus a third reviewer (PVC) was consulted. Discussions and agreements were documented. A secondary manual search of the literature of the studies included was also carried out. EndNote X8 was used to manage the bibliographical references.
Study selection criteria. The selection criteria for studies focusing on the population, intervention, comparison, and outcomes (PICO) related to the research question were defined prior to the search. We included studies conducted with adult patients, diagnosed with RA (ACR 1987, ACR/EULAR 2010 criteria) and associated ILD diagnosed by high-resolution computed tomography (HRCT) of the chest (UIP or NSIP radiological patterns) and/or respiratory function tests (FVC and/or DLCO) and treated pharmacologically (with DMARDs, glucocorticoids, IS or antifibrotics). The comparison intervention was a placebo or any of the other treatments. The studies had to include one of the following outcomes: efficacy (improvement of ILD), pulmonary toxicity (worsening or onset of ILD) and outcomes, measured by survival or mortality rates during treatment. Changes in ILD were assessed by HRCT, FVC and/or DLCO. In terms of study design, meta-analyses, SR, RCTs and in the event of insufficient evidence to answer the research question, observational studies (cohorts, case-controls, and case series) were included. Isolated clinical cases were excluded, as well as acute pneumonitis as an outcome and non-pharmacological treatments.
Data extraction and quality assessment. The quality assessment of the selected studies was performed using OSTEBA7 critical appraisal card templates and the level of evidence was graded using SIGN (Scottish Intercollegiate Guidelines Network) levels of evidence.8
This review was not prospectively recorded in the PROSPERO International Prospective Register of Systematic Reviews.
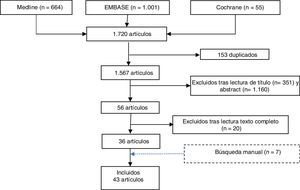
ResultsThe literature search identified 1,720 references. Once duplicates (153) had been eliminated, 1,511 references were screened by title and abstract and 56 articles were selected for evaluation. After full text reading, 20 studies were excluded, specifying the reason for exclusion (Appendix B annex 1). A further 7 studies were selected by manual search (Fig. 1).
A total of 43 studies were included: 7 SR, 2 RCTs (one of RTX and the other nintedanib), 5 cohort studies, 8 case-control studies and 21 case series (most RTX). The studies focussed on RA-associated ILD and aimed to evaluate the efficacy and safety of usual RA treatments on progression of ILD.
The quality of the studies included in this SR is generally average, although it varies with the different therapies. The most relevant data of the 7 SRs included are described in Table 1. Table 2 summarises the only two RCTs selected: one open-label, which investigates the effect of RTX on RA-associated ILD, 9 and another double-blind, which evaluates nintedanib in patients with fibrosing diseases.10 In the latter, there were 89 patients with RA and ILD, but we only have global results, not separated per disease.
Systematic reviews and meta-analysis.
| Author, year | Population, n | Results | Conclusions | Quality |
|---|---|---|---|---|
| Conway, 201411 | 22 RCT with 8,584 patients with RA.RR of MTX vs. comparator. | -RR non-infectious respiratory complications: 1.02 (.65–1.60),-RR acute pneumonitis: 7.81 (1.76–34.72)-RR death due to lung disease: 1.53 (.46−5.01). | No greater risk of non-infectious respiratory events or death due to lung disease. | High |
| Conway, 201612 | 8 RCT with a total of 4,579 patients: 2,274 with LEF and 2,305 with comparator (MTX) | - RR total respiratory adverse effects: .99 (.56−1.78).- RR infectious adverse effects:1.02 (.58−1.82).- RR non-infectious adverse effects: .64 (.41−.97). | There is no increase in respiratory adverse effects in patients with RA treated with LEF in double-blind RCT. | High |
| Huang, 201913 | 7 studies with 22,981 patients and 28 clinical cases - 40 patients. | TNFi greater risk of ILD events and mortality, compared with RTX and ABA. | This risk of TNFis is greater at older age and with pre-existing ILD. | Medium |
| Nelson, 201514 | 21 studies, 8 RCTs: 4,149 patients treated with ABA with exposure of 12132 patients/year. | During the combined long-term period, ILD was reported in 11 patients (IR = .11 (.06−.20). | There was no increase in the ILD incidence rate in patients who received ABA. | Medium |
| Hadjinicolaou 201115 | 1. RTX: 65 studies. 1 RCT with 316 patients.2. TCZ: 3 RCT, 589 patients.3. GOLI: 5 phase 3 RCTs | 1. RTX: 121 cases of ILD, in haematological processes.2. TCZ: 1% ILD (6 cases).3. GOLI: 3 cases.4. ABA: no case. | Association between use of TCZ, RTX and GOLI and development of ILD in patients with RA. | Medium |
| Roubille, 201416 | 88 case reports: RA with induced or exacerbated ILD. Exposure to biologics and non-biologics.Evaluate toxicity. | -Toxicity with biologics: 43 (31 with TNFi, mortality 35.5%; 12 non-TNFi ).-Toxicity non- biologics: 96 (34 LEF, mortality: 18%; 62 MTX, mortality 13%). | Rare drug induced ILD (1%).High mortality: 13% MTX, 18% LEF, 35.5% TNFi. Frequent respiratory infections (TB). | Low |
| Pérez-Álvarez, 201117 | Most are clinical cases.2 post-marketing studies.2 retrospective studies. | Onset or exacerbation of ILD occurred primarily with anti-TNF (58 ETN and 56 INF). | Anti-TNF should not be used in patients with pre-existing ILD. | Low |
Randomised clinical trials.
