Many patients diagnosed with rheumatoid arthritis (RA) report relief of symptoms after consuming certain foods. Diet plays a vital role in rheumatoid arthritis-related inflammation regulation. This study investigates the relationship between dietary inflammation index (DII) scores and RA disease activity.
Materials and methodsForty-one RA patients were enrolled in the study. The general inflammatory index of the diet was analyzed by recording the 24-h food consumption of the patients, and the nutrients were analyzed using the Nutrition Information Systems Package Program. Dietary inflammatory indices were calculated for each patient using the patients’ macro and micronutrient intake levels. RA disease activity was assessed using the Disease Activity Score-28 (DAS-28).
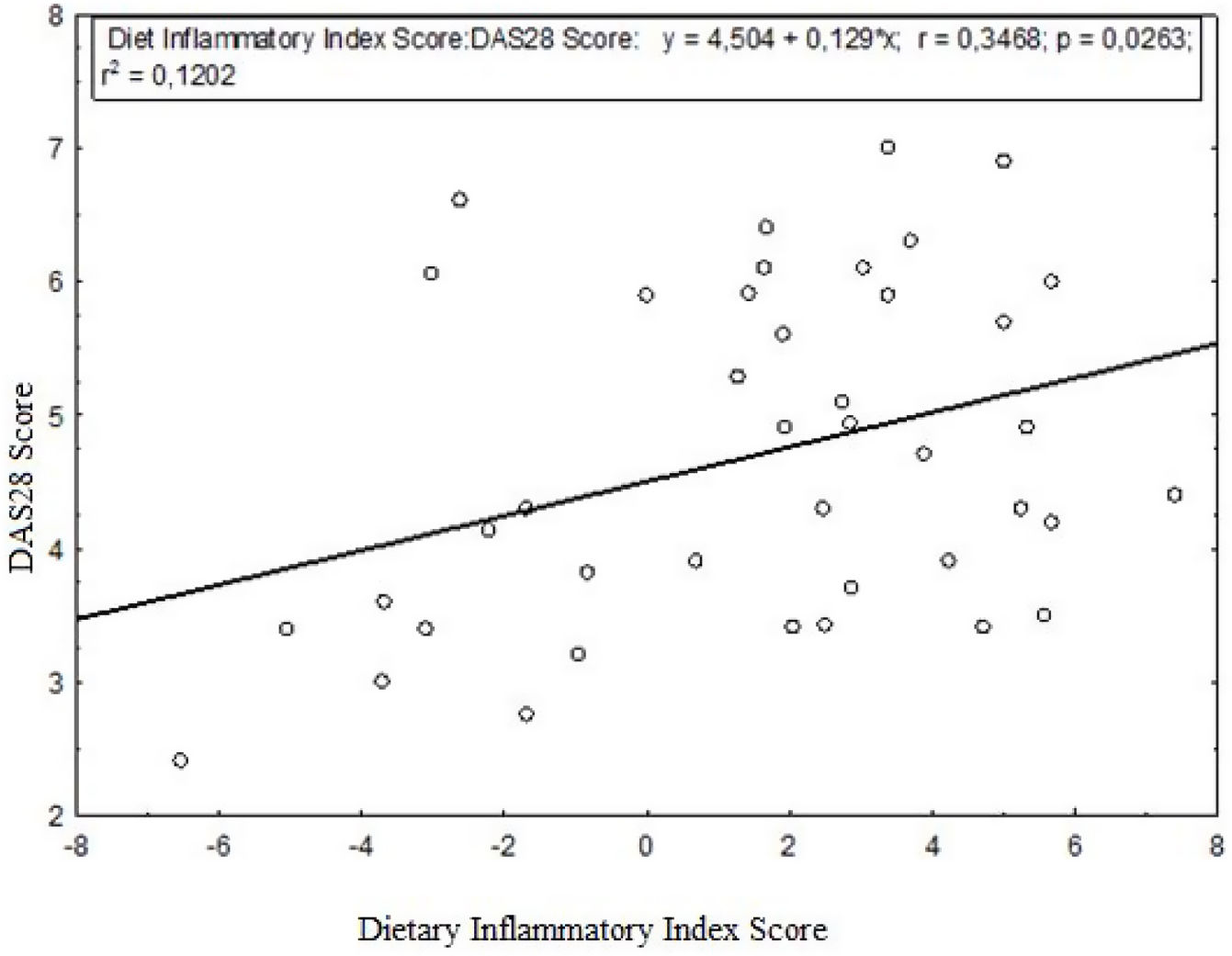
ResultsThe DAS-28 score was lower in the anti-inflammatory diet group compared to the pro-inflammatory diet group (p=0.163). A weak but significant relationship was found between diet inflammation index score and DAS-28 (r=0.3468, p=0.0263). The effect of the dietary inflammatory index on the DAS-28 was 12.02%. Dietary iron, vitamin C, niacin, and magnesium intakes were statistically significantly higher in the quartile group that received an anti-inflammatory diet than in the quartile group that received a pro-inflammatory diet. The intake of some micronutrients, such as iron, zinc, magnesium, and folic acid, was significantly lower than the recommended values in all RA quartile groups.
ConclusionOur results suggest that reducing inflammation through the diet may have a weak but significant effect in controlling disease activity in RA patients.
