Patients with diffuse connective tissue diseases frequently develop interstitial lung disease, which carries a worse prognosis and shortens survival. High-resolution computed tomography is the first-choice test, and is competitive with histopathology, however, the cost and radiation may limit its use, particularly for screening. Lung ultrasound is a rapid, accessible, reproducible, and inexpensive study that is useful for diagnosis of interstitial lung disease. Furthermore, extensive training is not required to identify the alterations associated with these lung diseases. B lines and pleural irregularities compose the ultrasonographic interstitial syndrome, although, it must be kept in mind that it is not specific, and it is necessary to rule out haemodynamic, cardiovascular, and infectious abnormalities. This review highlights the elevated prevalence of this lung condition in the main rheumatological diseases, with emphasis on the usefulness of pulmonary ultrasound.
Las enfermedades difusas del tejido conectivo con frecuencia desarrollan enfermedad pulmonar intersticial, lo que conlleva peor pronóstico y acorta la supervivencia. La tomografía axial computarizada de alta resolución es la prueba diagnóstica de elección, ya que ésta, es muy competitiva con la histopatología; sin embargo, el costo y la radiación pueden limitar su empleo, particularmente como escrutinio. El ultrasonido pulmonar, estudio rápido, de acceso fácil, reproducible y de menor costo resulta muy atractivo para determinar la existencia de enfermedad pulmonar intersticial. Adicionalmente, se requiere de poca experiencia para determinar las alteraciones correlacionables con estos padecimientos pulmonares. Las líneas B y las irregularidades pleurales conforman el denominado síndrome intersticial ultrasonográfico, aunque debemos tener en mente que no es específico y estamos obligados a considerar anormalidades hemodinámicas, cardiovasculares e infecciosas. En esta revisión, exponemos la alta prevalencia de esta patología pulmonar en los principales padecimientos reumatológicos, con énfasis en la utilidad del ultrasonido pulmonar, su facilidad de realización y alto desempeño diagnóstico.
Interstitial lung diseases (ILD) are a heterogeneous group of parenchymal lung diseases with similar pathophysiological mechanisms, clinical presentation, and radiological imaging.1 They are characterised by damage and thickening of the interstitium causing impaired gas exchange; the most common symptoms are progressive dyspnoea and persistent non-productive cough.2 ILD can be caused by various primary diseases (sarcoidosis, pulmonary lymphangioleiomyomatosis, alveolar proteinosis), environmental exposure, drug or radiotherapy toxicity, it can be associated with diffuse connective tissue disease (DCTD) or have no identifiable cause; in this case the diseases are referred to as idiopathic interstitial pneumonias (IIP).3
The ATS/ERS international multidisciplinary classification of IIP was made in 20024 and updated in 2013; the most recent update classifies the diseases into major IIP, rare IIP (lymphoid IIP and idiopathic pleuroparenchymal fibroelastosis), and unclassifiable IIP. Major IIP are further subdivided into chronic fibrosing (idiopathic pulmonary fibrosis and nonspecific interstitial pneumonias), smoking-related (desquamative interstitial pneumonia and ILD associated with respiratory bronchiolitis), and acute/subacute (cryptogenic organising pneumonia and acute interstitial pneumonia).5 Although there is no classification specifically for ILD-DCTD, the histopathological/radiological patterns described in IIP can be observed in ILD-DCTD, and therefore the same patterns are used to describe them.6,7
Worldwide, most studies highlight idiopathic pulmonary fibrosis and sarcoidosis as the 2 most frequent causes of ILD, responsible for almost half of cases, while those associated with DCTD are the third most frequent cause (7%–19.7%).8 In the Mexican population, a prospective study identified DCTD as the main cause of ILD (58% of 110 patients).9
Relevance of interstitial lung disease in diffuse connective tissue diseasesPatients with DCTD are at increased risk of developing ILD. A Taiwanese national population-based cohort study by the group of Ng et al., described in newly diagnosed patients with systemic sclerosis (SSc) a hazard ratio of 172.6 for developing ILD, compared to sex- and age-matched patients without DCTD; similarly, the hazard ratio was 119, 84.9, 32.8, and 8.29 for patients with dermatomyositis, polymyositis, systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA), respectively.10
The impact on morbidity and mortality is also very relevant: ILD is the leading cause of death in patients with SSc;11 in idiopathic inflammatory myopathies it is associated with poorer quality of life and worse prognosis, especially in patients with antibodies against MDA5 and aminoacyl tRNA synthetase;12 in RA with ILD mortality is 2–10 times higher, compared to controls with RA without ILD matched for sex and age.13
Diagnosis of interstitial lung disease associated with diffuse connective tissue diseasesLung biopsy is the gold standard to diagnose ILD-DCTD; it can determine the histological pattern according to the ATS/ERS classification and rule out other differential diagnoses. However, due to the invasiveness of the method and the high morbidity and mortality associated with the procedure, especially high in patients with DCTD, it is usually avoided or limited for diagnostic doubt or differential diagnosis.14 High-resolution computed axial tomography (HRCT) is now the diagnostic method of choice. It is more sensitive than chest X-ray and distinguishes radiological patterns, which have an acceptable correlation with the histological pattern, of utmost importance for therapeutic decision-making and prognosis.7,15
The major drawback of HRCT is the need to expose the patient to ionising radiation. For this reason, even in rheumatological diseases with a high prevalence of ILD such as idiopathic inflammatory myopathies, some experts do not recommend it as a screening method.16 The search for low-cost, easily accessible methods to detect ILD that do not expose the patient to ionising radiation has led to increased interest in lung ultrasound (LUS).17
Lung ultrasoundLichtenstein and Axler first described LUS in 1993.18 In subsequent years, its use gradually became widespread mainly in intensive care and emergency wards, because it proved more sensitive than conventional chest teleradiography in detecting entities such as pneumothorax, pulmonary consolidation, interstitial syndrome, and pleural effusion.19–21 Bedside LUS of the critically ill patient can rapidly identify the cause of acute respiratory failure as outlined in the BLUE protocol,22 and serve as a guide to the cause of acute circulatory failure in conjunction with echocardioscopy through the FALLS protocol.23 It was only in more recent years that the usefulness of LUS in the diagnosis of ILD started to be studied.