| Author, year | Design | Population (n) | Intervention | Comparator | Objectives | Results |
|---|---|---|---|---|---|---|
| Matteson, 20129 | Open pilot study. | RA-ILD (10)Follow-up of 48 weeks. | RTX1 g, days 1 and 15,+MTX. | --- | Clinical effect of RTX on RA-ILDDLCO/FVC/HRCT | 7 patients completed week 48: 2/7 improved, 4/7 stable and 1/7 worsened.No differences UIP-Non-UIP. |
| Flaherty, 201910 | RCTINBUILD Study. | SAD 663 patients.RA-UIP 89.Follow-up of 52 weeks. | Nintedanib 150 mgTwice/day42 patients. | Placebo(47 patients). | FVC | Improved FVC (in patients with a fibrotic pattern similar to IPF, the adjusted rate of reduction in FVC was -82.9 ml per year with nintedanib and -211.1 ml per year with placebo, with a difference of 128.2 ml (70.8−185.6); P < .001).Diarrhoea 66.9% (vs. 23.9% in placebo). |
Two other RCTs are currently underway in patients with RA-associated ILD: one in phase II with pirfenidone (NCT02808871) and another in phase III with abatacept (NCT03084419), but results are not yet available for either.
Tables 3 and 4 show the efficacy of conventional synthetic DMARDs (csDMARDs), glucocorticoids (GC) and IS on ILD in patients with RA. Table 5 shows the cohort and case-control studies of anti-TNF and non-anti-TNF biologics and Table 6 provides a summary according to levels of evidence.
Studies that evaluate efficacy and survival with csDMARDs.
| Author, year | Population, n | Design | Objectives | Results | Conclusions |
|---|---|---|---|---|---|
| Kiely, 201918 | RA-ILD: 2692MTX: 1578No MTX: 1114 | Prospective | Onset of new or exacerbation of pre-existing ILD. | Exposure to MTX not associated with ILD OR = .85, (.49–1.49), P = .578.Exposed to MTX: 2.5% ILD,Not exposed to MTX: 4.8% ILD. | MTX not associated with a greater risk of ILD; on the contrary, it can delay its onset |
| Rojas-Serrano, 201219 | RA-ILD 40 | Retrospective4 months. | Efficacy (lung function). | MTX was the most used DMARD (45%), improved FVC at 4 months. | Improved FVC after csDMARD. |
| Rojas-Serrano, 201720 | RA-ILD 78Cases (MTX): 52Controls: 26 | RetrospectiveFollow-up: 1,956.5 months. | Prognostic factors and survival. | Factors associated with mortality:- Extent of ILD (HRCT).- Advanced age: HR: 1.04; (1.003−1.09).- Male sex: HR: 2.78; (.98−7.9). | Better survival MTX (multivariate analysis).UIP no worse prognosis. |
| Detorakis, 201721 | RA-ILD 42RA without ILD 40 | Prospective | Efficacy and safety anti-TNF agents vs. MTX. | Negative correlation between use of MTX and extent of ILD. | There was no de novo ILD or exacerbation of pre-existing ILD with MTX. |
| Dixon, 201022 | RA-ILD 367 | Retrospective.Anti-TNF (299) vs. csDMARD (68). | Mortality. | Mortality Anti-TNF: 21%.Mortality csDMARD: 7%. | Less mortality csDMARD (MTX). |
Studies that evaluate efficacy and mortality due to glucocorticoids and immunosuppressants.