Muchos pacientes diagnosticados con artritis reumatoide (AR) informan un alivio de los síntomas después de consumir ciertos alimentos. La dieta juega un papel vital en la regulación de la inflamación relacionada con la artritis reumatoide. Este estudio investiga la relación entre las puntuaciones del índice de inflamación dietética (DII) y la actividad de la enfermedad de AR.
Materiales y métodosSe inscribieron en el estudio 41 pacientes con AR. El índice inflamatorio general de la dieta se analizó registrando el consumo de alimentos de los pacientes durante 24 horas y los nutrientes se analizaron mediante el Programa de Paquetes de Sistemas de Información Nutricional. Los índices inflamatorios dietéticos se calcularon para cada paciente utilizando los niveles de ingesta de macro- y micronutrientes de los pacientes. La actividad de la enfermedad de AR se evaluó mediante la puntuación de actividad de la enfermedad-28 (DAS-28).
ResultadosLa puntuación DAS-28 fue menor en el grupo de dieta antiinflamatoria en comparación con el grupo de dieta proinflamatoria (p=0,163). Se encontró una relación débil pero significativa entre la puntuación del índice de inflamación de la dieta y el DAS-28 (r=0,3468, p=0,0263). El efecto del índice inflamatorio dietético sobre el DAS-28 fue del 12,02%. La ingesta dietética de hierro, vitamina C, niacina y magnesio fue estadísticamente significativamente mayor en el grupo del cuartil que recibió una dieta antiinflamatoria que en el grupo del cuartil que recibió una dieta proinflamatoria. La ingesta de algunos micronutrientes, como hierro, zinc, magnesio y ácido fólico, fue significativamente menor que los valores recomendados en todos los grupos del cuartil de AR.
ConclusiónNuestros resultados sugieren que la reducción de la inflamación a través de la dieta puede tener un efecto débil pero significativo en el control de la actividad de la enfermedad en pacientes con AR.
Rheumatoid arthritis (RA) is a chronic inflammatory and autoimmune disease characterized by systemic inflammation and joint destruction. A prevalence of 0.4–1% worldwide has been reported in various populations. Treatment of RA includes suppression of synovitis and systemic inflammation to avoid long-term complications such as joint deformity and disability and possibly prolong survival. The goal of treatment is to achieve sustained disease remission, which often requires adjustments in the therapeutic approach. Disease-modifying antirheumatic drugs (DMARDs) are effective in slowing or stopping the progression of RA disease and form the basis of RA treatment.1,2 Patients diagnosed with RA expect unique dietary advice from their physicians. Many patients report that different foodstuffs improve or worsen their disease symptoms.3 The dietary inflammation index was developed in 2009 and is a method used to precisely measure the inflammatory potential of diet that contributes to many chronic conditions. Dietary inflammation index; assesses the inflammatory potential of the diet based on the balance of pro- and anti-inflammatory properties of dietary components, including macronutrients, vitamins, minerals, flavonoids, and specific foodstuffs. The Western diet pattern (highly concentrated in red meat, high-fat dairy products, and refined grains) is associated with high inflammatory markers. The Mediterranean diet (high consumption of whole grains, fruit and green vegetables, fish, low consumption of red meat and butter, moderate alcohol, and olive oil) is associated with low levels of inflammatory markers.4 In people with RA, it causes an increase in the metabolic index and nutritional needs and reduces food intake due to chronic inflammation. In addition, physical difficulties in purchasing and cooking food, chewing difficulties due to jaw joint involvement, chewing and swallowing difficulties due to decreased secretion in patients with Sjögren's syndrome, nausea, and loss of appetite in those with side effects due to drug treatment adversely affect the nutritional status of RA patients.5
Dietary models evaluated in the medical nutrition therapy of patients with rheumatoid arthritis include Mediterranean, anti-inflammatory, and gluten-free.6 The anti-inflammatory diet is similar to the Mediterranean diet, with a higher content of potential anti-inflammatory components than the Mediterranean diet. It is thought that pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α are effective by reducing gene expression.7 In patients with rheumatoid arthritis, the Mediterranean diet has been reported to reduce pain, increase physical function, and reduce disease activity scores.8 Gluten appears to be a clinically inflammatory glycoprotein. Case reports show that a gluten-free diet can improve symptoms in patients with rheumatoid arthritis resistant to conventional drug therapy. Gluten activates Toll-like receptor 4 and stimulates the production of various pro-inflammatory cytokines such as IL-6, IL-1, and especially IL-17. High levels of anti-gliadin antibodies have been detected in the intestinal fluid of RA patients compared to healthy individuals. This finding supports the possible antigenic effect of gluten.9 More profound knowledge about the relationship between general diet and RA may assist in the remission of RA, a lifelong disease.
Our aim in this study is to investigate the nutritional status of patients with RA and whether a higher dietary inflammatory index contributes to disease activity.
Materials and methodsA total of 41 RA patients aged 18 years and over, 34 women and 7 men, who were being followed up in the Rheumatology Department of Aydın Adnan Menderes University Research and Teaching Hospital, were included in the study. Patients diagnosed with RA were included according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria. Demographic data of the patients, laboratory results (complete blood count, Erythrocyte Sedimentation Rate (ESH), C-reactive protein (CRP), renal and hepatic function tests), and disease activation score (DAS-28) were recorded. Demographic and nutritional data were collected from the patients through face-to-face interviews with the specialist dietitian, and general information was recorded in the patient data sheet. This sheet was used to collect the general information of the participants. General information includes education level, age, gender, body weight (kg), height (m), smoking, alcohol, and general eating habits. This descriptive and cross-sectional study was approved by the Ethics Committee of Faculty of Medicine (2021/151). An informed consent form was obtained from the patients before the procedure.