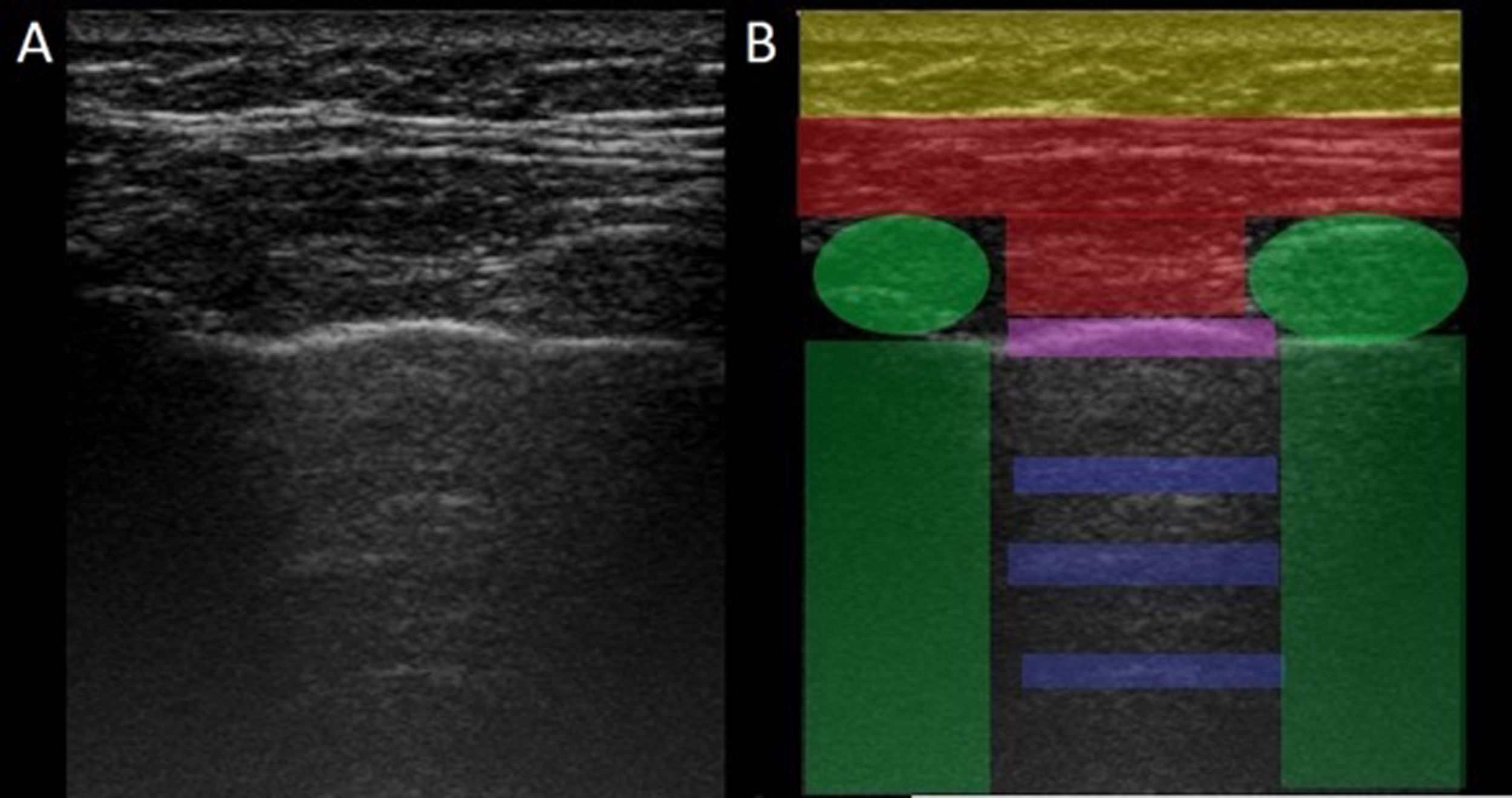
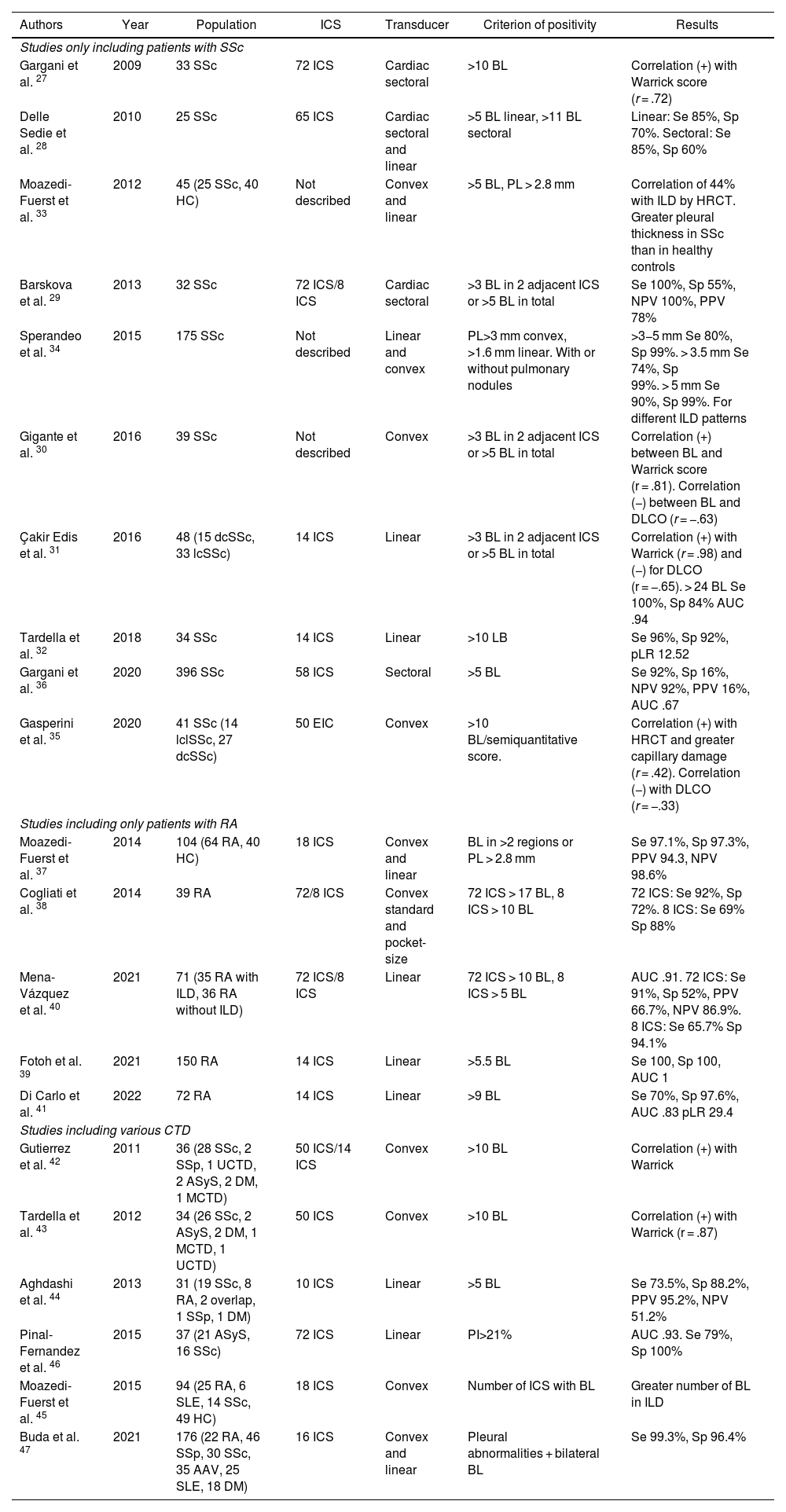
LUS is performed through the intercostal spaces with the probe perpendicular to the thoracic surface in a longitudinal position, resting its proximal and caudal ends on the upper and lower ribs that delimit the intercostal space. The bony landmarks are the bony costal ridges. The visceral and parietal pleura are depicted as a single line located 0.5 cm below the costal ridges, called the "pleural line". The appearance of the 2 costal images with the intervening pleural line is known as the "bat sign" (Fig. 1).