| Author, year | Design and population | Treatment | Results | Conclusions |
|---|---|---|---|---|
| Ota, 201723 | Case series: 17 patients with RA-ILD, exacerbation (admission).Follow-up: 474 days. | GCTacrolimusCPMCyclosporine | Efficacy: IS therapy IS suppressed deterioration in lung function, with improved ground- glass opacity.Those with less fibrosis on HRCT: better response. | CPM group good prognosis.For severe cases with low respiratory function, intensive therapy including CPM could improve prognosis (limitations). |
| Fischer, 201324 | Case series, CTD (125)18 RA-ILD.2.5 years follow-up. | MMF | Efficacy: aggregated efficacy result: MMF stabilizes/improves lung function (FVC, DLCO). | Good GC sparer.Improved FVC.Well tolerated. |
| Kelly, 201625 | Retrospective of 1 cohort of 290 patients RA-ILD,Follow-up >12 years | PrednisoneAZAMMF | Mortality: 186 deaths (110 due to respiratory cause).RR of death due to respiratory cause:- GC: 2.10 (1.7−2.9)- AZA: 1.62 (1.2−2.1)- MMF: 1.03 (.7−1.5) | MMF associated with lower RR of all-cause death and lower RR respiratory cause death, compared with AZA and GC. |
| Scott, 201426 | Cases-control154 RA-ILD (cases)36 controls. | Oral prednisone >3 months. | Mortality: RR of death:cases: 1.65 (1.2−2.3) P = .002,control group 1.07 (.7−1.6) NS. | Greater RR death with GC (respiratory). |
Cohort and case-control studies of anti-TNF and non-anti-TNF biologics.
| Author, year | Design | Population (n) | Exp. G. | Control G. | Objectives | Conclusions |
|---|---|---|---|---|---|---|
| Druce, 201727 | CohortBSRBB | RA-ILD (352) | RTX | TNFi | Mortality in RA-ILD who received RTX or TNFi, as 1st biologic. | Higher mortality with TNFi than RTX. |
| Dixon, 201022 | CohortBSRBB | RA-ILD (367) | 299 Anti-TNF | 68 csDMARDs | Mortality due to ILD. | Greater mortality with anti-TNF (21%) than DMARDs (7%). |
| Curtis, 201528 | Case-control | RA (13795) | Anti-TNF | ABA,RTX andTCZ | Incidence of ILD and its exacerbation of ABA, RTX, TCZ, compared with anti-TNF. | There were no significant differences. |
| Nakashita 201429 | Case-control, | RA (163) | TNFi | TCZ, ABA. | Risk of ILD onset / exacerbation. | TNFi: potential risk ILD events, especially if pre-existing ILD. |
| Nakashita, 201230 | Case-control | RA-ILD (60) | Anti-TNF(INF, ETN, ADA) | TCZ, ABA. | Exacerbation ILD. | Greater exacerbation rate with. |
| Detorakis, 201721 | Cohort | RA (168) | Anti-TNF | MTX | Efficacy and safety anti-TNF vs. MTX. | ILD incidence or exacerbation not increased with anti-TNF. |
| Palmer, 201431 | Case-control, | RA-ILD (188)RA without ILD (188) | Anti-TNF | RTX | Mortality. | Greater mortality with anti-TNF than RTX. |
| Odden, 201032 | Case series | RA-ILD (23) | Anti-TNF | --- | Evaluate FVC, 1 year before and 6 months after anti-TNF. | No significant differences short term FVC. |
Table evaluating the quality of results.
| Summary of the evidence | LE |
|---|---|
| Drug induced ILD in patients with RA is rare (1%) (Roubille 2014)16 | 1+ |
| Patients with RA and ILD have no greater risk of non-infection respiratory complications or mortality due to respiratory cause, when they receive MTX than when they receive other csDMARDs or biologics (Conway, 2014)11 | 1++ |
| RA patients treated with MTX do not present a higher incidence of ILD and it could even delay its onset (Kiely 2019)18 | 2+ |
| Patients with RA-associated ILD who receive MTX have higher survival (Rojas-Serrano 2017)20 | 2+ |
| Patients with RA treated with LEF have no greater risk of ILD or lung complications (Conway 2016)12 | 1++ |
| Patients with RA-associated ILD treated with GC have higher all-cause and respiratory-cause mortality (Scott 2014)26 | 2++ |
| Immunosuppressive therapy can be effective for RA-associated ILD exacerbations (Ota 2017)23 | 3 |
| RA associated ILD has higher mortality with TNFi than with csDMARDs (MTX or LEF) (Roubille 2014)16 | 1+ |
| TNFi could be associated with a greater risk of ILD-related complications and mortality in RA patients, especially if they are of advanced age and with pre-existing ILD (Huang 2019, Koo, 2015)13,33 | 1+ |
| Patients with RA and associated ILD have higher mortality than patients without ILD (Palmer 2014)31 | 2+ |
| Patients with RA and associated ILD are more likely to be treated with biologics than patients without ILD (Palmer 2014)31 | 2+ |
| Patients with RA-associated ILD have higher survival when treated first-line with RTX than with TNFi (Druce, 2017)27 | 2+ |
| RTX stabilized functional and radiological parameters in more than half the patients with RA and progressive ILD (Matteson, 2012)9 | 1+ |
| Most of the patients with RA and stable ILD remained stable with RTX (Kabia 2015)34 | 3 |
| Abatacept does not increase the incidence of ILD and stabilizes/improves pre-existing ILD parameters in patients with RA (Nelson 2015)14 | 1+ |
| The risk of worsening of pre-existing ILD in patients with RA is less with ABA than with TNFi (Nakashita 2016)35 | 3 |
The prevalence of ILD in RA is variable. A recent prospective multicentre study18 found that 3.7% of patients with recent onset RA had ILD, associated with older age, smoking, male gender, activity, seropositivity and delayed DMARD treatment. Following our review, a retrospective study36 was published in which development of ILD was found to correlate with these same factors, with a short duration of RA and increased lactate dehydrogenase (LDH) levels, but not with the use of csDMARDs in RA. RA treatment-induced ILD is more rare (<1%).16 The outcome of a pre-existing ILD may be different depending on the drug used.