In the 24-h diet recall form, the food and amounts consumed by the participants in the last 24h were recorded. A dietitian checked it. The 24-h diet records of individuals were analyzed with the Nutrition Information Systems Package Program (BEBIS). Average daily intake of macro and micronutrients such as dietary protein, total fat, carbohydrate (CHO), free sugar, polyunsaturated fatty acids (PUFA), monounsaturated fatty acids (MUFA), linoleic acid (LA), omega-3 fatty acids. Purchase quantities were calculated as a result of the analysis.
The intake status of 29 nutrients and nutrients used in the study was determined. DII was calculated using the parameters of 29 nutrients (e.g., energy, carbohydrate, fiber, protein). From the participants’ daily intake of food and nutrients, “[(the participant's daily consumption amount of that nutrient or nutrient-average global daily consumption amount)/the standard deviation value of that nutrient or nutrient X overall inflammatory effect score]” for each nutrient individually calculated. DII values, which determine the inflammatory load of the participants’ diet, were determined by adding up all the calculated scores. After the calculations were made for the participants, groups were evaluated according to the DII value. Inflammatory index scores were calculated from individuals’ 24-h daily food consumption records. Negative inflammatory index scores represented an anti-inflammatory diet with positive components for health. Positive inflammatory index scores, on the other hand, represented a pro-inflammatory diet. In this study, individuals were divided into quarter groups according to their dietary inflammatory index scores; the groups 1. quartile (Q1), 2. quartile (Q2), 3. quartile (Q3), and 4. quartile (Q4) were compared. The first quartile (Q1) represents the anti-inflammatory diet. The inflammatory load of the diet increases as the quartiles increase. 4. quartile (Q4) indicates a pro-inflammatory diet.10 European Food Safety Authority (EFSA) reference data were used to compare patients’ dietary macro- and micronutrients.11
The Kolmogorov–Smirnov test determined the distributions of quantitative variables. Descriptive statistics were given as X¯±SD for normally distributed variables and median (25–75 percentile) for non-normally distributed variables. Comparison of groups, normally distributed variables ANOVA one-way analysis of variance in independent group comparison, Kruskal–Wallis H test was used for non-normally distributed variables. Independent analysis of categorical variables Chi-square analysis method was applied. A p-value<0.05 was considered statistically significant. Independent samples t test was used in the comparison of two independent groups. Spearman's correlation test was used to determine the relationship between quantative variables.
ResultsWe included 41 patients diagnosed with RA in our study, 83% of whom were women. All patients were taking medication for the treatment of RA, including corticosteroids. The groups were homogeneously distributed regarding age, gender, smoking status, and diabetes. The mean age of the individuals was 53.1±11.7 years in the Q1 group on the anti-inflammatory diet and 58.7±10.1 years in the Q4 group on the pro-inflammatory diet. For the diagnosis of RA, the disease history was 5.3±3.97 years in the Q1 group and 12.3±7.3 years in the Q4 group. The DAS-28 score evaluation showed 4.1±1.53 in the Q1 group on an anti-inflammatory diet and 4.7±1.13 in the Q4 group on a pro-inflammatory diet. Furthermore, mean serum CRP level evaluations showed 3.0 in the Q1 group on an anti-inflammatory diet and 17.4 in the Q4 group on the pro-inflammatory diet.
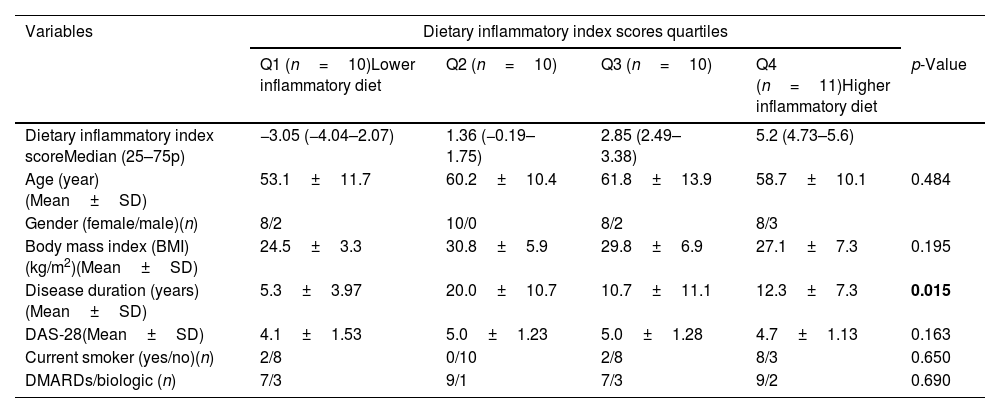
Of the patients in our study, 9% had a history of diabetes. After the dietary inflammatory index quartile groups were evaluated for the body mass index (BMI), there was no statistically significant association between the groups (p=0.195). In the evaluation of rheumatoid arthritis for disease duration, the group on an anti-inflammatory diet and the quartile group on a pro-inflammatory diet were significantly different (p=0.015). DAS-28 score was lower in the quartile group receiving an anti-inflammatory diet than in the quartile group receiving a pro-inflammatory diet, but this difference was not statistically significant (p=0.163). There was no significant difference between all quartile groups in terms of the treatment (Disease-modifying anti-rheumatic drug and biologic agents) they received (p=0.69) (Table 1).