Image of normal ultrasound performed in a healthy patient, obtained with GE Logiq 3 equipment with a 10–14 MHz multifrequency linear transducer, in the third right intercostal space, with the patient in the supine decubitus position and the transducer in longitudinal direction. A) Original image. B) Skin and subcutaneous cellular tissue highlighted in yellow; in red, myofascial layers; in green, ribs and the acoustic shadow they generate; in purple, pleural line, and in blue, A lines.
In the normal lung, because of the large difference in acoustic impedance between the air and the surrounding soft tissues, the pleura behaves as an almost perfect reflector of the ultrasound waves; it produces multiple reverberation phenomena between the pleural line and the probe. The pleural line is visualised as hyperechoic, shiny, thin, and regular, with smooth sliding motion synchronous with respiration called "lung sliding". Below the pleural line there are characteristic horizontal artefacts: "A-lines", which are replicas of the pleural line arranged equidistant from each other due to ultrasound reverberation at the pleural line and artefacts resulting from the myofascial layers of the chest wall due to minor reverberation phenomena and mirror effects.24
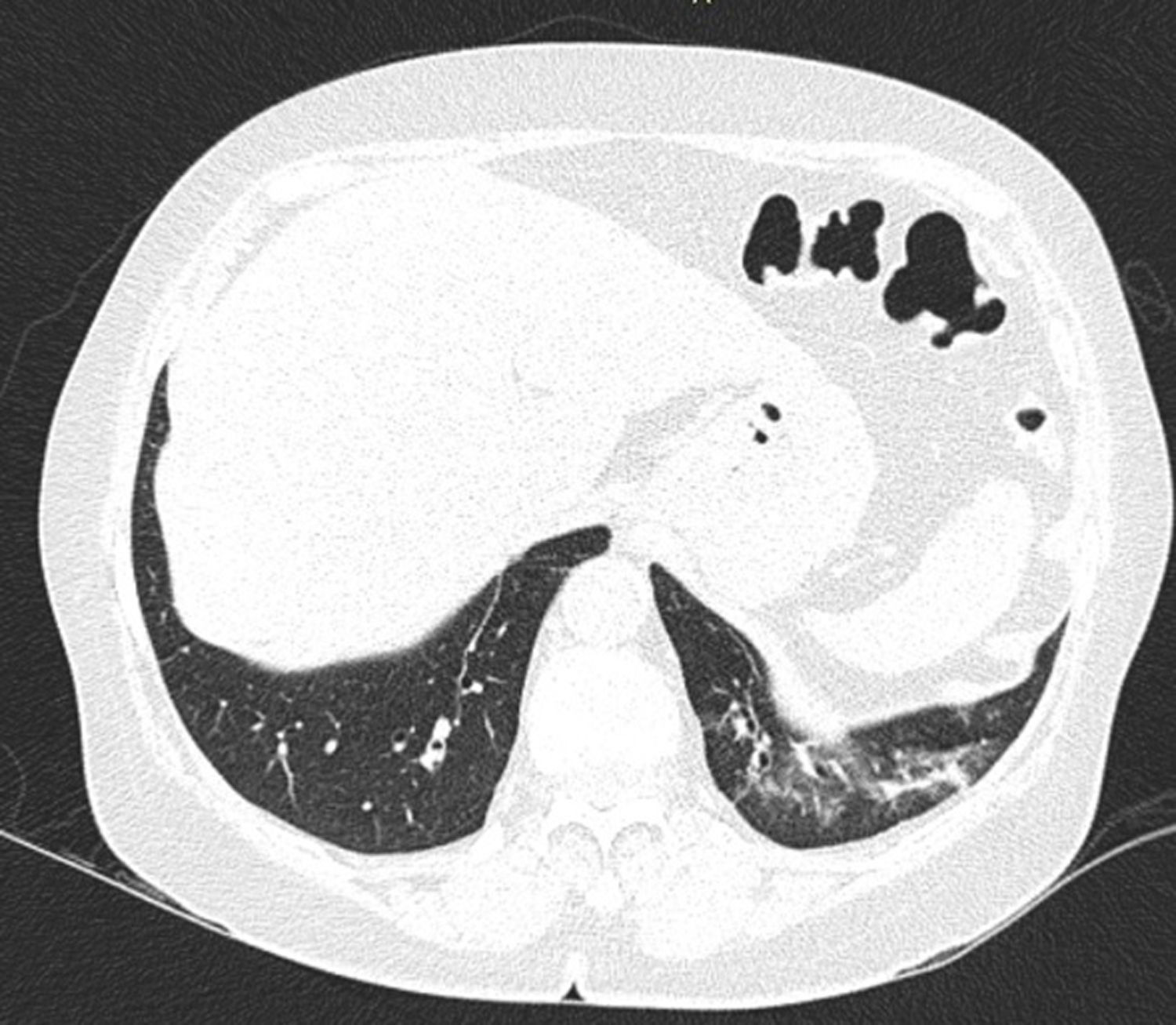
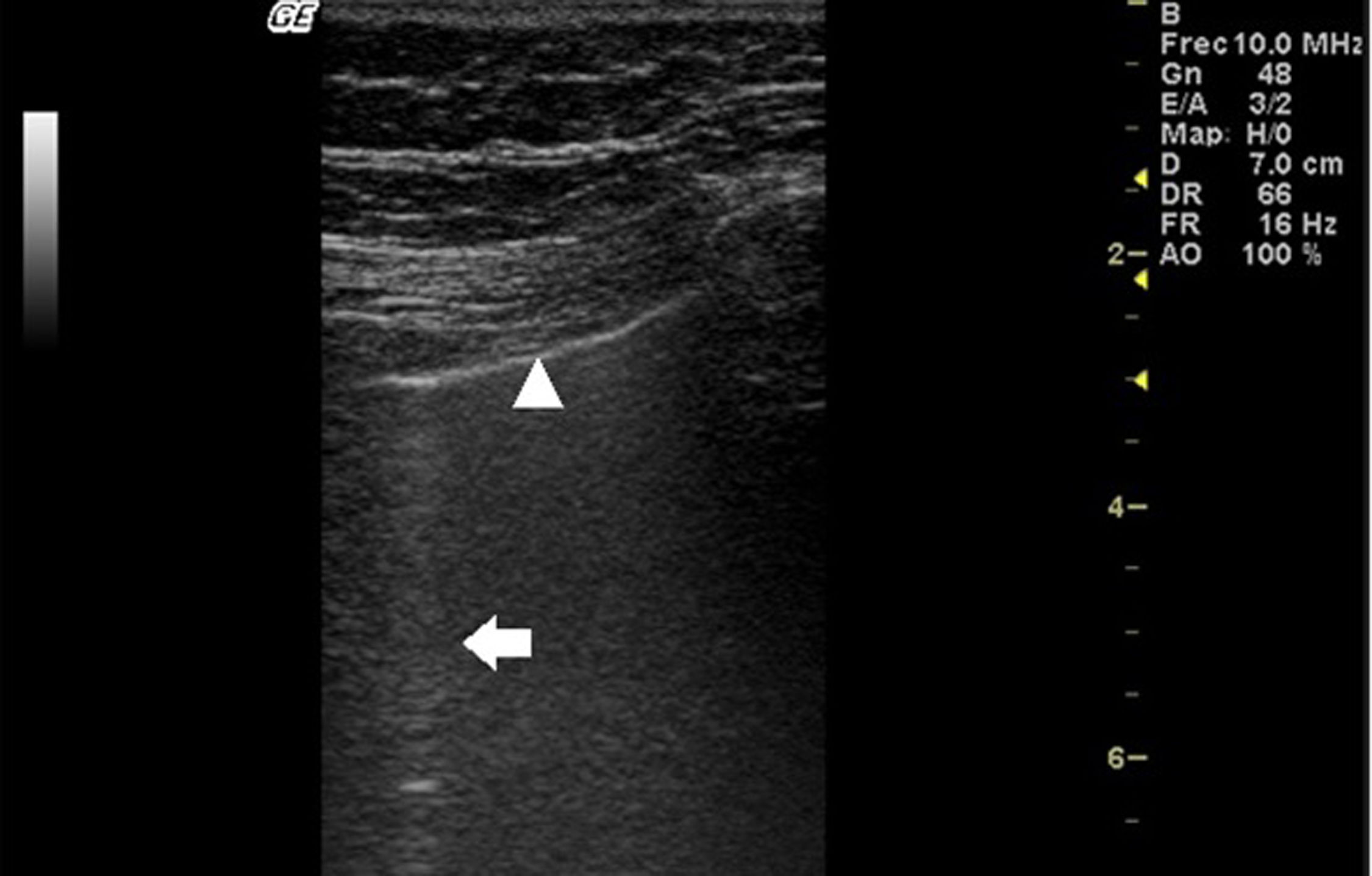
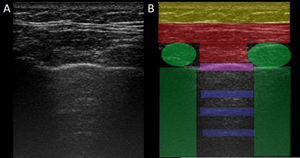
