Rheumatoid arthritis (RA) is associated with a 1.3–3-fold increase in mortality, being the major cause of death from cardiovascular complications (40%–50%). Therefore, the initial approach should include cardiovascular risk (CVR) assessment using algorithms adapted for this population. Although, SCOREM is an important advance, there are data indicating that subclinical atherosclerosis may be underdiagnosed.
ObjectiveTo estimate the strength of association between carotid ultrasound and SCOREM in this population, as well as the implication of disease activity.
MethodologyCross-sectional, observational, analytical study performed at the General Hospital of Ciudad Real, Spain, between June 2013 and May 2014. The evaluation of CVR was performed and, according to SCOREM, the population was divided into low and high (medium, high and very high) risk. We studied the presence of subclinical atherosclerosis in low-risk patients.
ResultsOf the total of 119 RA patients, 73.1% had traditional risk factors. Thirty-eight patients were excluded because of a previous cardiovascular event, diabetes mellitus and/or nephropathy. Atheromatous plaque was observed in 14.63% of the low-risk population. The factor with the strongest association to the presence of subclinical atherosclerosis was a moderate or high activity of RA measured by the simplified disease activity index with an odds ratio of 4.95 (95% CI: 1.53–16.01).
ConclusionsAlthough there was an acceptable correlation between the presence of subclinical atherosclerosis and SCOREM, there was a considerable proportion of atheromatous plaques in low-risk patients. Disease activity was the risk factor most closely associated with increased CVR.
La artritis reumatoide (AR) presenta una mortalidad de 1,3 a 3 veces superior a la población general; la principal causa de muerte son las complicaciones cardiovasculares (40-50%). En el abordaje inicial se debe incluir la valoración del riesgo cardiovascular (RCV) mediante algoritmos adaptados para esta población. Si bien el SCOREm constituye un avance importante, hay datos que indican que podría infradiagnosticar la ateroesclerosis subclínica.
ObjetivoEstimar la fuerza de asociación entre la ecografia carotídea y el SCOREm en esta población, así como la implicancia de la actividad de la enfermedad.
MetodologíaEstudio observacional, transversal y analítico, realizado en el Hospital General de Ciudad Real durante el periodo junio de 2013-mayo de 2014. Se realizó la valoración del RCV y según el SCOREm se dividió a la población en riesgo bajo y alto (medio, alto y muy alto). Se estudió la presencia de ateroesclerosis subclínica en los pacientes de riesgo bajo.
ResultadosDel total de 119 pacientes con AR, el 73,1% presentaba factores de riesgo tradicionales. Se excluyeron 38 pacientes por evento cardiovascular previo, diabetes mellitus y nefropatía. Se objetivó placa ateromatosa en el 14,63% de la población de riesgo bajo. El factor con mayor asociación con la presencia de aterosclerosis subclínica fue el grado de actividad moderada/alta de la AR medida mediante el SDAI, con un OR de 4,95 (IC 95%: 1,53-16,01).
ConclusionesAunque existe una aceptable asociación entre la presencia de aterosclerosis subclínica y el SCOREm, hay una proporción no despreciable de pacientes clasificados de riesgo bajo con placas ateromatosas. La actividad de la enfermedad fue el factor de riesgo más asociado al incremento del RCV.
Rheumatoid arthritis (RA) affects from .5%–1% of the population, presents with a 1.3–3-fold increase in mortality compared with that of the general population, and its 40%–50%1–4 mortality rate from cardiovascular origin is a notable factor.
