Adult-onset Still's Disease (AOSD) is a systemic inflammatory disorder. There is no definitive AOSD activity indicator. Two of the currently used disease activity scores are the Modified Pouchot Activity Score (mPoS), and Systemic Feature Score (SFS). Another scoring system has been recently introduced, named the Still Activity Score (SAS).
AimsIn this single-center cross-sectional study, we aimed to compare the performance of the SAS with the mPoS and SFS, both of which have been used for a long time for measuring disease activity in patients with AOSD.
Method69 patients aged 18 or older were screened in the study who attended the Ankara University Faculty of Medicine between 2010 and 2020 with a diagnosis of AOSD. We compared SAS, SFS and mPoS with physician global assessment (PhGA) in patients with AOSD.
ResultsOf 69 patients screened, 45 patients with AOSD who fulfilled the Yamaguchi criteria were analyzed. The results showed no significant difference in SAS between patients with PhGA<6 and PhGA≥6, but mPoS and SFS scores were higher in the PhGA≥6 group (p=0.053, p=0.001, p=0.007, respectively). There was a significant correlation between mPoS and PhGA (p=0.018).
ConclusionThis is the first study to evaluate the SAS score, which is used for patients with AOSD. SAS is user-friendly but may not be as sensitive as mPoS and SFS for assessing disease activity in AOSD.
La enfermedad de Still de inicio en la adultez (AOSD) es un trastorno inflamatorio sistémico. No existe un indicador definitivo de actividad para la AOSD. Dos de las puntuaciones de actividad de la enfermedad que se utilizan actualmente son la puntuación de actividad modificada de Pouchot (mPoS) y la puntuación de características sistémicas (SFS). Recientemente, se ha introducido otro sistema de puntuación denominado puntuación de actividad de Still (SAS).
ObjetivosEn este estudio transversal de un solo centro, nuestro objetivo fue comparar el rendimiento de la SAS con la mPos y la SFS, ambos utilizados durante mucho tiempo para medir la actividad de la enfermedad en los pacientes con AOSD.
MétodoSe examinaron 69 pacientes de 18 años o más en el estudio que acudieron a la Facultad de Medicina de la Universidad de Ankara entre 2010 y 2020 con un diagnóstico de la AOSD. Comparamos la puntuación de actividad de Still, la mPoS y la SFS con la evaluación global del médico (PhG) en los pacientes con AOSD.
ResultadosDe los 69 pacientes evaluados, se analizaron 45 pacientes con AOSD que cumplían los criterios de Yamaguchi. Los resultados no mostraron una diferencia significativa en la SAS entre los pacientes con PhGA<6 y aquellos con PhGA≥6, pero las puntuaciones de mPos y SFS fueron más altas en el grupo con PhGA≥6 (p=0,053; p=0,001 y p=0,007, respectivamente). Hubo una correlación significativa entre la mPos y la PhGA (p=0,018).
ConclusiónEste es el primer estudio que evalúa la puntuación SAS, que es fácil de usar en los pacientes con AOSD. La SAS es fácil de usar, pero puede no ser tan sensible como la mPos y la SFS en la evaluación de la actividad de la AOSD.
Adult-onset Still's Disease (AOSD) is a systemic inflammatory disorder characterized by fever, arthralgia, a salmon-colored rash, sore throat, lymphadenopathy, hepatosplenomegaly, leukocytosis, elevated acute phase reactants and hyperferritinemia.1,2 Although AOSD has bimodal age peaks, it is mostly prevalent between 16 and 35 years of age.2,3 Macrophage activation syndrome, fulminant hepatitis and interstitial pulmonary fibrosis are potentially fatal complications of AOSD.2
Accurately assessing the severity of the disease is challenging due to the unpredictable and rapidly changing symptoms of AOSD. The accurate and reliable assessment of disease activity is crucial for the effective management of AOSD.4 Two of the currently used disease activity scores are the Modified Pouchot-Activity Score (mPoS)4 and the Systemic Feature Score (SFS).5 mPoS evaluates disease activity using 12 clinical and/or laboratory parameters. Rau et al. showed that the mPoS score could distinguish high disease activity in AOSD from sepsis and low disease activity AOSD.6 Numerous studies used mPOS as one of the primary metrics to assess activity level in patients with AOSD.7,8 SFS consists of 5 clinical and 5 laboratory parameters, and each is given a score of 1 (present) or 0 (not present). There is limited data regarding disease activity assessment using SFS. In 2022, Kalyoncu et al. introduced a new and practical score named the Still Activity Score (SAS) to assess disease activity in AOSD patients.9 SAS includes only four parameters and is extremely simple to implement in daily practice.