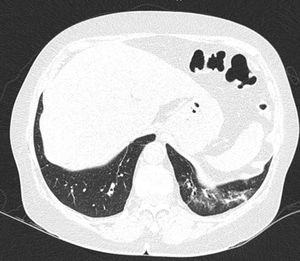
In ILD, there is a decrease in acoustic impedance between the lung air and the soft tissues of the chest wall, because part of the space initially occupied by air volume is replaced by infiltration of inflammatory cells or connective tissue, leading to pleural line disturbances and vertical artefacts called "B-lines". B-lines are indicative of increased subpleural lung density prior to consolidation and may be caused by the presence, not exclusively, of interstitial disease.25 The pleural line becomes irregular and thickened and may appear blurred and fragmented. B-lines are shown as vertical hyperechoic streak-like artefacts arising from the pleural line and extending to the end of the screen without fading, blurring the A-lines, and moving synchronously with the pleural sliding. The presence of multiple B-lines is the defining ultrasound sign of "interstitial syndrome".26Fig. 2 shows a CT scan of a patient with RA and ILD; Fig. 3 shows the ultrasound findings.
Lung ultrasound performed on the same patient as in Fig. 2 with GE Logiq 3 equipment, with 10–14 MHz multifrequency linear transducer, with the patient in a seated position and transducer in longitudinal direction. The arrow points to a B line. The triangle points to the pleural line, which is irregular in appearance. Note the absence of A lines.