Studies like those of Solomon et al. and Maradit-Kremers et al. have reported the increase in the risk of acute myocardium infarct (RR: 2.07–3.17) and stroke (RR: 1.48) in patients with RA,5,6 comparable to that described in type 2 diabetes mellitus (OR: 2.7–3.11).7,8
Although the traditional risk factors are important in the pathogenesis of atherosclerosis, they do not fully explain the reason for the increase in cardiovascular events (CVE) described in this patient group.9–11 Cardiovascular disease is therefore currently considered to be an extra articular symptom of RA.12–16 Medium to long-term cardiovascular risk (CVR) estimation is therefore necessary,17 as this would enable prevention activities to be prioritised and for the intensity with which these factors must be treated to be defined.18,19
In 2010, EULAR reached a consensus regarding the modified SCORE (SCOREM) CVR assessment scale, which consisted in multiplying by a conversion factor of 1.5 the result obtained with SCORE for those patients with 2 of the following 3 criteria: duration of the disease higher or equal to 10 years, rheumatoid factor (RF) or the determination of anti cyclic citrullinated peptide (ACCP) antibodies and the presence of extra articular symptoms.20 However, it has been observed that this tool and the factors under consideration under diagnose this risk: up to between 12% and 30% of subclinical atherosclerosis have been identified by carotid ultrasound scan and the development of short to medium term CVE in patients classified as low/intermediate risk,21–26 which has resulted in further attention being paid in recent years to other clinical features linked with RA which could be more associated with an increase in the risk of subclinical atherosclerosis. The most outstanding of these are: the level of activity of the disease, the inflammation markers, the level of compromise or articular erosion or the use of corticoids.27–33
The aim of this study was to calculate the role disease activity plays in the development of subclinical atherosclerosis in patients with RA of low cardiovascular risk.
Materials and MethodsA cross-sectional, observational, analytical study (blind to the ultrasound scan study) was performed for patients with RA (according to ACR 1987 or ACR/EULAR 2010 criteria) at the General University Hospital of Ciudad Real (HGUCR) during the period from June 2013-May 2014.
Patients over18 who agreed to participate in the study and who signed the informed consent form were included. Patients with non-affiliated arthritis or overlap syndromes, recent clinical history or diagnosis of previous CVE, diabetes mellitus or kidney failure (glomerular filtration rate<60ml/min).
A complete analysis was performed which included serological and activity markers (speed of globular sedimentation, reactive C protein, rheumatoid factor and anti CCPs), together with a metabolic study for the evaluation of CVR (baseline blood sugar level, glycosylated haemoglobin, uricaemia, renal, lipid and thyroid profiles). The degree of activity was calculated through the SDAI (number of swollen joints, number of painful joints, overall patient assessment, overall assessment of the physician and CRP levels). Activity records fro the previous year were collected through the SDAI, including those made at the start of the study and an average was obtained with regard to the activity.
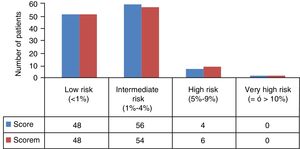
The patients with RA were divided into low CVR and high CVR (patients with medium, high or very high risk) according to the SCOREM.
Finally, a carotid ultrasound scan study was performed by a radiologist who was always unaware into which group each patient belonged. An ultrasound scan of the brand Toshiba Aplio XG, model Ssa-790A was used, with a lineal transductor of 7–10MHz, focusing on the measurement of the intima-media thickness (IMT). It was considered pathological if the IMT was >.9mm and with the presence of the atheromatous plaque (AP) (focal thickening >1.5mm), according to standard protocol.34–36
The information obtained was inserted into a Microsoft Excel database. The variables were assessed using frequency measurements and central tendency/dispersion measurements and the strength of the association of the variables were assessed with the odds ratio and its confidence interval at 95%. STATA 12.0 was used for analysis and the calculation of Yates correction was performed with the IBM SPSS 22.0 programme. The study protocol was approved by the Clinical Research Ethics Committee of the University Hospital of Ciudad Real.
ResultsDuring the period from June 2013 to May 2014 of a total of 119 patients with RA (63.87% females; 36.13% males) agreed to take part in the study (see Table 1).