Demographic and anthropometric characteristics of participants according to dietary inflammatory index quartiles.
| Variables | Dietary inflammatory index scores quartiles | ||||
|---|---|---|---|---|---|
| Q1 (n=10)Lower inflammatory diet | Q2 (n=10) | Q3 (n=10) | Q4 (n=11)Higher inflammatory diet | p-Value | |
| Dietary inflammatory index scoreMedian (25–75p) | −3.05 (−4.04–2.07) | 1.36 (−0.19–1.75) | 2.85 (2.49–3.38) | 5.2 (4.73–5.6) | |
| Age (year)(Mean±SD) | 53.1±11.7 | 60.2±10.4 | 61.8±13.9 | 58.7±10.1 | 0.484 |
| Gender (female/male)(n) | 8/2 | 10/0 | 8/2 | 8/3 | |
| Body mass index (BMI) (kg/m2)(Mean±SD) | 24.5±3.3 | 30.8±5.9 | 29.8±6.9 | 27.1±7.3 | 0.195 |
| Disease duration (years)(Mean±SD) | 5.3±3.97 | 20.0±10.7 | 10.7±11.1 | 12.3±7.3 | 0.015 |
| DAS-28(Mean±SD) | 4.1±1.53 | 5.0±1.23 | 5.0±1.28 | 4.7±1.13 | 0.163 |
| Current smoker (yes/no)(n) | 2/8 | 0/10 | 2/8 | 8/3 | 0.650 |
| DMARDs/biologic (n) | 7/3 | 9/1 | 7/3 | 9/2 | 0.690 |
Q: quartile, SD: standard deviation, DAS-28: disease activity score-28, DMARD: disease-modifying anti-rheumatic drug.
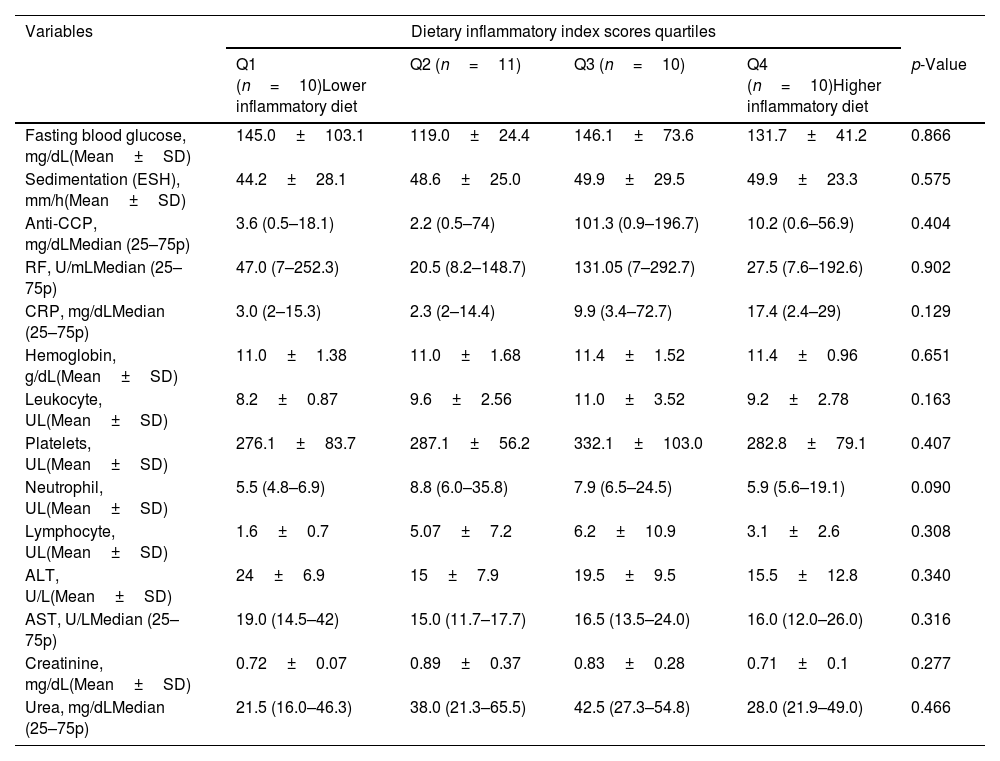
Sedimentation and CRP values were lower in the quartile group receiving an anti-inflammatory diet than in the quartile group receiving a pro-inflammatory diet, but this difference was statistically insignificant (p=0.575, p=0.129). The difference between the RA quartile groups regarding other blood laboratory parameters was also insignificant (Table 2).