AimIn this single-center cross-sectional study, we aimed to compare and evaluate the performance of the SAS score with mPoS and SFS, both of which have been used for a long time in measuring disease activity for patients with AOSD.
Methods69 patients aged 18 or older were screened in the study who attended the Ankara University Faculty of Medicine between 2010 and 2020 with a diagnosis of AOSD. Of 69 patients, 45 patients who met the Yamaguchi criteria were included in the study. The study excluded patients with active infection, non-AOSD rheumatic diseases, and a prior history of cancer. The medical records of patients were reviewed for demographic characteristics, clinical features including fever, arthritis, arthralgia, number of joints involved, myalgia, rash, sore throat, lymphadenomegaly, hepatosplenomegaly, pleural and pericardial effusions, uveitis, encephalitis, epilepsy, aseptic meningitis and pulmonary fibrosis. Laboratory data of the patients, such as leukocytes, neutrophils, hemoglobin, platelets, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin, liver function tests, triglycerides, antinuclear antibody (ANA), rheumatoid factor (RF), and anti-cyclic citrullinated peptide (CCP), were also recorded. The initial and maintenance treatments of all patients, response to medication and relapse were all noted.
The physician's global assessment (PhGA) was recorded at the patient's initial visit to our clinic. Due to the lack of a gold-standard method for evaluating disease activity, PhGA was used as the primary criterion. PhGA scores range from 0 to 10. Those with a PhGA score ≥6 are categorized as having high disease activity.10 Patients were divided into 2 groups according to PhGA and were evaluated according to three disease activity scores (mPoS, SFS and SAS).
The mPoS comprises one point for each of 12 typical disease parameters: fever, evanescent rash, sore throat, arthritis, myalgia, pleuritis, pericarditis, pneumonitis, lymphadenopathy, hepatomegaly or abnormal liver function tests, leukocyte count>15,000/μl, and serum ferritin>3000μg/l. If the mPoS score is 4 and above, it is accepted as high disease activity; if lower, it is considered low disease activity.5,11 The mPoS was chosen for comparison with the SAS due to its superior ability to differentiate between disease activity and sepsis.6 SFS is a scoring system used to assess disease activity in systemic-onset juvenile idiopathic arthritis and AOSD by evaluating five clinical (fever, rash, enlargement of lymph nodes, enlargement of liver or spleen size, and serositis) and five laboratory parameters (ESR≥20mm/h, CRP≥10mg/l, white blood cell count≥12×109/l, hemoglobin≤11g/dl, platelet count≥400×109/l). SAS is a scoring system used to assess disease activity in AOSD by evaluating four clinical (fever, arthralgia) and laboratory (ferritin, neutrophil count) parameters.9 Those with an SAS score ≥4 are considered to have high disease activity, and those with a SAS score <4 are considered to have low disease activity.9
This study was conducted in accordance with the Declaration of Helsinki Ethical Principles and was approved by the ethics committee of Ankara University Faculty of Medicine (İ01-49-23).
Statistical analysisCategorical data are presented using frequencies and percentages. Quantitative data are presented as median and interquartile range (IQR). In the comparison of categorical data for high and low disease activity groups, the Chi-square test or Fisher's exact test was used based on suitability. Intergroup differences were assessed using the Mann–Whitney U test. Spearman's correlation test was used for analysis of the relationships between CRP, ferritin, mPOS, SAS and SFS scores. A value of p<0.05 was considered statistically significant. The data were analyzed using SPSS version 21 software (SPSS, Chicago, USA).