Conventional synthetic DMARDs and immunosuppressantsStudies in RA and ILD patients treated with csDMARDs are scarce and contradictory, which has led to restriction of their use in this complication.
Methotrexate (MTX). Considered a prototype of drug-induced pulmonary toxicity, MTX has been avoided until a few years ago in the treatment of RA patients with ILDs. We now have evidence from two SRs11,16 and two cohort studies,18,20 which address the relationship of MTX with ILD onset, progression, and mortality. No association was found between MTX exposure and onset of ILD (OR: .85; (.49–1.49); P = .58) in a prospective study conducted by Kiely et al.18 in 2,692 patients with recent-onset RA (1,578 exposed to MTX and 1,114 not exposed). In the patients exposed to MTX, ILD onset was longer than in those not exposed (OR: .41; (.23–.75); P = .004). Conway et al.11 found in an MA of 22 studies with 8,584 RA patients, that MTX produced a small increase in the risk of total respiratory complications (RR: 1.10 (1.02–1.19), due to the increased risk of infections (RR: 1.11 (1.02–1.21). However, they found no increase in the risk of non-infectious respiratory complications (RR 1.02 (.6–1.60), or death from lung disease (RR 1.53 (.46−5.01). MTX produced an increased risk of acute pneumonitis (RR: 7.81 (1.76–34.72), but not of chronic interstitial pneumopathy (IP).
The risk of hospital admission due to ILD in RA patients treated with biologics decreased with MTX exposure (HR: .06 (80.06-.469); P < .001), irrespective of concomitant biologic therapy (anti-TNF vs. non-anti-TNF), in a retrospective study using data from US mutual health insurance companies.28 MTX was also not found to be associated with the onset or exacerbation of ILD in RA in a Greek study comparing the outcome of treatment of RA over 1 year with anti-TNF agents and csDMARDs in patients with ILD (42 and 44) and without ILD (40 and 44, respectively).21 In contrast, the multivariate analysis showed a negative correlation of MTX use with extent of ILD and degree of air entrapment.
The survival of patients with RA-ILD, adjusted for confounding variables, was higher with MTX (median 70 months) than without MTX (log Rank P < .0005), in a retrospective study by Rojas-Serrano et al.20 The 56 cases (25 with associated LEF) and the 26 controls (4 AZA, 9 LEF, 4 SSZ and 9 without DMARDs) had received the same GC regimen.
In an MA, Roubille et al.16 found lower mortality of patients with ILD in RA with MTX (13%) than with TNFi (35.5%).
Leflunomide (LEF). After it was marketed, cases of ILD in RA patients treated with LEF began to be published, especially in Japan. In 2016, Suissa et al.37 published an extensive case-control study nested in an RA cohort, in which they admit that the 2-fold increase in ILD associated with LEF could reveal a treatment channelling bias of patients with pre-existing ILD to LEF. Conway et al.12 also published an SR and an MA of 8 RCTs (only 1 in an Asian population) of LEF in RA. They included 4579 patients, of whom 2274 received LEF and 2305 a comparator (usually MTX) and found 6 cases of pneumonitis and 4 deaths from lung causes, all in the comparator group. LEF was not associated with an increased risk of total respiratory adverse effects (RR: .99 (.56−1.78), infectious (RR: 1.02 (.58−1.82) or non-infectious adverse effects (RR: .64 (.41−.97)), or an increased risk of death from lung disease (RR: 1.53 (.46−5.01). The abovementioned SR of cases of RA-associated ILD16 included 12 studies with 34 cases of ILD in RA treated with LEF. In this study, Roubille et al. estimated a fatal outcome in 18% of patients with LEF, compared to 13% of those with MTX.