Laboratory findings of patients according to dietary inflammatory index quartiles.
| Variables | Dietary inflammatory index scores quartiles | ||||
|---|---|---|---|---|---|
| Q1 (n=10)Lower inflammatory diet | Q2 (n=11) | Q3 (n=10) | Q4 (n=10)Higher inflammatory diet | p-Value | |
| Fasting blood glucose, mg/dL(Mean±SD) | 145.0±103.1 | 119.0±24.4 | 146.1±73.6 | 131.7±41.2 | 0.866 |
| Sedimentation (ESH), mm/h(Mean±SD) | 44.2±28.1 | 48.6±25.0 | 49.9±29.5 | 49.9±23.3 | 0.575 |
| Anti-CCP, mg/dLMedian (25–75p) | 3.6 (0.5–18.1) | 2.2 (0.5–74) | 101.3 (0.9–196.7) | 10.2 (0.6–56.9) | 0.404 |
| RF, U/mLMedian (25–75p) | 47.0 (7–252.3) | 20.5 (8.2–148.7) | 131.05 (7–292.7) | 27.5 (7.6–192.6) | 0.902 |
| CRP, mg/dLMedian (25–75p) | 3.0 (2–15.3) | 2.3 (2–14.4) | 9.9 (3.4–72.7) | 17.4 (2.4–29) | 0.129 |
| Hemoglobin, g/dL(Mean±SD) | 11.0±1.38 | 11.0±1.68 | 11.4±1.52 | 11.4±0.96 | 0.651 |
| Leukocyte, UL(Mean±SD) | 8.2±0.87 | 9.6±2.56 | 11.0±3.52 | 9.2±2.78 | 0.163 |
| Platelets, UL(Mean±SD) | 276.1±83.7 | 287.1±56.2 | 332.1±103.0 | 282.8±79.1 | 0.407 |
| Neutrophil, UL(Mean±SD) | 5.5 (4.8–6.9) | 8.8 (6.0–35.8) | 7.9 (6.5–24.5) | 5.9 (5.6–19.1) | 0.090 |
| Lymphocyte, UL(Mean±SD) | 1.6±0.7 | 5.07±7.2 | 6.2±10.9 | 3.1±2.6 | 0.308 |
| ALT, U/L(Mean±SD) | 24±6.9 | 15±7.9 | 19.5±9.5 | 15.5±12.8 | 0.340 |
| AST, U/LMedian (25–75p) | 19.0 (14.5–42) | 15.0 (11.7–17.7) | 16.5 (13.5–24.0) | 16.0 (12.0–26.0) | 0.316 |
| Creatinine, mg/dL(Mean±SD) | 0.72±0.07 | 0.89±0.37 | 0.83±0.28 | 0.71±0.1 | 0.277 |
| Urea, mg/dLMedian (25–75p) | 21.5 (16.0–46.3) | 38.0 (21.3–65.5) | 42.5 (27.3–54.8) | 28.0 (21.9–49.0) | 0.466 |
Q: quartile, SD: standard deviation, RF: rheumatoid factor, CRP: C-reactive protein, ALT: alanine transaminase, AST: aspartate amino transferase.
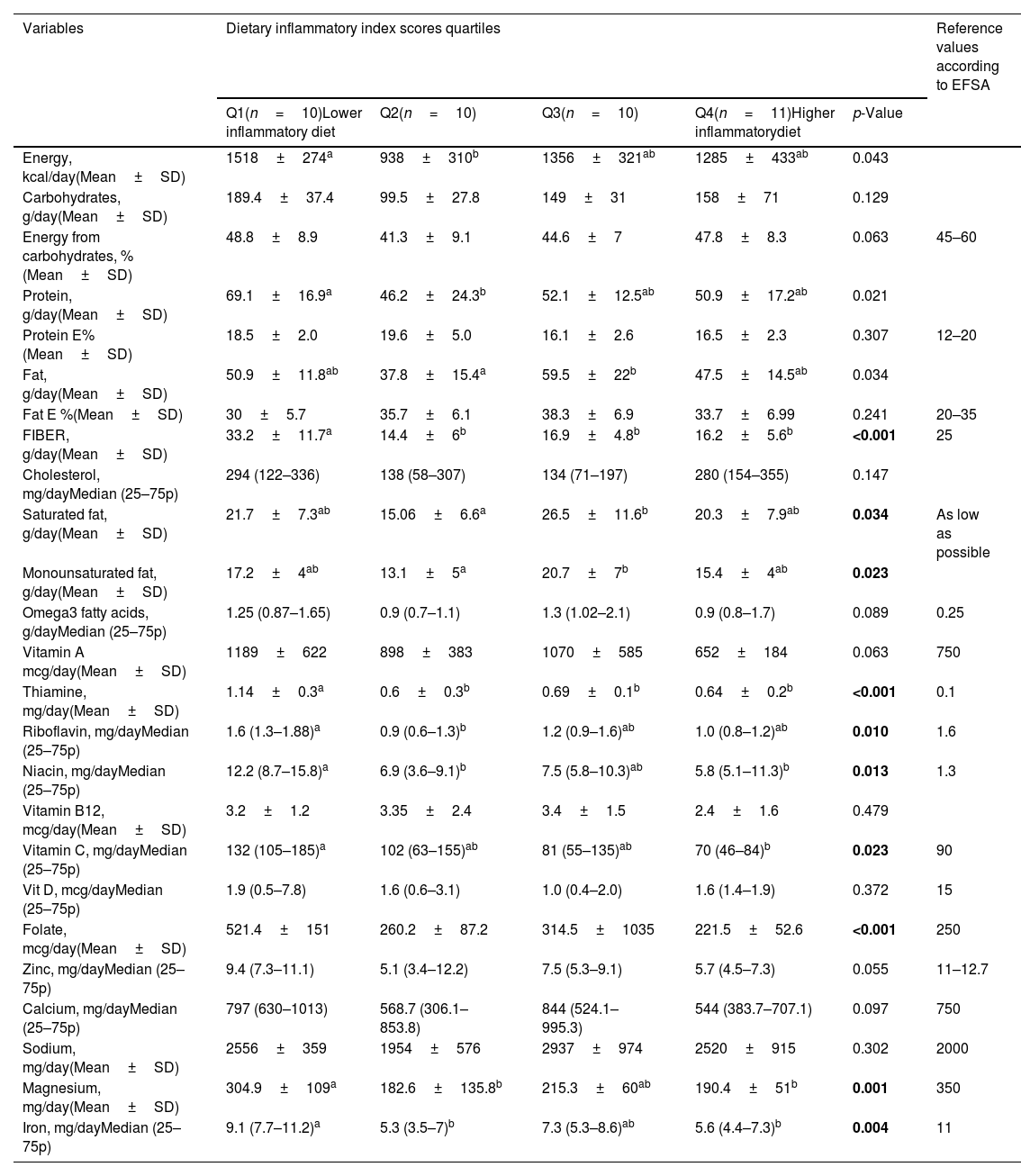
In the evaluation of dietary macro- and micronutrient analysis among dietary inflammatory quartile groups, dietary iron (Fe) intake was higher in the Q1 group receiving the anti-inflammatory diet than in the Q4 group receiving the pro-inflammatory diet (p=0.004). It was found that the Q1 and Q2 groups receiving an anti-inflammatory diet had higher magnesium intake than the Q4 group receiving a pro-inflammatory diet (p=0.001). As a result of the comparison of vitamin C and niacin in the Q1 group receiving an anti-inflammatory diet, the intake level of vitamin C (p=0.023) and niacin (p=0.013) was higher than the Q4 group receiving the pro-inflammatory diet. Dietary riboflavin intake in the Q1 quartile group receiving the anti-inflammatory diet was higher than in the Q4 quartile group receiving the pro-inflammatory diet (p=0.010).
The Q1 quartile group receiving the pro-inflammatory diet had a significantly higher dietary protein intake than the Q2 quartile group. However, there was no significant difference between the protein intake levels of the quartile group who received anti-inflammatory and pro-inflammatory diets (p=0.021) (Table 3). In comparing the quartile group taking an anti-inflammatory diet and the quartile group receiving a pro-inflammatory diet, dietary iron, vitamin C, folic acid, niacin, riboflavin, and magnesium intakes were found to be statistically significantly higher in the quartile group receiving an anti-inflammatory diet. The intake of some micronutrients, such as iron, zinc, riboflavin, thiamine and magnesium was significantly lower than the recommended values in all RA quartile groups (Table 3).
Dietary macro and micronutrient intake levels of patients according to dietary inflammatory index quartiles.
| Variables | Dietary inflammatory index scores quartiles | Reference values according to EFSA | ||||