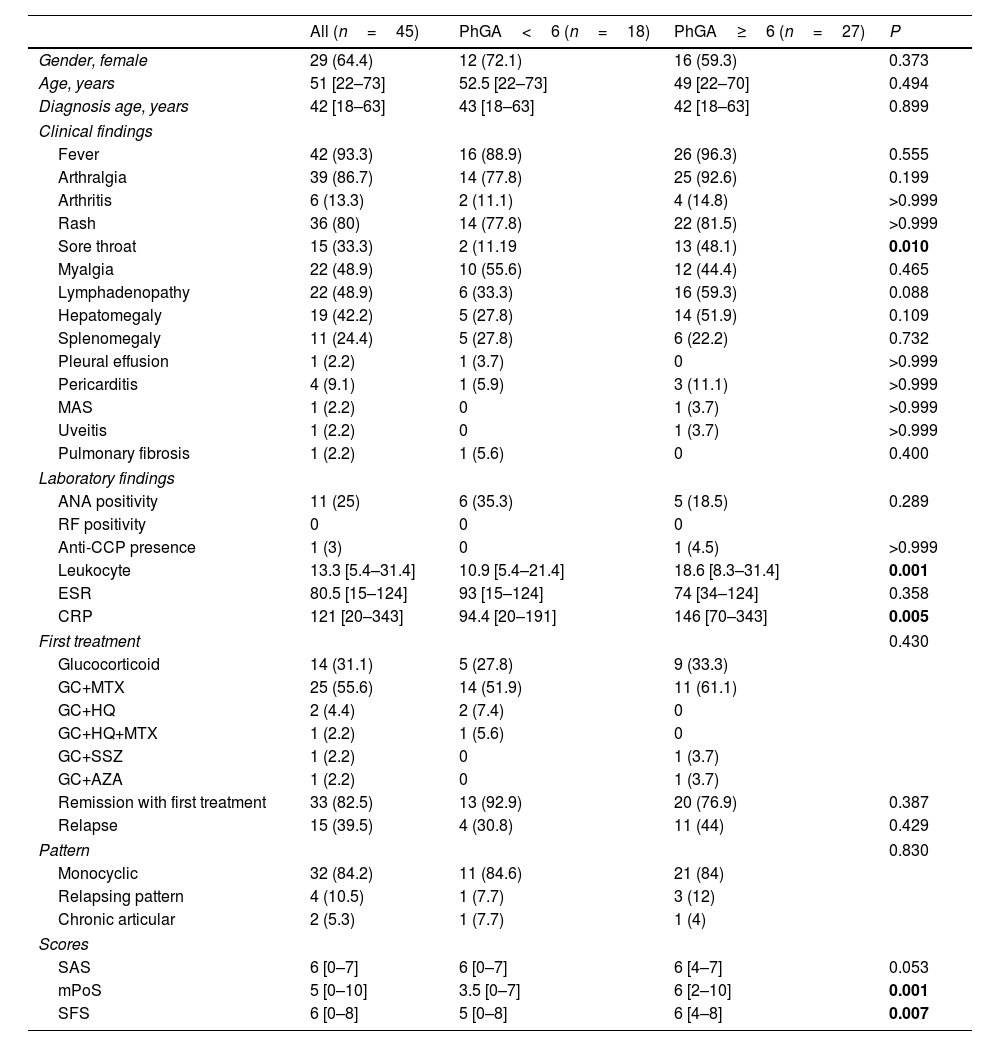
ResultsThe study included 45 patients with AOSD who fulfilled the Yamaguchi criteria. The median age of the patients at diagnosis was 42 years (range: 22–73), and 29 (64.4%) of the patients were female (Table 1). Fever (93.3%) was the most common sign, followed by arthralgia (86.7%) and a salmon-colored rash (80%) (Table 1). ANA positivity was present in 25% of patients with AOSD. No patient tested positive for RF (Table 1).
Characteristics of AOSD patients.