The study by Rojas-Serrano et al.,20 which showed increased survival in RA-ILD of patients treated with MTX, observed that combination with LEF (25/58) did not worsen survival.
Glucocorticoids (GC) and Immunosuppressants (IS). Glucocorticoids (GC) and Immunosuppressants (IS). There is little evidence of the effect of IS on RA-associated ILD. They are sometimes used in the treatment of exacerbations of ILD with good response according to some studies, whose authors conclude that intensive therapy with GC and cyclophosphamide (CPM) could improve prognosis in severe cases, with low respiratory function.
The effect of oral GC on mortality in RA-ILD was evaluated in a case-control study,26 which found that patients who received oral prednisone for more than 3 months had a higher relative risk of death than those who did not (RR: 2.06 (1.1–3.8); P = .02), especially from respiratory causes (RR: 2.75 (1.6–4.7); P = .0002). The predominance of the UIP pattern in the GC treatment group may account for some of the excess mortality.
In recent years, mycophenolate mofetil (MMF) has replaced CPM and azathioprine (AZA) in the treatment of ILD associated with systemic autoimmune diseases (SADs). Fisher et al.24 retrospectively analysed the effects of MMF on the progression of ILD in 125 SAD patients, of whom 18 had RA. In general, MMF was well tolerated and allowed the dose of CG to be reduced. In RA-associated ILD, the previous trend of progressive deterioration changed to an improvement in FVC after starting MMF, although it did not reach statistical significance. MMF was also associated with an RR of death from any cause, and from respiratory causes, lower than AZA and prednisone, in a retrospective UK study25 of 290 RA-ILD patients followed up over more than 12 years. These results are consistent with the results of the PANTHER study38on idiopathic pulmonary fibrosis (IPF), which showed an increase in mortality associated with the use of prednisone and AZA, compared with placebo.
Regarding Tacrolimus, with no indication in our setting for treating patients with RA, we only found the anecdotal description of 10 cases of RA39 that developed lung lesions during treatment (with fatal outcome in 2), and therefore we cannot draw any conclusions.
Anti-TNF biological DMARDsWe found 3 SRs13,16,17 that deal with the effect of anti-TNF therapies on patients with RA-associated ILD, but the methodology used calls for caution in interpreting the results.
The SR by Roubille et al.16 were based on published clinical cases. They found that TNFi are associated with a low incidence of ILD (1%), but high mortality (35.5%).
In a more recent SR of cohort, case-control and case report studies, Huang et al.13 analysed the onset or worsening of ILD (ILD event) in RA patients treated with TNFi compared to non-TNFi biologics. TNFis were implicated in 85% of detected ILD events. Events and mortality were associated with older age and concomitant use of AZA. They found that patients with RA-associated ILD treated with TNFi may be at higher risk of adverse events and mortality from ILD than those treated with ABA or RTX, especially at older age and with pre-existing ILD.
An SR conducted in Spain on patients with SAD who developed ILD secondary to the use of biologics included clinical cases, retrospective and post-marketing studies.17 Only 52 cases of ILD had sufficient information, of which 38 had RA. Of these, 13 patients died, who were of more advanced age, had pre-existing ILD and immunosuppressive treatment more frequently than the 25 survivors. The authors conclude that anti-TNF therapy should not be used in patients with pre-existing ILD.
There are several cohort and case-control studies involving anti-TNFs compared with csDMARDs21,22 or non-anti-TNF bDMARDs.27–31 Based on data from the British Society for Rheumatology Biologics Register, Dixon et al.22 conducted an observational study to assess the influence of anti-TNF therapy on overall mortality, and specifically that due to ILD, in patients with RA and pre-existing ILD. A prevalence of ILD of 2.6% in patients with RA was found, being higher in those treated with anti-TNF therapy (299/9294) than with csDMARDs (68/2454) (2.9% vs. 1.8%; P = .02). This prevalence supports the suspicion that only a fraction of patients with this complication are identified in the clinic. No significant differences were found between either group for standardized mortality rate or ILD mortality, although the latter was numerically higher with anti-TNF (15/70) than with csDMARDs (1/14) (21% vs. 7%; NS). Pre-existing ILD was a strong predictor of all-cause mortality in both cohorts. The authors conclude that, despite the results, it should not be assumed that any patient with RA-associated ILD can be treated with anti-TNF therapy. Another study, based on the Spanish registry of biological therapies (BIOBADASER),40 also found no significant difference in the incidence of ILD or mortality from this complication in patients treated with anti-TNF therapy compared to those treated with csDMARDs in another cohort (EMECAR).