|---|---|---|---|---|---|---|
| Q1(n=10)Lower inflammatory diet | Q2(n=10) | Q3(n=10) | Q4(n=11)Higher inflammatorydiet | p-Value | ||
| Energy, kcal/day(Mean±SD) | 1518±274a | 938±310b | 1356±321ab | 1285±433ab | 0.043 | |
| Carbohydrates, g/day(Mean±SD) | 189.4±37.4 | 99.5±27.8 | 149±31 | 158±71 | 0.129 | |
| Energy from carbohydrates, %(Mean±SD) | 48.8±8.9 | 41.3±9.1 | 44.6±7 | 47.8±8.3 | 0.063 | 45–60 |
| Protein, g/day(Mean±SD) | 69.1±16.9a | 46.2±24.3b | 52.1±12.5ab | 50.9±17.2ab | 0.021 | |
| Protein E%(Mean±SD) | 18.5±2.0 | 19.6±5.0 | 16.1±2.6 | 16.5±2.3 | 0.307 | 12–20 |
| Fat, g/day(Mean±SD) | 50.9±11.8ab | 37.8±15.4a | 59.5±22b | 47.5±14.5ab | 0.034 | |
| Fat E %(Mean±SD) | 30±5.7 | 35.7±6.1 | 38.3±6.9 | 33.7±6.99 | 0.241 | 20–35 |
| FIBER, g/day(Mean±SD) | 33.2±11.7a | 14.4±6b | 16.9±4.8b | 16.2±5.6b | <0.001 | 25 |
| Cholesterol, mg/dayMedian (25–75p) | 294 (122–336) | 138 (58–307) | 134 (71–197) | 280 (154–355) | 0.147 | |
| Saturated fat, g/day(Mean±SD) | 21.7±7.3ab | 15.06±6.6a | 26.5±11.6b | 20.3±7.9ab | 0.034 | As low as possible |
| Monounsaturated fat, g/day(Mean±SD) | 17.2±4ab | 13.1±5a | 20.7±7b | 15.4±4ab | 0.023 | |
| Omega3 fatty acids, g/dayMedian (25–75p) | 1.25 (0.87–1.65) | 0.9 (0.7–1.1) | 1.3 (1.02–2.1) | 0.9 (0.8–1.7) | 0.089 | 0.25 |
| Vitamin A mcg/day(Mean±SD) | 1189±622 | 898±383 | 1070±585 | 652±184 | 0.063 | 750 |
| Thiamine, mg/day(Mean±SD) | 1.14±0.3a | 0.6±0.3b | 0.69±0.1b | 0.64±0.2b | <0.001 | 0.1 |
| Riboflavin, mg/dayMedian (25–75p) | 1.6 (1.3–1.88)a | 0.9 (0.6–1.3)b | 1.2 (0.9–1.6)ab | 1.0 (0.8–1.2)ab | 0.010 | 1.6 |
| Niacin, mg/dayMedian (25–75p) | 12.2 (8.7–15.8)a | 6.9 (3.6–9.1)b | 7.5 (5.8–10.3)ab | 5.8 (5.1–11.3)b | 0.013 | 1.3 |
| Vitamin B12, mcg/day(Mean±SD) | 3.2±1.2 | 3.35±2.4 | 3.4±1.5 | 2.4±1.6 | 0.479 | |
| Vitamin C, mg/dayMedian (25–75p) | 132 (105–185)a | 102 (63–155)ab | 81 (55–135)ab | 70 (46–84)b | 0.023 | 90 |
| Vit D, mcg/dayMedian (25–75p) | 1.9 (0.5–7.8) | 1.6 (0.6–3.1) | 1.0 (0.4–2.0) | 1.6 (1.4–1.9) | 0.372 | 15 |
| Folate, mcg/day(Mean±SD) | 521.4±151 | 260.2±87.2 | 314.5±1035 | 221.5±52.6 | <0.001 | 250 |
| Zinc, mg/dayMedian (25–75p) | 9.4 (7.3–11.1) | 5.1 (3.4–12.2) | 7.5 (5.3–9.1) | 5.7 (4.5–7.3) | 0.055 | 11–12.7 |
| Calcium, mg/dayMedian (25–75p) | 797 (630–1013) | 568.7 (306.1–853.8) | 844 (524.1–995.3) | 544 (383.7–707.1) | 0.097 | 750 |
| Sodium, mg/day(Mean±SD) | 2556±359 | 1954±576 | 2937±974 | 2520±915 | 0.302 | 2000 |
| Magnesium, mg/day(Mean±SD) | 304.9±109a | 182.6±135.8b | 215.3±60ab | 190.4±51b | 0.001 | 350 |
| Iron, mg/dayMedian (25–75p) | 9.1 (7.7–11.2)a | 5.3 (3.5–7)b | 7.3 (5.3–8.6)ab | 5.6 (4.4–7.3)b | 0.004 | 11 |
Q: quartile, SD: standard deviation, EFSA: European Food Safety Authority, DRV: Within rows, means followed by the same letter are significantly different (a, b, ab) (p<0.05).
DAS-28 score change is affected by the dietary inflammatory index at a rate of 12.02% (Fig. 1). A weak but significant relationship was found between DAS-28 and dietary inflammatory index (r=0.3468, p=0.0263) (Fig. 1).
DiscussionIt is known that genes, environmental factors, and hormones play a role in the development of rheumatoid arthritis. The activity of primary female sex hormones explains the more frequent occurrence of the disease, especially in women.10 In addition, many environmental factors, such as trauma, infections, and nutrition, mainly smoking, also affect the development of RA.12 The anti-inflammatory activity of some nutrients has been reported in various primary and clinical studies. It has been reported that polyunsaturated fatty acids