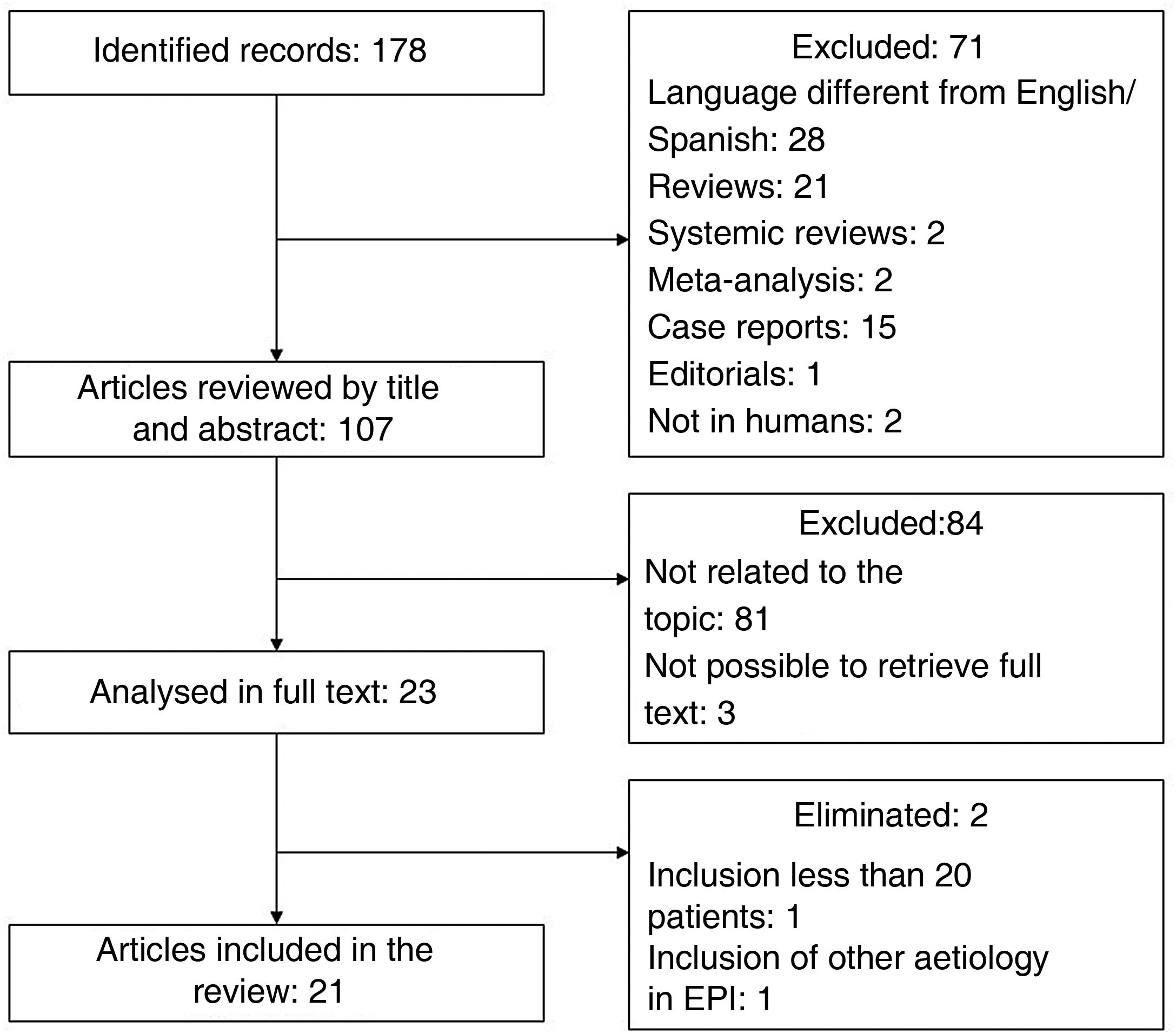
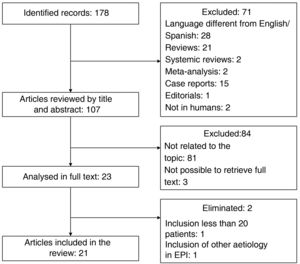
To identify relevant articles evaluating the usefulness of LUS in patients with DCTD, we conducted a search in the specialised health meta-search engine PubMed. The MeSH terms used were "Ultrasonography", "Lung Diseases, Interstitial", and "Connective Tissue Diseases". The search strategy was constructed with the descriptors and their synonyms using the Boolean operators "OR" and "AND"; the complete strategy can be found in the appendix. Only original articles were included. Duplicates, review articles, posters, and conference abstracts, articles with a title/abstract not related to the topic, language other than English or Spanish, and those where it was not possible to obtain the full text were excluded. Articles with small populations (fewer than 20 patients) and those that included other aetiologies of ILD in addition to DCTD were eliminated. The search performed on 01/04/22 yielded 178 records; after applying the exclusion and elimination criteria (process detailed in the flow chart shown in Fig. 4), a total of 21 articles were included. Table 1 gives a summary of the most relevant characteristics.
Summary of the articles included.
| Authors | Year | Population | ICS | Transducer | Criterion of positivity | Results |
|---|---|---|---|---|---|---|
| Studies only including patients with SSc | ||||||
| Gargani et al. 27 | 2009 | 33 SSc | 72 ICS | Cardiac sectoral | >10 BL | Correlation (+) with Warrick score (r = .72) |
| Delle Sedie et al. 28 | 2010 | 25 SSc | 65 ICS | Cardiac sectoral and linear | >5 BL linear, >11 BL sectoral | Linear: Se 85%, Sp 70%. Sectoral: Se 85%, Sp 60% |
| Moazedi-Fuerst et al. 33 | 2012 | 45 (25 SSc, 40 HC) | Not described | Convex and linear | >5 BL, PL > 2.8 mm | Correlation of 44% with ILD by HRCT. Greater pleural thickness in SSc than in healthy controls |
| Barskova et al. 29 | 2013 | 32 SSc | 72 ICS/8 ICS | Cardiac sectoral | >3 BL in 2 adjacent ICS or >5 BL in total | Se 100%, Sp 55%, NPV 100%, PPV 78% |
| Sperandeo et al. 34 | 2015 | 175 SSc | Not described | Linear and convex | PL>3 mm convex, >1.6 mm linear. With or without pulmonary nodules | >3−5 mm Se 80%, Sp 99%. > 3.5 mm Se 74%, Sp 99%. > 5 mm Se 90%, Sp 99%. For different ILD patterns |
| Gigante et al. 30 | 2016 | 39 SSc | Not described | Convex | >3 BL in 2 adjacent ICS or >5 BL in total | Correlation (+) between BL and Warrick score (r = .81). Correlation (−) between BL and DLCO (r = −.63) |
| Çakir Edis et al. 31 | 2016 | 48 (15 dcSSc, 33 lcSSc) | 14 ICS | Linear | >3 BL in 2 adjacent ICS or >5 BL in total | Correlation (+) with Warrick (r = .98) and (−) for DLCO (r = −.65). > 24 BL Se 100%, Sp 84% AUC .94 |
| Tardella et al. 32 | 2018 | 34 SSc | 14 ICS | Linear | >10 LB | Se 96%, Sp 92%, pLR 12.52 |
| Gargani et al. 36 | 2020 | 396 SSc | 58 ICS | Sectoral | >5 BL | Se 92%, Sp 16%, NPV 92%, PPV 16%, AUC .67 |
| Gasperini et al. 35 | 2020 | 41 SSc (14 lclSSc, 27 dcSSc) | 50 EIC | Convex | >10 BL/semiquantitative score. | Correlation (+) with HRCT and greater capillary damage (r = .42). Correlation (−) with DLCO (r = −.33) |
| Studies including only patients with RA | ||||||
| Moazedi-Fuerst et al. 37 | 2014 | 104 (64 RA, 40 HC) | 18 ICS | Convex and linear | BL in >2 regions or PL > 2.8 mm | Se 97.1%, Sp 97.3%, PPV 94.3, NPV 98.6% |
| Cogliati et al. 38 | 2014 | 39 RA | 72/8 ICS | Convex standard and pocket-size | 72 ICS > 17 BL, 8 ICS > 10 BL | 72 ICS: Se 92%, Sp 72%. 8 ICS: Se 69% Sp 88% |
| Mena-Vázquez et al. 40 | 2021 | 71 (35 RA with ILD, 36 RA without ILD) | 72 ICS/8 ICS | Linear | 72 ICS > 10 BL, 8 ICS > 5 BL | AUC .91. 72 ICS: Se 91%, Sp 52%, PPV 66.7%, NPV 86.9%. 8 ICS: Se 65.7% Sp 94.1% |
| Fotoh et al. 39 | 2021 | 150 RA | 14 ICS | Linear | >5.5 BL | Se 100, Sp 100, AUC 1 |
| Di Carlo et al. 41 | 2022 | 72 RA | 14 ICS | Linear | >9 BL | Se 70%, Sp 97.6%, AUC .83 pLR 29.4 |
| Studies including various CTD | ||||||
| Gutierrez et al. 42 | 2011 | 36 (28 SSc, 2 SSp, 1 UCTD, 2 ASyS, 2 DM, 1 MCTD) | 50 ICS/14 ICS | Convex | >10 BL | Correlation (+) with Warrick |
| Tardella et al. 43 | 2012 | 34 (26 SSc, 2 ASyS, 2 DM, 1 MCTD, 1 UCTD) | 50 ICS | Convex | >10 BL | Correlation (+) with Warrick (r = .87) |
| Aghdashi et al. 44 | 2013 | 31 (19 SSc, 8 RA, 2 overlap, 1 SSp, 1 DM) | 10 ICS | Linear | >5 BL | Se 73.5%, Sp 88.2%, PPV 95.2%, NPV 51.2% |
| Pinal-Fernandez et al. 46 | 2015 | 37 (21 ASyS, 16 SSc) | 72 ICS | Linear | PI>21% | AUC .93. Se 79%, Sp 100% |
| Moazedi-Fuerst et al. 45 | 2015 | 94 (25 RA, 6 SLE, 14 SSc, 49 HC) | 18 ICS | Convex | Number of ICS with BL | Greater number of BL in ILD |
| Buda et al. 47 | 2021 | 176 (22 RA, 46 SSp, 30 SSc, 35 AAV, 25 SLE, 18 DM) | 16 ICS | Convex and linear | Pleural abnormalities + bilateral BL | Se 99.3%, Sp 96.4% |
AAV: Anti-neutrophil cytoplasmic antibody-associated vasculitis; ASyS: Antisynthetase syndrome; AUC: Area under the curve; BL: B-lines; dcSSc: Diffuse cutaneous systemic sclerosis; DM: Dermatomyositis; HC: Healthy controls; HRCT: High-resolution computed axial tomography; ICS: Intercostal spaces; lcSSc: Limited cutaneous systemic sclerosis; MCTD: Mixed connective tissue disease; NPV: Negative predictive value; PI: Pleural irregularity; PL: Pleural line; pLR: Positive likelihood ratio; PPV: Positive predictive value; pSS: Primary Sjögren’s syndrome; RA: Rheumatoid arthritis; r: rho; Se: Sensitivity; SLE: Systemic lupus erythematosus; Sp: Specificity; SSc: Systemic sclerosis; UCTD: Undifferentiated connective tissue disease.