A Greek cohort study21 compared the efficacy and safety of anti-TNF therapy with that of csDMARDs (MTX) in 170 RA patients with and without ILD. Anti-TNF agents were not associated with the development or progression of ILD, but instead had a beneficial effect (wall thickening and respiratory function tests) in patients with pre-existing ILD.
Two studies investigated the mortality of patients with ILD treated with anti-TNF therapy.27,31 A multicentre study31 found that mortality due to respiratory causes was higher in patients with ILD than in those without ILD (10% vs. 6%; NS) and higher with anti-TNF therapy than with RTX (15% vs 8%; NS). Although statistical significance was not reached, the study results suggest a trend for patients with ILD to have higher mortality and to receive biologics than patients without ILD, and to have longer survival if they receive RTX rather than anti-TNF.
Druce et al. compared 5-year mortality rates and causes in RA and ILD patients treated first-line with RTX (43) or TNFi (309).27 The all-cause mortality rate was 94.8 with TNFi and 53.0 with RTX per 1000 patients/year. RTX compared with TNFi had an HR adjusted for confounders at 5 years of .49 (.23–1.06). Although not statistically significant, this study also suggests that patients with RA-associated ILD who received first-line RTX had a longer survival than those receiving TNFi.
In another study, conducted by Curtis et al.28 from US mutual insurance data, the incidence and exacerbation of ILD in a cohort of RA patients treated with non-anti-TNF bDMARDs compared to anti-TNF therapy was investigated. No difference in the risk of developing ILD or hospitalization due to pre-existing ILD was found among the cohorts, but patients with pre-existing ILD (419 patients with 499 episodes) were more likely to receive RTX (19.8% vs. 8.5%) and less likely to receive anti-TNF therapy (46.5% vs. 59.8%) than controls (10,800 patients without ILD). The risk of hospitalisation decreased with exposure to MTX (HR: .06; 95% CI:.06-.46; P < .001) and increased in men and previous hospitalisations due to asthma or ILD (P < .05). Therefore, there appear to be initial differences between patients receiving anti-TNF therapy or bDMARDs with another mechanism of action, but not in the incidence of ILD or onset of complications.
Nakashita et al. conducted two retrospective studies in patients with RA and ILD treated with biologics.29,30 In both studies, the extent of lung involvement was measured in 3 stages using HRCT. In the first study30 58 patients, 46 cases (with TNF blockers) and 12 controls (9 TCZ and 3 ABA). After one year of follow-up, the exacerbation rate of pre-existing ILD was high for TNF blockers (30.04%) and zero for non-TNFi bDMARDs (0%) (P = .024).
They then assessed the risk of onset/exacerbation of ILD in 163 patients with RA starting a biologic (102 TNFi and 51 non- TNFi), 58 with pre-existing ILD (30 grade I, 22 grade II and 6 grade III) and 105 without ILD.29 After one year of follow-up, ILD events occurred in 17 patients (10%), more frequently in the patients treated with TNFi.
In a retrospective study of 23 patients with RA and ILD treated with anti-TNF agents32 no statistically significant differences in FVC data were found at 6 months of treatment compared to baseline, suggesting that anti-TNF agents do not worsen lung function in the short term in patients with RA-associated ILD.
Non-anti-TNF bDMARDsTocilizumab (TZC). TZC is an interleukin-6 (IL-6) receptor blocker useful in the treatment of joint symptoms and some systemic manifestations of RA. While excessive production of IL-6 has been associated with fibrosis in ILD,29 several clinical cases of de novo onset or exacerbation of ILD following initiation of treatment with TZC have been published.
An SR conducted in 2010 to identify non-infectious pulmonary complications with new biological agents used in rheumatological diseases included 3 RCTs of TCZ with 589 patients diagnosed with RA.15 Six (1%) patients developed non-infectious pulmonary adverse events, including 3 exacerbations of pre-existing ILD (with fatal outcome), 1 recent onset ILD and 1 idiopathic pulmonary fibrosis.
Curtis et al.28 found no statistically significant difference in the incidence of ILD or hospital admissions of patients with pre-existing ILD in 59 patients treated with TCZ compared to 232 who received anti-TNF agents, although the number of biologics prior to TCZ was higher (2.1 ± 1.1 vs. 1.4 ± .7; P < .0001). Nakashita and collaborators also found no increase in the development or exacerbation of ILD in 36 patients treated with TCZ,29 but they did with TNFi.
In a post-marketing surveillance study41 that analysed cumulative safety data from 7,901 Japanese patients treated with TCZ, the incidence of ILD in the treated group was 10 cases/1,000 patients/year, clearly higher than the estimated incidence of the disease (4 and 4.5/1,000 patients/year).