have antioxidant and anti-inflammatory effects, and vitamin D has a protective effect on the development of RA.13 In our study, there was no difference in the number of smokers in the Q1 group receiving an anti-inflammatory diet and the Q4 group receiving a pro-inflammatory diet. It was determined that 35% of the patients in the study were smokers. Our study found no significant difference in dietary unsaturated fat intake levels. Omega 3 fatty acids were above the reference intake level in all groups. All study group's dietary vitamin D intake levels were below the reference levels.
In a study of 184 patients with a diagnosis of rheumatoid arthritis, a linear trend was found from dietary inflammatory index groups to pro-inflammatory areas, with a decrease in lean mass, weight, and energy intake. The increased rheumatoid arthritis disease activity score (DAS-28), ESR, and CRP levels were detected.14 In a study conducted with newly diagnosed rheumatoid arthritis patients and healthy controls, They stated that inflammatory dietary intake might act as a potential risk factor contributing to the development and severity of RA. It has also been reported that patients with high DII have higher serum inflammatory (hs-CRP and TNF) and clinical markers (tender joint score and DAS-28 score). Therefore, they thought that dietary modification to lower the DII score might be beneficial to improve clinical outcomes in such patients.15 In our study, ESR and CRP values were high with the increase in the pro-inflammatory effect potential of the diet, but it was not statistically significant. The regression analysis showed that the inflammatory effect of the general diet in rheumatoid arthritis patients had a 12.02% effect on the activity of the disease. A weak but significant relationship was found between DAS-28 and DII. The DAS-28 score was lower in the group receiving an anti-inflammatory diet. Studies have reported that plasma inflammatory adipokines such as leptin, adiponectin, and visfatin released from white adipose tissue were higher in RA than in healthy individuals. In addition, obesity is reported to be a risk factor for RA patients.16–18 In our study, the mean BMI of the patients with the highest DAS-28 score was between 29.8 and 30.8. A study evaluating daily energy intake reported that women with movement restrictions had lower energy intake than healthy individuals.19
In Bekar et al.’s study with rheumatoid arthritis patients, the daily dietary energy intake (1641.2±447.58kcal/day) of patients in the patient group was lower than healthy individuals (1816.2±535.62kcal/day) was not statistically significant. It has been reported that people with rheumatoid arthritis provide 44.8% of daily dietary energy from carbohydrates, 37.5% from fats, and 14.4% from proteins.20 In our study, the daily energy intakes of the quartile group, which received an anti-inflammatory and pro-inflammatory diet, were found to be 1518±274kcal/day and 1285±433kcal/day, respectively. According to the EFSA (European Food Safety Authority) reference values, it is recommended to provide 45–60% of the daily energy of individuals from carbohydrates, 12–20% from proteins, and 20–35% from fats for adequate and balanced nutrition. Furthermore, it is also recommended to increase omega-3 (n−3) fatty acids in the diet.11 Our study evaluated the percentage of daily dietary energy from carbohydrates, fat, and protein. The anti-inflammatory quartile group's carbohydrate, fat, and protein values were 48.8%, 30.5%, and 18.5%, respectively. We found 47.8%, 33.7%, and 16.5% for the pro-inflammatory quartile group.