The first study exploring the validity of LUS to diagnose ILD associated with SSc was published by Gargani et al. in 2009. This included 33 patients with a diagnosis of SSc who underwent LUS with a sectoral transducer, using a 72 intercostal space (ICS) scanning protocol in the anterior and posterior thorax, in addition to a CT scan, pulmonary function tests (PFT) and determination of lung capacity and diffusing capacity for carbon monoxide (DLCO). They defined as positive the finding of 10 or more B-lines in the sum of the ICS assessed; 51% of patients were positive for this criterion and found a positive correlation between the ultrasound score (number of B-lines) and the Warrick score (score assessing the severity and extent of ILD by CT) (r = .72). They also described an inverse correlation between the number of B-lines and DLCO values (r = .60). Of note, the time taken for LUS was short (<10 min), feasible, and reproducible in all patients.27 They later published a second study, comprising 23 of the 33 patients from the original cohort, who underwent HRCT and LUS with sectorial and linear transducer; the protocol was with 62 ICS, which demonstrated a good intraclass correlation for the number of B-lines detected by both transducers (.68). In addition, they confirmed the correlation between the number of B-lines and the Warrick score. This time, sensitivity (Se) and specificity (Sp) were calculated, and the tomography result was used as the gold standard to diagnose ILD; different cut-off points were defined for the number of B-lines identified with the sectoral (>5) and linear (>11) transducer, because the width of the latter may increase the B-line count. With these cut-off points, the sectoral transducer showed Se 85% and Sp 70%, comparable to the performance of the linear transducer (Se 85% and Sp 60%).28
In a design similar to that of Gargani et al., Barskova et al. performed LUS with cardiac transducer and the same ICS, HRCT, transthoracic echocardiogram, and PFT protocol in 55 patients with SSc. They defined LUS positive for ILD as the presence of more than 3 B-lines in contiguous ICS or more than 5 B-lines in total. They confirmed the feasibility of LUS as it could be used in all patients and described acceptable intra- and interobserver variability: 5.1% and 7.4%, respectively. They found a concordance rate for diagnosis of ILD by HRCT and LUS of 83%; all discordant cases were false positives. With HRCT as the reference standard, 88% of patients were diagnosed with ILD. To determine the usefulness of LUS as a diagnostic method, they performed ROC curve and area under the curve (AUC) calculation with a result of .94. The Se and Sp of LUS were 100% and 55%, respectively, the positive predictive value (PPV) was 78% and the negative predictive value (NPV) was 100%.29
In 2016, a study with 39 patients was published that corroborated the correlation between the number of B-lines and the Warrick score, as well as the inverse correlation of these with DLCO. It should be noted that, unlike previous studies, a convex transducer was used, no posterior chest regions were scanned and capillaroscopy findings were included in the analysis; patients with more capillaroscopy damage showed more B-lines.30 In the trend of scanning fewer ICS and with linear transducer, we can encompass the studies published by Çakir Edis et al. in 2016 with 48 patients and Tardella et al. in 2018 with 34 patients. Both used a LUS protocol of 14 ICS. The former highlighted that even in a simplified protocol, a high correlation between the number of B lines and the Warrick tomographic score (r = .89) was maintained, as well as an inverse correlation with DLCO and forced vital capacity. They calculated an AUC of .94, using the same criteria to consider ILD by USP as Barskova et al., with observation of an even higher yield: Se of 100%, Sp of 84%, PPV of 90.6%, and NPV of 100%.31 Tardella et al. sought to describe the utility of USP to diagnose clinically relevant ILD, which they defined as a Warrick score >7 as the cut-off point correlating with altered PFT in a previous study. With the criterion of more than 10 B-lines, they observed Se of 96.3%, Sp of 92.3%, and likelihood ratio of 12.52.32
Detection of pleural abnormalities to diagnose ILDMoazedi-Fuerst et al. described in 2012 a higher frequency of abnormalities on LUS in SSc patients, including pleural line thickening and fragmented appearance, compared to healthy controls.33 The work of Sperandeo et al. examined this area in more depth and performed LUS with convex transducer in anterior and lateral chest, HRCT, and PFT in 175 patients with SSc. Analysis of LUS focused on detecting pleural line alterations; when >3 mm was found, it was qualified as thickening, and >5 mm as severe thickening. To establish this cut-off point, LUS was previously performed on 200 healthy controls, obtaining a mean pleural line thickness of 1.4 ± 1.1 mm. The usefulness of LUS for detecting different patterns of ILD by tomography was tested; for the reticular pattern an AUC of .95, Se of 80%, and Sp of 99% was described, and for the reticulonodular pattern with honeycombing, an AUC of .99, Se of 74% and Sp of 99%. The best performance was for honeycombing alone: AUC of .99, Se of 90.1%, and Sp of 99.34
Lung ultrasound for prognosisIn 2020, the results of a study of 39 patients with SSc were published; the number of B-lines assessed at baseline correlated with the change in DLCO observed at 12 months, potentially predictive of worsening ILD.35 In the same year, Gargani et al. emphasised the possible prognostic utility of LUS. They included 396 patients with SSc who underwent LUS in a protocol of 58 ICS with sectoral transducer. After a mean follow-up of 28 months, 50 patients developed ILD or had worsening of already diagnosed ILD. The number of B-lines in the posterior chest was shown to be of moderate utility in predicting either of these events; an AUC of .67 was calculated or the cut-off point of more than 5 B-lines showed Se of 92%, Sp of 16%, NPV of 92%, and PPV of 16%. The presence of more than 5 B-lines in the posterior chest conferred a hazard ratio (HR) of 5.5 for developing or worsening of ILD. In multivariate analysis it remained relevant and statistically significant (HR 3.37), even higher than the HR conferred by topoisomerase-1 antibody positivity (HR 2.98).36