A series of 12 patients with RA-associated ILD (11 UIP) treated with TCZ in a Japanese hospital was published recently,42 in which significant differences in biomarkers of joint activity (MMP-3) but not lung activity (KL-6 and LDH) were observed 6 months after administration of TCZ with respect to baseline values.
The evidence is scanty and study results are not uniform, therefore we must be cautious when drawing conclusions. Controlled studies are required to assess the efficacy of TCZ in patients with RA and ILD.
Rituximab (RTX). The observation of follicular B-cell hyperplasia and interstitial plasma cell infiltrates in lung biopsy specimens from RA and ILD patients provides histological justification for anti-CD20 therapy.43 However, further evidence of efficacy and safety in clinical practice is needed to recommend RTX therapy in these patients. The SR by Hadjinicolaou et al.15 included a RCT of RTX in RA in which ILD developed in 1 of the 136 patients in the active arm (.32%) and none of the 149 controls.
Subsequently, a prospective open-label pilot study was conducted with RTX9 in 10 patients with active and progressive RA and ILD (FVC > 50% and DLCO > 30%), who had not received bDMARDs or IS in the previous 8 weeks. RTX1 g was administered at weeks 0, 2, 24 and 26. There were 3 losses (1 infusion reaction and 2 deaths, from pneumonia and hip fracture). Of the 7 evaluable patients (FVC, DLCO or HRCT) at week 48, 5 were stable, 1 had worsened and 1 improved. Although no significant improvement was observed, RTX achieved active and progressive ILD stabilisation in treated patients.
As previously mentioned, two studies on mortality23,27 found better outcomes with RTX than with TNFi, although statistical significance was not reached. One found a lower mortality rate in the 43 patients who received RTX (HR .49; 95%CI: .23–1.06) than in the 309 who received TNFi as their first biologic.27 Previously, in a retrospective multicentre study,31 higher overall and respiratory mortality had been observed in patients who had ILD (22% and 10%) than in those who did not (14% and 6%); and among patients with ILD, in those who received anti-TNF therapy (31% and 15%) than in those who received RTX (8% and 4%).
Curtis et al.28 found no difference, in a retrospective study, in the risk of developing ILD or ILD complications during treatment with RTX, compared to anti-TNF therapy. However, patients were more likely to have received RTX if they had pre-existing ILD than if they did not (19.8% vs. 8.5%; P < .0001), unlike with anti-TNF therapy (46.5% vs. 59.8%; P < .0001).
Yusof et al. conducted a retrospective study44 in a cohort of 700 RA patients treated with RTX, 56 (8%) with pre-existing ILD. In 10 years of follow-up only 1/700 patients developed ILD (.4%). Of those with pre-existing ILD, 9 (16%) died from progression of the ILD. In 44 of the patients it was possible to assess the evolution of lung function (FVC and DLCO): 14 (31.8%) worsened, 23 (52%) remained stable and 7 (16%) improved with RTX. In another retrospective multicentre study, Duarte and collaborators45 evaluated the effectiveness and safety of RTX in patients with SAD (30 RA). Overall, they found lung function (FVC and DLCO) had stabilized at 12 months following treatment in both regimens and on the HRCT in the patients with NSIP. Infections were frequent and there were 4 treatment discontinuations and 2 deaths. According to the authors of these studies, RTX appears to be an acceptable treatment option, even in monotherapy, for patients with RA and ILD. In a recent Italian study46 a statistically significant difference in post-treatment FVC and DLCO values was found between cases (14 RTX) and controls (14 without RTX), despite the small sample size. These results coincide with the abovementioned pilot, prospective, open study conducted on 10 patients,9 and with numerous series presented at conferences. In general terms, the drug achieves stabilisation or improvement of respiratory function parameters in 70% of cases, without differences in response according to histopathological pattern, and is a relatively safe treatment (although usually associated with an increase in respiratory or urinary infections, the majority not severe).
Abatacept (ABA). ABA, a fusion protein that inhibits T lymphocyte co-stimulation, has been favourably positioned by GUIPCAR2 in the treatment of RA patients who develop ILD.
A low incidence of ILD induced or exacerbated by biologics in patients with RA was confirmed in two SRs.14,16 In the SR of clinical cases of ILD due to DMARds by Roubille et al.16 only one case was found to be related to ABA. In the extensive SR by Nelson et al.,14 a lower Incidence Rate (IR) was observed in an MA of 8 RCTs of ABA in RA (.09 (.01–.31) in the short term and .11 (.06−.20) during the combined extension period) than in health insurance studies (1.1 (.1–4.1) in MarketScan and 4.0 (1.6–8.2) MediCare). Furthermore, in this SR, observational studies and case reports showed that there was no increase in the incidence of ILD and that the parameters assessed improved in all but one case of pre-existing ILD. Therefore, despite the limitation of the data, it appears that ABA does not increase the incidence of ILD and may be useful in the management of patients with RA and associated ILD.