Clinical Characteristics of the 119 Patients With Rheumatoid Arthritis of the HGUCR.
| Characteristics | N | % or interval |
|---|---|---|
| Female | 76 | 63.8 |
| Average age (years) | 57.43 | 29–85 |
| Body mass index (weight [kg]/height2[m]) | 27.47 | 16.44–49.48 |
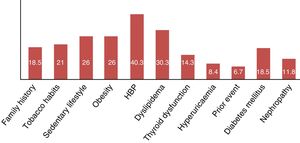
| Family history of cardiovascular event | 22 | 18.48 |
| Sedentrism | 31 | 26.05 |
| Active smoker | 25 | 21 |
| High blood pressure | 48 | 40.33 |
| Dyslipidemia | 36 | 30.25 |
| Thyroid dysfunction | 17 | 14.28 |
| Hyperuricaemia | 10 | 8.4 |
| Known vascular disease | ||
| Previous cardiovascular event | 8 | 6.72 |
| Type 2 diabetes | 4 | 3.36 |
| Nephropathy | 2 | 1.68 |
| Rheumatoid arthritis | ||
| Time of evolution ≥10 years | 56 | 47.05 |
| RF (+) | 58 | 48.74 |
| ACCP (+) | 82 | 68.9 |
| Extra articular symptoms | 28 | 23.53 |
| Average CRP (NV<.5mg/dl) | 1.13 | 0–14.4 |
| Average ESR (NV: men<10mm/h, women<20mm/h) | 22.63 | 2–98 |
| Current treatment | ||
| Corticoids | 5 | 4.2 |
| Corticoids+DMARD | 76 | 63.87 |
| Corticoids+DMARD+biologic agent | 14 | 11.77 |
| DMARD+biologic agent | 6 | 5 |
| Biologic agent+corticoids | 9 | 7.56 |
| Other combinations | 9 | 7.56 |
| SDAI (remission or low activity | 79 | 66.39 |
ACCP: anti cyclic citrullinated peptide; DMARD: disease-modifying anti-rheumatic drug; RF: rheumatoid factor; kg: kilograms; m: metres; CRP: C-reactive protein; SDAI: simplified disease activity index; NV: normal value; ESR: erythrocyte sedimentation rate.
In the population under study there was a high prevalence of the presence of any CVR factor (84.87%) and a 73.1% presence of standard risk factors. Furthermore, 6.72% had presented a CVE when the study began.
With regard to the clinical RA profile, a prevalence of 79.83% positive serological markers (RF or ACCP) was observed, together with disease course above 10 years in 47% of our sample. Furthermore, 66% received appropriate disease control, both globally via the SDAI (remission or low activity) and through the presence of inflammatory activity biological markers. The most common treatment was the use of corticoid therapy at a 5–10mg/day dose of prednisone or equivalent and disease modifying anti-rheumatic drugs (principally methotrexate).
Eleven patients (9.24%) were excluded at the beginning of the study because they presented with known vascular disease (8 previous events, 4 diabetes mellitus and 2 nephropathies), and therefore a total of 108 patients remained upon whom the study analysis and SCOREM calculation was performed (48 low risk and 60 intermediate, high and very high risk) (Fig. 1).
Following the specific analytical study results (baseline blood sugar level, lipid and thyroid profiles, creatinine, glomerular filtration, glycosylated haemoglobin, uric acid, acute phase reactants, RF, ACCP, etc.) 27 patients were excluded: 18 due to altered glycosylated haemoglobin and 12 due to glomerular filtration <60ml/min (3 patients with both findings), which elevated the prevalence of CVR factors in our total population sample (Fig. 2).
Of the remaining 81 patients (46 low risk and 25 intermediate, high and very high risk), 10 patients did not attend the carotid ultrasound scan, and the study analysis was finally performed on a total of 71 patients (41 low risk and 30 intermediate, high and very high risk) (Table 2). General recommendations were given to all patients regarding healthy lifestyles and high/very high-risk patients were administered with treatments depending on their CVR profile, with subsequent follow-up by the primary care physician.