Omega fatty acids are essential components of the diet and are commonly known as omega-3, omega-6, and omega-9. The richest dietary sources of omega fatty acids are vegetable and fish oils. Omega-3 and omega-6 are essential fatty acids that must be externally taken. Omega-3, α-linolenic acid (ALA) is of vegetable origin, and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are abundantly in fish.21 It has been reported that omega-3, polyunsaturated fatty acids (PUFA), especially EPA and DHA, may have protective effects by reducing the formation of pro-inflammatory eicosanoids in RA disease or healing effect on the disease process.22 It has been reported that omega-3, polyunsaturated fatty acids (PUFA), and especially EPA and DHA may be protective against RA by reducing the formation of proinflammatory eicosanoids or may have healing effects on the disease process.23 In Tadeschi et al. study, it has been reported that greater fish consumption associated with lower disease activity in RA.24 Classically, the intake of anti-inflammatory fatty acids such as n-3 fatty acids and γ-linolenic acid has been reported to reduce inflammation and disease activity in RA patients.25 In our study, the dietary omega-3 intakes of the patients were above the EFSA dietary reference value in the whole patient quartile group. Patients reported that they rarely consumed fish. Dietary monounsaturated fatty acid (MUFA) intake levels were compared. The MUFA intake level was higher in the quartile group receiving an anti-inflammatory diet than in the quartile group receiving a pro-inflammatory diet, but this difference was not statistically significant.
In a study of female rheumatoid arthritis patients with a normal BMI, daily intakes of energy and micronutrients, including dietary calcium, folic acid, zinc, magnesium, and vitamin B6, were significantly lower than dietary reference values.26 In our study, when we compared the dietary reference values in patients with RA, daily dietary magnesium, zinc, and iron intakes were low in all quartile groups. Folate was found to be low only in the pro-inflammatory quartile group.
DAS-28 is the most commonly used method for clinical assessment of disease activity in RA. The DAS-28 score is a composite score that includes the condition of 28 joints (tenderness and swelling), markers of inflammation along with erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP), and a person's perceived health using a visual analog scale (VAS). In the evaluation of the DAS-28 score, ≤2.6, indicates low disease activity, >3.2 and ≥5.1, moderate disease activity and >5.1 high disease activity.27 In our study, patients in anti-inflammatory, pro-inflammatory, and other quartile groups had moderate disease activity in terms of DAS-28 score. We compared the groups receiving anti-inflammatory and pro-inflammatory diets regarding the history of RA disease. We found that individuals with a more extended disease history took a more pro-inflammatory diet. There was no significant difference between the quartile groups in terms of RA treatment (disease-modifying anti-rheumatic drug and biologic agents). Regarding its limitations, our study is cross-sectional and reports data from a single institution.
In this study, we observed that patients with a long-term diagnosis of rheumatoid arthritis generally tended to follow a poor diet with a high inflammatory index. The reason for this is decreased appetite due to the duration of the disease, complaints related to the gastrointestinal system, and long-term use of a wide variety of drugs reasons may be considered. In addition, it is thought that they are malnourished in terms of micronutrients such as iron, zinc, and vitamin D, and they are fed with low calories due to movement restriction. In this respect, making adjustments to patients’ diets can be recommended. We think the anti-inflammatory effect of the diet might be effective in the new onset period of the disease. Individuals with chronic and long-term diseases pose a greater risk for nutritional deficiencies. As a result of our study and literature review, this patient group should increase the frequency of fish consumption, and those with low micronutrients such as zinc, iron, and vitamin D should be supplemented. There seem to be limited studies on this subject in the literature. Therefore, we believe that our study is essential for both being the first study in Turkiye and its contribution to the literature.
Ethics committee approvalThe study protocol was approved by the Adnan Menderes University Medical Faculty Ethics Committee (date: 09.09.2021, No: E-53043469-050.04.04-75261). The study was conducted in accordance with the principles of the Declaration of Helsinki.
FundingThe authors received no financial support for the research and/or authorship of this article.
Conflict of interestThe authors declare that they have no conflict of interest.