Lung ultrasound in rheumatoid arthritisMoazedi-Fuerst et al. published a pilot study in this population in 2014. They included 64 patients with RA without respiratory symptoms, performed PFTs that were normal in all subjects, LUS was performed in 18 regions, using a convex transducer to assess B-lines and a linear transducer for pleural line characteristics. They defined ILD by LUS as the presence of B-lines in 2 or more regions, as well as pleural thickening >2.8 mm. Eighteen (28%) of the 64 RA patients were positive for this criterion; when all patients underwent CT scans, diagnosis of ILD was confirmed in 17 of the 18 identified by LUS. In parallel, 40 healthy volunteers were studied; LUS abnormalities were described in only 3 (7%); HRCT was not performed in this group for ethical reasons. They calculated an Se of 97.1%, Sp of 97.3%, PPV of 94.3%, and NPV of 98.6%.37 In the same year, Cogliati et al. confirmed the usefulness of LUS, describing that diagnostic yield does not decrease significantly when performed by a physician with little training (2 sessions of 3 h to detect B-lines), or when using pocket-size ultrasound equipment compared to an expert physician using standard equipment, or with a simplified ICS protocol (8 regions in anterior and lateral thorax) versus a comprehensive protocol of 72 ICS.38
In 2021, in a case-control study, Fotoh et al. studied 75 patients with RA + ILD previously diagnosed by HRCT and 75 patients with RA without ILD, matched for sex and age. They performed LUS with a 14 ICS protocol; AUC analysis showed exceptional performance (AUC = 1) when setting the cut-off point >5.5 B-lines with Se of 100% and Sp of 100%. At one-year follow-up, 33% of patients in the ILD + RA group had died; in multivariate analysis they identified an odds ratio of 1.53 for mortality in patients with more than 28 B-lines. They also studied a serum marker of lung damage (Krebs von den Lungen-6) and found high concordance (r = .96) between its levels and the B-line count per LUS.39 In the same year and with the same design, but with fewer patients (35 RA + ILD and 36 RA without ILD), a simplified index was described, in which they only considered the B-line count in 8 ICS, which was developed by scanning 72 ICS by LUS selecting only the spaces that showed the highest correlation with ILD.40
The most recent study addressing ILD in RA was published in 2022 and focused on detecting clinically relevant ILD, which they defined as percentage of lung area affected by fibrosis >10.7% on HRCT using a computer-aided method. In this protocol scanning 14 ICS they found good yield, with AUC of .83; the highest diagnostic yield cut-off point (more than 9 B-lines) obtained Se of 70%, Sp of 97% and likelihood ratio of 29.41
Lung ultrasound in groups with various rheumatic diseasesShortly after the usefulness of USP to diagnose ILD in patients with SSc had been demonstrated, studies began in other cohorts, with a variety of rheumatic diseases. The first such study was published in 2011, enrolling 36 patients with SSc, anti-synthetase syndrome, dermatomyositis, mixed connective tissue disease, and undifferentiated connective tissue disease, in which they first described the LUS protocol exploring 14 ICS. From the comprehensive assessment and after selecting the most accessible and most frequently abnormal ICS, they confirmed that in this heterogeneous group of patients there was also a correlation between LUS score and CT-observed damage score; they also demonstrated good correlation between the comprehensive and simplified ICS protocol, whereas the latter is faster to perform (8.3 vs. 23.3 min).42 In 2012, Tardella et al. confirmed the correlation between LUS and HRCT scores in another similar group of patients in which they scanned 50 ICS.43 With fewer patients and a diversity of diseases (31 patients with SSc, RA, dermatomyositis, overlap syndrome), in 2013 Aghdashi et al. determined Se of 73% and Sp of 88.2% by exploring only 10 ICS; they took more than 5 B-lines as a cut-off point.44 In 2015, two additional studies were published that also showed high Se and Sp of LUS in a similar population. Finally, in 2021 Buda et al. reported one of the studies with the largest number of patients and diversity; they included 180 patients with RA, SLE, SSc, anti-neutrophil cytoplasmic antibody-associated vasculitis, and Sjögren's syndrome. By combining pleural line abnormalities with the presence of B-lines, they obtained Se of 99.3% and Sp of 96.4% to diagnose ILD.47
Other potential causes of ultrasonographic interstitial syndrome in patients with rheumatic diseasesWith the existing evidence on the usefulness of USP in DCTD and ILD, it is essential to recognise that the changes identified are not entirely specific. The interstitial syndrome that involves the appearance of multiple B-lines, with a variable cut-off point depending on the equipment and type of transducer used, translates as non-specific tissue abnormalities, such as changes in the deposition of extracellular matrix, increased vascular permeability, and oedema or increased cellularity, regardless of whether it is an inflammatory context. In this sense, diseases such as congestive heart failure and infectious processes may cause imaging changes indistinguishable from those described in ILD.25,48
Rheumatic diseases are related to deficits in immune function due to both disease-specific mechanisms and treatment with conventional synthetic and biological disease-modifying antirheumatic drugs. In inflammatory arthropathies, the use of methotrexate and leflunomide has been shown to increase the risk of infections from 20% to 40%–60% with the use of glucocorticoids. Biological disease-modifying antirheumatic drugs, primarily tumour necrosis factor inhibitors, increase the risk of bacterial pneumonia especially in the first year of use.49 In SSc, 30% of the causes of death not attributable to the disease are of infectious aetiology,50 while in SLE infectious lung diseases are the main cause of hospitalisation, with a 36% increase in mortality associated with severe pneumonia in intensive care units.51,52