Clinical Features of the Population Distributed According to Their Cardiovascular Risk Through the Application of the SCOREM.
| Features (Number of patients) | High risk according to SCOREM (30) | Low risk according to SCOREM (41) | P |
|---|---|---|---|
| Average age (years)a | 61.2 | 45 | <.001* |
| Female (50)a | 15 | 35 | .002* |
| Family history (13) | 8 | 5 | .12 |
| Active smoker (20)a | 10 | 10 | .4 |
| Sedentary lifestyle (15) | 8 | 7 | .33 |
| High blood pressure (19)a | 12 | 7 | .03* |
| Dyslipidemia (11)a | 7 | 4 | .12 |
| Average BMI | 27.71 | 26.96 | .47 |
| Thyroid alteration (10) | 2 | 8 | .14 |
| Hyperuricaemia (5) | 2 | 3 | .91 |
| Disease progression>10 years (28)a | 12 | 16 | .93 |
| RF(+) (38)a | 18 | 20 | .35 |
| ACCP (+) (48)a | 23 | 25 | .05* |
| Extra articular symptoms (15)a | 7 | 8 | .69 |
| Altered CRP (37) | 18 | 19 | .25 |
| Altered ESR (47) | 19 | 28 | .66 |
| SDAI (mod and high) (19) | 13 | 6 | .009* |
ACCP: anti cyclic citrullinated peptide; RF: rheumatoid factor; BMI: body mass index; CRP: C-reactive protein; SDAI: simplified disease activity index; ESR: erythrocyte sedimentation rate.
The variables taken into account for estimation of the SCOREM were more prevalent in the high-risk group. The variables in the high risk group with a statistically significant difference (P<.05) were age, gender, HBP, ACCP and activity measured by SDAI.
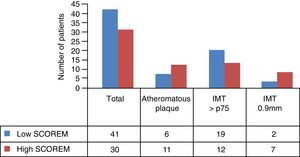
Average IMT in the low risk group measured by SCOREM was .65mm and .80mm in the high-risk group. The other ultrasound features per risk groups are shown in Fig. 3.
Analysis of the correlation between the CVR stratification by SCOREM and the findings from the ultrasound scan related that, according to our results, stratifying as a high risk (intermediate, high and very high) increases the risk of presenting with atheromatous plaque by 3 times, with a P=.037. However, there is a notable existence of 14.63% patients classified as low risk (6/41) who present with subclinical atherosclerosis due to the presence of atheromatous plaque. This represents 35.39% (6/17) of the total AP identified in our study population (Table 3).
Association Between SCOREM and Atheromatous Plaque Type Subclinical Atherosclerosis.
| Atheromatous plaque | OR and CI 95% | P | ||
|---|---|---|---|---|
| +(17) | −(54) | |||
| High risk SCOREM (30) | 11 | 19 | 3.37 (1.07–10.56) | .037 |
| Low risk SCOREM (41) | 6 | 35 | ||
Specificity: 64.81% (CI 95%: 51.48–76.18); Power: 67.43%; Sensitivity: 64.71% (CI 95%: 41.3–82.69); VPN: 85.37% (CI 95%: 71.56–93.12); VPP: 36.67% (CI 95%: 21.87–54.49).
Results are similar if calculation of sensitivity, specificity, VPP and VPN are made using the cut off point of the IMT of ≥.9mm (Table 4). However, results vary if we take the percentile p75 of the IMT as reference, in the healthy Spanish population, as the sensitivity of this tool drops to 38.71%, which determines that 46.34% of patients classified as low risk by SCOREM present with a IMP higher than the IMT p75 depending on gender and age. In the light of these factors, the specificity obtained (55%–64.81%) expresses a limitation in the capacity this test has in detecting those who do not have the sought-after condition (true negatives).