| All (n=45) | PhGA<6 (n=18) | PhGA≥6 (n=27) | P | |
|---|---|---|---|---|
| Gender, female | 29 (64.4) | 12 (72.1) | 16 (59.3) | 0.373 |
| Age, years | 51 [22–73] | 52.5 [22–73] | 49 [22–70] | 0.494 |
| Diagnosis age, years | 42 [18–63] | 43 [18–63] | 42 [18–63] | 0.899 |
| Clinical findings | ||||
| Fever | 42 (93.3) | 16 (88.9) | 26 (96.3) | 0.555 |
| Arthralgia | 39 (86.7) | 14 (77.8) | 25 (92.6) | 0.199 |
| Arthritis | 6 (13.3) | 2 (11.1) | 4 (14.8) | >0.999 |
| Rash | 36 (80) | 14 (77.8) | 22 (81.5) | >0.999 |
| Sore throat | 15 (33.3) | 2 (11.19 | 13 (48.1) | 0.010 |
| Myalgia | 22 (48.9) | 10 (55.6) | 12 (44.4) | 0.465 |
| Lymphadenopathy | 22 (48.9) | 6 (33.3) | 16 (59.3) | 0.088 |
| Hepatomegaly | 19 (42.2) | 5 (27.8) | 14 (51.9) | 0.109 |
| Splenomegaly | 11 (24.4) | 5 (27.8) | 6 (22.2) | 0.732 |
| Pleural effusion | 1 (2.2) | 1 (3.7) | 0 | >0.999 |
| Pericarditis | 4 (9.1) | 1 (5.9) | 3 (11.1) | >0.999 |
| MAS | 1 (2.2) | 0 | 1 (3.7) | >0.999 |
| Uveitis | 1 (2.2) | 0 | 1 (3.7) | >0.999 |
| Pulmonary fibrosis | 1 (2.2) | 1 (5.6) | 0 | 0.400 |
| Laboratory findings | ||||
| ANA positivity | 11 (25) | 6 (35.3) | 5 (18.5) | 0.289 |
| RF positivity | 0 | 0 | 0 | |
| Anti-CCP presence | 1 (3) | 0 | 1 (4.5) | >0.999 |
| Leukocyte | 13.3 [5.4–31.4] | 10.9 [5.4–21.4] | 18.6 [8.3–31.4] | 0.001 |
| ESR | 80.5 [15–124] | 93 [15–124] | 74 [34–124] | 0.358 |
| CRP | 121 [20–343] | 94.4 [20–191] | 146 [70–343] | 0.005 |
| First treatment | 0.430 | |||
| Glucocorticoid | 14 (31.1) | 5 (27.8) | 9 (33.3) | |
| GC+MTX | 25 (55.6) | 14 (51.9) | 11 (61.1) | |
| GC+HQ | 2 (4.4) | 2 (7.4) | 0 | |
| GC+HQ+MTX | 1 (2.2) | 1 (5.6) | 0 | |
| GC+SSZ | 1 (2.2) | 0 | 1 (3.7) | |
| GC+AZA | 1 (2.2) | 0 | 1 (3.7) | |
| Remission with first treatment | 33 (82.5) | 13 (92.9) | 20 (76.9) | 0.387 |
| Relapse | 15 (39.5) | 4 (30.8) | 11 (44) | 0.429 |
| Pattern | 0.830 | |||
| Monocyclic | 32 (84.2) | 11 (84.6) | 21 (84) | |
| Relapsing pattern | 4 (10.5) | 1 (7.7) | 3 (12) | |
| Chronic articular | 2 (5.3) | 1 (7.7) | 1 (4) | |
| Scores | ||||
| SAS | 6 [0–7] | 6 [0–7] | 6 [4–7] | 0.053 |
| mPoS | 5 [0–10] | 3.5 [0–7] | 6 [2–10] | 0.001 |
| SFS | 6 [0–8] | 5 [0–8] | 6 [4–8] | 0.007 |
Abb: PhGA: physician global assessment, MAS: macrophage activation syndrome, ANA: antinuclear antibody, RF: rheumatoid factor, Anti-CCP: anti-cyclic citrulline protein, ESR: erythrocyte sedimentation rate, CRP: C-REACTIVE PROtein, GC: glucocorticoid, MTX: methotrexate, AZA: azathioprine, SSZ: sulfasalazine, SAS: Still activity score, mPoS: Modified Pouchot Activity Score, SFS: Systemic Feature Score.
According to the PhGA score, 18 patients were classified as having low disease activity, and 27 were classified as having high disease activity. The leukocyte count and CRP levels of the two groups were significantly different (respectively p=0.001, and p=0.005).
We compared SAS, SFS and mPoS with PhGA for patients with AOSD. The results showed no significant differences in the SAS scores between patients with PhGA<6 and PhGA≥6, but the mPoS and the SFS scores were higher in patients with PhGA≥6 (p=0.053, p=0.001, p=0.007 respectively) (Table 1).
Patients with an mPoS score of 4 or above were considered to have high disease activity. According to mPoS, 32 patients had high disease activity when we divided the patients into those with high and those with low disease activity. There was an association between the mPoS and PhGA (p=0.018).