LUS has demonstrated Se of 77% to detect pneumonia in emergency departments, 25% higher than conventional chest X-ray.53 There are assessment protocols with different lung ultrasound scan areas that can achieve a Se score of up to 97%. The main changes observed include consolidations, interstitial syndrome, and pleural line abnormalities.54
These ultrasound patterns are variable depending on the disease course duration and the pathogen involved. Interstitial syndrome has been specifically described in Chlamydia pneumonia, Pneumocystis, measles, and influenza virus. In the last 2 years and following the impact of the COVID-19 pandemic, LUS has become more prominent in timely disease detection, procedure management, and decision making in hospital respiratory care settings. In early stages SARS-CoV-2 infection usually manifests with multifocal B-lines, which over time become confluent and may later form subpleural consolidations.55
Another differential to consider in ultrasonographic interstitial syndrome includes the changes produced by pulmonary congestion in heart failure. In rheumatic diseases, primarily RA, there is ample evidence of the risk associated with cardiovascular morbidity and mortality. Patients with RA have twice the risk of heart failure as the general population, even in the absence of ischaemic heart disease.56 Endothelial dysfunction, myocardial inflammation, and accelerated atherosclerosis are part of the pathophysiological process of heart failure associated with RA and other chronic autoimmune and inflammatory diseases such as SLE, SSc, and mixed connective tissue disease.57
Comparative studies between pulmonary involvement due to heart failure and acute respiratory failure syndrome using LUS showed that confluent B-lines are frequently observed in both, with absence of pleural line abnormalities and consolidations in the case of heart failure,58 and therefore clinical picture and patient characteristics are still mandatory for appropriate diagnostic assessment.
ConclusionsLUS is established as a useful tool to diagnose ILD in various DCTDs; it is easily accessible and inexpensive, with diagnostic yield close to the current gold standard, with the advantage of avoiding exposure to ionising radiation. It has demonstrated higher sensitivity than other low-cost methods, such as chest radiography and PFT. There is currently no consensus on the standard scanning protocol, however, several studies have reported comparable performance between extended protocols of more than 50 ICS and the less time-consuming simplified protocols of 14 and 10 ICS. An additional advantage is that even when the study is performed by staff with little training time, diagnostic yield is not significantly affected. B-lines are the artefacts most studied, but considering pleural changes and subpleural nodules in the assessment seems to increase Se and Sp.
One of the main disadvantages to consider is that a positive LUS still requires a CT scan to identify the imaging pattern due to the correlation with histological patterns and the therapeutic and prognostic implications that this entails. Likewise, there are other entities that can cause ultrasonographic interstitial syndrome with the characteristic increase in B-lines and were systematically excluded in all studies, and therefore they should be considered in real practice as possible causes of false positives.
Given the diagnostic yield and the advantages and disadvantages outlined above, we consider that LUS is very useful as a screening method for ILD in patients with DCTD. There is less evidence of its prognostic utility, and its potential as a follow-up tool in patients with established ILD is still to be assessed.
Conflict of interestsThe authors have no conflict of interests to declare.
(((((Connective Tissue Diseases[MeSH Terms]) OR (Connective Tissue Diseases[Title/Abstract])) OR (Disease, Connective Tissue[Title/Abstract])) OR (Diseases, Connective Tissue[Title/Abstract])) AND (((((((((((((((Lung Diseases, Interstitial[MeSH Terms]) OR (Lung Diseases, Interstitial[Title/Abstract])) OR (Diffuse Parenchymal Lung Disease[Title/Abstract])) OR (Interstitial Lung Diseases[Title/Abstract])) OR (Diffuse Parenchymal Lung Diseases[Title/Abstract])) OR (Interstitial Lung Disease[Title/Abstract])) OR (Lung Disease, Interstitial[Title/Abstract])) OR (Pneumonia, Interstitial[Title/Abstract])) OR (Interstitial Pneumonia[Title/Abstract])) OR (Interstitial Pneumonias[Title/Abstract])) OR (Pneumonias, Interstitial[Title/Abstract])) OR (Pneumonitis, Interstitial[Title/Abstract])) OR (Interstitial Pneumonitides[Title/Abstract])) OR (Interstitial Pneumonitis[Title/Abstract])) OR (Pneumonitides, Interstitial[Title/Abstract]))) AND ((((Ultrasonography[MeSH Terms]) OR (Ultrasonography[Title/Abstract])) OR (Diagnostic Ultrasound[Title/Abstract] OR Diagnostic Ultrasounds[Title/Abstract] OR Ultrasound, Diagnostic[Title/Abstract] OR Ultrasounds, Diagnostic[Title/Abstract] OR Ultrasound Imaging[Title/Abstract] OR Imaging, Ultrasound[Title/Abstract] OR Imagings, Ultrasound[Title/Abstract] OR Echotomography[Title/Abstract] OR Ultrasonic Imaging[Title/Abstract] OR Imaging, Ultrasonic[Title/Abstract] OR Sonography, Medical[Title/Abstract] OR Medical Sonography[Title/Abstract] OR Ultrasonographic Imaging[Title/Abstract] OR Imaging, Ultrasonographic[Title/Abstract] OR Imagings, Ultrasonographic[Title/Abstract] OR Ultrasonographic Imagings Echography[Title/Abstract] OR Diagnosis, Ultrasonic[Title/Abstract] OR Diagnoses, Ultrasonic[Title/Abstract] OR Ultrasonic Diagnoses[Title/Abstract] OR Ultrasonic Diagnosis[Title/Abstract] OR Echotomography, Computer[Title/Abstract] OR Computer Echotomography[Title/Abstract] OR Tomography, Ultrasonic[Title/Abstract] OR Ultrasonic Tomography[Title/Abstract]))).