Association Between SCOREM and Subclinical Atherosclerosis Type IMT≥.9mm.
| IMT≥.9mm | OR and CI 95% | P/Yates’ correction | ||
|---|---|---|---|---|
| +(9) | −(62) | |||
| SCOREM high risk (30) | 7 | 23 | 5.93% (1.13–31.01) | .035/.0514 |
| SCOREM low risk (41) | 2 | 39 | ||
Specificity: 62.9% (CI 95%: 50.46–73.84); Sensitivity: 77.78% (CI 95%: 45.26–93.68); VPN: 95.12% (CI 95%: 83.86–98.65); VPP: 23.33% (CI 95%: 11.79–40.93).
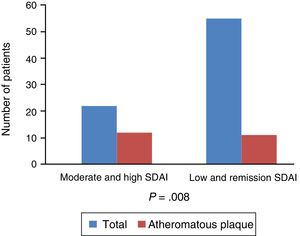
Regarding the link with the level of activity and subclinical atherosclerosis, there is a notably strong association, with an OR 4.95 and P=.008, between the presence of atheromatous plaque and the degree of activity of the disease measured globally with clinical and analytical parameters by the SDAI and not just due to the elevation of the acute phase reactants. The presence of disease activity would condition an increase in the risk of presenting a pathological IMT: IMT>.9mm (OR 2.5; CI 95%: .59–10.55; P=.21) or IMT>p75 (OR 2.2; CI 95: .75–6.4; P=.14) with a fairly acceptable confidence interval (Fig. 4).
The factors relating to the RA which are taken into consideration by EULAR for modification of the SCORE proposal present an association with the increase in the risk of subclinical atherosclerosis, although this is not statistically significant: evolution time >10 years (P=.19), RF (P=.95), ACCP (P=.73) and extra articular symptoms (P=.34).
DiscussionSCOREM and the Under Diagnosis of Cardiovascular RiskDespite attempts to design algorithms for an appropriate CVR stratification in the RA population (Reynolds scale and QRisk II scale) and the modifications proposed by EULAR to the SCORE, these tools contiunue presenting a lower than expected sensitivity and specificity (68%–87% and 55%–76%, respectively), which leads to up to 32% of the CVE occurring in the population classified as low risk according to these tools.26,37–41 Even those factors considered up until now as determinant in the increase of CVR (course of the disease >10 years, RF [+], ACCP [+], etc.) may not have the expected impact on CVR stratification.21,42,43
In our study we observed the presence of atheromatous plaques in 14.63% of the population classed as low risk according to SCOREM, with an average IMT of .6530mm, values which are much higher than those expected in the healthy Spanish population of low risk according to the SCORE44 (3% of atheromatous plaques and an average IMT of .56mm [SD ±.1078mm]). These data provide SCOREM with sensitivity of 64.71% (CI 95%: 41.3–82.69) taking as reference the presence of atheromatous plaque and of 38.71% (CI 95%: 23.73–56) if we take the IMT>p75, according to age and gender in the Spanish population. This means to say that although the SCOREM could be acceptably associated with the presence of subclinical atherosclerosis (AP and IMT>.9mm) in patients with RA, a considerable percentage of patients in the low risk group exist who are not detected by this tool. Up until now, studies on subclinical atherosclerosis focusing on the low risk group are scarce and our results are in keeping with them, which is principally that SCOREM as a tool presents with low level specificity. In the study by Corrales et al.25,45,46 performed on the Spanish population (Santander, Northern Spain), the presence of subclinical atherosclerosis was identified (IMT≥.9mm or presence of AP) through carotid ultrasound in up to 13%–33% of the population classed according to the SCOREM as low risk. Moreover, a Dutch study by Arts et al.,47 performed on a cohort of 1050 patients with RA over approximately 10 years recorded 149 CVE, of which 32% were presented by the low risk group. These data confirm the results of other studies which postulate the final low impact of SCOREM in intermediate and low risk group patients.21–26 The use of other tools or techniques with higher sensitivity are needed to enable us to provide a more accurate and realistic stratification. Due to this, as in the study by Gonzalez-Gay et al.,48 the use of carotid ultrasound is recommended in those patients with low/intermediate risk for their reclassification.