Other studies, with a lower level of evidence, have found similar results. Nakashita et al. reported, from a case-control study with 25 ABA controls29 and a series of 16 ABA cases,35 that ABA has less risk of producing new cases of ILD or worsening of pre-existing ILD than TNFi in Japanese RA patients. An extensive case series of RA and ILD patients collected in numerous centres in Spain, has led to different communications. When retrospectively comparing47 patients treated with ABA, 30 treated with RTX and 25 treated with TCZ, they found improvement at 12 months on HRCT in 36.4% of the patients with ABA, 28.6% with RTX and 8.3% with TCZ. In 2019 they reported the results of the largest series of RA and ILD treated with ABA.48 They analysed the clinical, functional respiratory (FVC and DLCO) and radiological (HRCT) progress of 181 patients treated with ABA (81 in monotherapy) during a median follow-up of 12.1 (6.2–24.1) months and observed stabilisation in most of the parameters analysed in a substantial percentage of the patients. Kurata et al. found in another series of 49 RA cases49 treated with different drugs, that the use of ABA can behave as a protective factor.
In contrast, an extensive retrospective US study (11,219 subjects) to assess the incidence of ILD and complications of pre-existing ILD in RA patients receiving their second or subsequent biologic, 28 in which 109 of the 419 patients with pre-existing ILD received ABA, found no difference in the incidence of ILD and complications of pre-existing ILD between the different biologics.
Targeted synthetic DMARDs (tsDMARDs)The safety of these drugs at the lung level has not been evaluated in any RCT and there is scanty available information. A post-marketing surveillance drug study50 conducted in 2,882 Japanese patients on tofacitinib identified 14 cases of ILD (.5%), of whom 3 died. In a description of 4 cases of RA with ILD treated with tofacitinib51 the authors state that the patients improved and had no pulmonary exacerbation during follow-up (8–12 months), but they do not provide pulmonary assessment data.
AntifibroticsLittle information is available on the effect of antifibrotics in patients with RA-associated ILD. The INBULID RCT10 was recently published, comparing nintedanib against placebo in fibrosing connective tissue diseases (CTD) (663 patients, of whom 89 were RA-ILD). In patients with a fibrotic pattern similar to that of IPF, the adjusted rate of decrease in FVC was -82.9 ml per year with nintedanib and -211.1 ml per year with placebo, with a difference of 128.2 ml (95%CI: 70.8–185.6; P < .001). There is a phase II clinical trial with pirfenidone in patients with RA and ILD (NCT02808871), for which we do not yet have results, which is why it has been excluded from the review. According to the considerations of Fisher and Distler,52 pirfenidone and nintedanib could stop the progression of ILD in patients with RA, and with SAD, as they have already demonstrated in patients with IPF.
This review has limitations. In this type of work, publication bias and even potential confusion by indication cannot be ruled out. Many of the included studies did not have a comparator group, which makes it difficult to assign a causal relationship. Finally, many patients received different DMARDs, and therefore the precise contribution of each treatment to the progression of ILD cannot be defined. Despite these limitations, our review provides evidence useful for the routine clinical practice of rheumatologists. On the one hand, MTX does not worsen progression of ILD and it would not be necessary to discontinue it in the treatment of these patients. On the other hand, RTX and ABA have shown promising results in RA-associated ILD and should be considered as a priority option over anti-TNF therapy when these patients need to initiate or change a biologic due to RA activity or ILD progression. However, there is not enough evidence to recommend the discontinuation of an anti-TNF if the RA is in remission and the ILD is not progressing.
ConclusionsRA patients who develop ILD have higher mortality. Their therapeutic management is a challenge for the clinician. No IS are currently used to treat RA, but they have shown benefit in exacerbations of ILD, especially CPM.
It does not appear necessary to discontinue MTX in patients with RA-ILD, as there is evidence that it does not increase the incidence or exacerbations of ILD and improves survival. When these patients need biological therapy, ABA or RTX is preferable to anti-TNF therapy (with more potential risk) or tsDMARDs (due to lack of evidence). Antifibrotics could be a promising treatment for more progressive forms of ILD, although more controlled studies in RA are needed. Future lines of research could focus on the combination of antifibrotic and immunosuppressive therapies in RA-ILD.
FundingWe have received no funding.
Conflict of interestsThe authors have no conflict of interests to declare.
Ethical responsibilitiesNone.
Please cite this article as: Carrasco Cubero C, Chamizo Carmona E, Vela Casasempere P. Revisión sistemática sobre el impacto de los fármacos en la enfermedad pulmonar intersticial difusa asociada a Artritis Reumatoide. Reumatol Clin. 2021;17:504–513.