When SAS score of 4 or higher is used to define high disease activity, 43 patients were labeled as having high disease activity. There was no significant correlation between the SAS score and the PhGA score in determining disease activity (p=0.155).
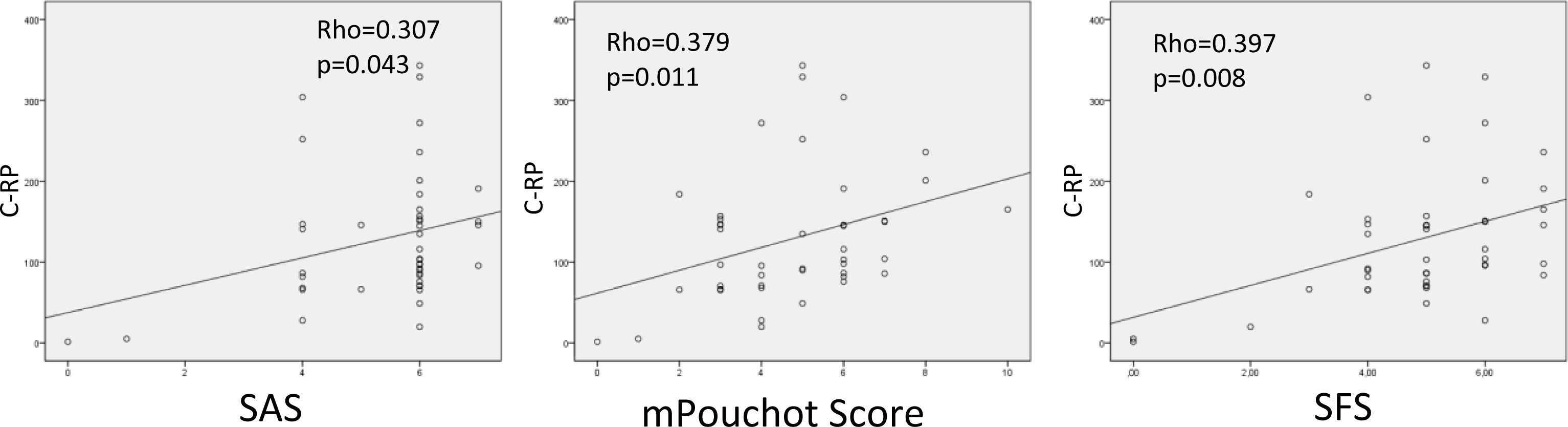
CRP levels were correlated with SAS, mPoS and SFS scores (p=0.043-Rho=0.307, p=0.011-Rho=0.379, and p=0.008-Rho=0.397, respectively) (Fig. 1).
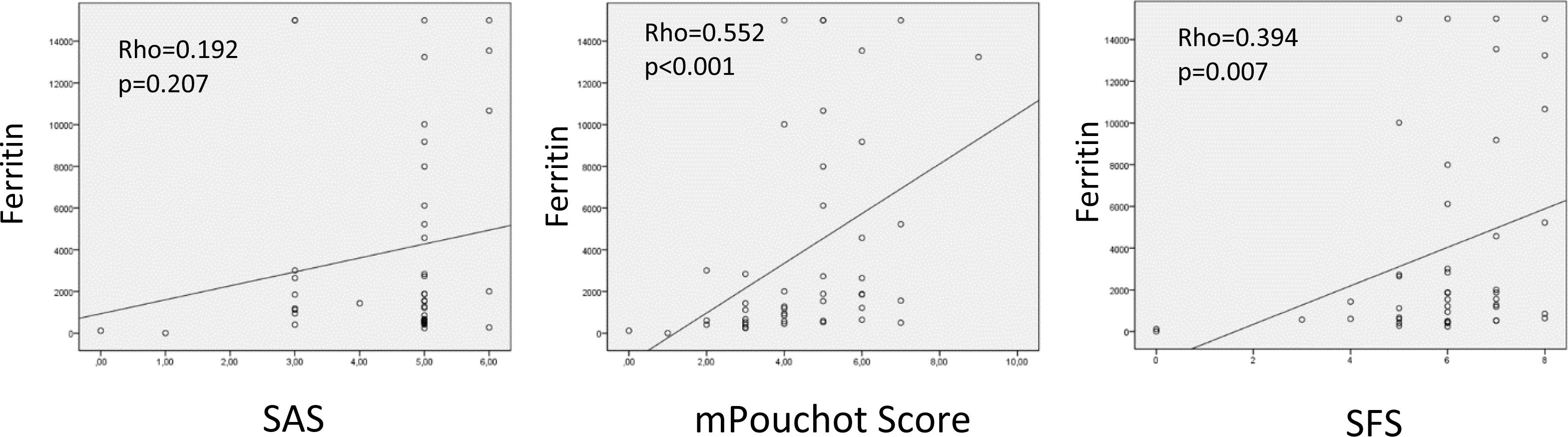
Ferritin levels were correlated with mPOS and SFS scores (p<0.001-Rho=0.552, p=0.001-Rho=0.394, respectively) (Fig. 2).