Disease Activity and Increase in Cardiovascular RiskIn our cohort the global level of disease activity through the SDAI was identified as a risk factor for the development of atheromatous plaque (OR 4.95; P=.008). In contrast, the variables proposed by EULAR for its incorporation in the SCORE (RF, ACCP, extra articular symptoms and duration >10 years) presented a tendency of association with the increase in risk of subclinical atherosclerosis, but it was not significant. These findings are similar to those described in the studies by Montes et al.49 and Arts et al.,50 in which neither the RF, ACCP, ESR, nor disease progression time were related to subclinical atherosclerosis or CVE. In contrast, the level of activity through DAS28 was significantly linked with the development of CVE (P=.002)50 and the presence of atheromatous plaques (P=.005).51 Furthermore, in the study by Myasoedova et al.,52 where the impact of the level of accumulated inflammatory activity in the development of CVE was evaluated in a series of 525 patients with RA vs 524 patients without RA and adjusted to age, gender and standard CVR factors, a similar incidence of CVE was observed between patients in remission compared with non RA patients (RR 0.90; CI 95%: .51–1.59). However, the patients with RA in intermediate activity and outbreaks accumulated during a year presented with an increase in CVR of 1.37 (CI 95%: 1.01–1.89) and of 2.42 (CI 95%: 1.14–5.14), respectively, compared with non-RA patients.
Finally, in the North American cohort of 24,989 patients with RA with a follow-up over 4 years, Solomon et al.53 observed that a high level of activity according to the CDAI involved a 60% risk increase of presenting with a CVE of those who were in remission (CI 95%: 23–80). This determinant influence of activity on the increase of CVR may be observed even at molecular and cellular level (pre-atherogenic), as was demonstrated in the Groot et al.54 study in which a direct and statistically significant relationship was identified between DAS28 and expression levels of the cellular adhesion molecules (VCAM-1 and Von Willebrand factor) and the end products of advanced glycation. This was also demonstrated by the Targónska-Stepniak et al.55 study which identified a direct relationship between serum amyloid A levels and the degree of activity through DAS28 (P<.0001) and the presence of atheromatous plaques through carotid ultrasound (P=.04).
The main limitation of our study was sample size (N=71). This led to a statistical power of 67.43%. However, he obtained relevant results with sufficient statistical significance.
ConclusionsAlthough there is an acceptable correlation between the presence of subclinical atherosclerosis using carotid ultrasound and the SCOREM stratification table proposed by EULAR, there is an underestimation of the risk in 14.63% of the population classified as low risk who present with atheromatous plaque. This leads to low sensitivity and specificity as a screening tool. As a result we recommend performing a carotid ultrasound in patients with RA who are not initially classified as high/very high risk according to the available stratification tables. This would enable us to re-stratify those patients initially considered to be low/intermediate risk and thus change our clinical, therapeutic and follow-up approach. Due to all of the above we believe it essential to create a specific tool for patients with RA which would enable the calculation of their real CVR, taking into account factors which are strongly related to CVR.
Since one of the risk factors most highly associated with the present of subclinical atherosclerosis was the level of inflammatory activity, the aim of our treat to target approach would have an impact on pain control, functional improvement and also reduce CVR in our patients.
Ethical DisclosuresProtection of people and animalsThe authors declare that no experiments using human beings or animals have been carried out for this research study.
Data confidentialityThe authors declare they have followed the protocols of their centre of work on patient data publication.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of InterestsThe authors have no conflict of interests to declare.
This study would not have been possible without the dedication and commitment of the authors and without the disinterested help of the rheumatology, radiodiagnostic and clinical analysis services of the University Hospital of Ciudad Real.
Please cite this article as: Ramírez Huaranga MA, Mínguez Sanchez MD, Zarca Diaz de la Espina MÁ, Espinosa Prados PJ, Romero Aguilera G. Qué papel juega la actividad de la enfermedad en el riesgo cardiovascular de la artritis reumatoide. Reumatol Clin. 2018;14:339–345.