DiscussionWhen we divided the patients into high and low disease activity groups based on PhGA, while the mPoS and SFS scores were higher in the high disease activity group, there was no significant difference in SAS scores between the groups. The SAS score could not distinguish patients with high disease activity and low disease activity in the same way as mPoS and SFS did. When identifying patients with high and low disease activity based on mPoS, this distinction was correlated with PhGA (p=0.018). However, when we divided patients into groups with low and high disease activity according to SAS score, this was not evident with PhGA (p=0.155).
AOSD is an inflammatory disease with different clinical and laboratory signs, no definitive diagnostic marker, and no gold standard test to demonstrate disease activity. SAS is a newly developed, and easy-to-use method for evaluating disease activity in AOSD.9 Our study is the first study to examine how well SAS can measure the activity of disease in a real life setting. The results of the present study indicate that the newly developed SAS scoring to evaluate disease activity in AOSD may not be as effective as other measures such as mPoS and SFS in identifying patients with high disease activity according to PhGA. This finding is particularly relevant since AOSD is a disease with no definitive diagnostic markers and no gold standard test to show disease activity. However, it should be borne in mind that the small number of patients included in this study may have contributed to the lack of statistical significance observed in some of the comparisons. However, in line with previous studies, mPoS and SFS were comparable with PhGA in our study for indicating disease activity.2,10,12,13
The findings of our study suggest that fever, arthralgia, and a salmon-colored rash are the most common signs in AOSD patients, which are consistent with previous studies.14 Additionally, elevated CRP levels are related to disease activity15–17 and, in line with the literature, we found that CRP levels were significantly associated with disease activity assessed by SAS, mPoS, and SFS scores. CRP levels were significantly correlated with mPOS and SFS scores, which is consistent with previous reports.2 In our study, the relationship between SAS score and CRP was significant in terms of SAS score showing disease activity.
In this study, ferritin levels were significantly associated with mPoS and SFS scores. In previous studies, high ferritin levels were found to be associated with disease activity.17 Benedotti et al. demonstrated that hyperferritinemia can predict high disease activity.18 A lower cut-off for ferritin level in the SAS score compared to other scoring systems may have contributed to this result.
The limitations of our study include the following, there is no gold standard test to show disease activity, PhGA is subjective as it is based on the evaluation of the treating physician, small sample size and the collection of data was retrospective.
In conclusion, our study is the first study to evaluate SAS score, which is user-friendly, for patients with AOSD. The study suggests that even though the SAS is easy to use, it may not be as sensitive as mPoS and SFS in assessing disease activity in patients with AOSD. Future studies with larger sample sizes are needed to assess the performance of SAS for AOSD.
Authors’ approvalAll the authors have read and agreed with the content and interpretation of the submitted article.
Authors’ contributionsAccording to the International Committee of Medical Journals Editors (ICMJE), the duties of each editor
Emine USLU-The conception and design of the study, interpretation of data, drafting the article, final approval of the version to be submitted.
Mücteba Enes YAYLA-Analysis and interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Didem ŞAHİN EROĞLU-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Büşra ATMACA HAKTANIYAN-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Nilgün Göveç GIYNAŞ-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Recep YILMAZ-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Ahmet İLBAY-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Yeter MAHMUTOĞLU-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Ahmet USTA-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Abdulbaki GAYDAN-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Tahsin Murat TURGAY-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Gülay KINIKLI-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Aşkın ATEŞ-Interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
FundingNo financial support was received from any institution in our study.
Conflict of interestThe authors declare that they have no conflict of interest.