Anti-neutrophil cytoplasmic autoantibodies (ANCA) associated vasculitis (AAV) is a small vessel vasculitis with insufficient epidemiological estimates in India. We aimed to determine demographic, clinical features, and laboratory diagnosis of AAV patients presenting to a large tertiary care centre in India.
Material and methods1289 patient samples were screened for ANCA by indirect immunofluorescence test (IIFT) and confirmation of ANCA target antigens was done by line immunoassay. Association between IIFT and LIA was determined in AAV.
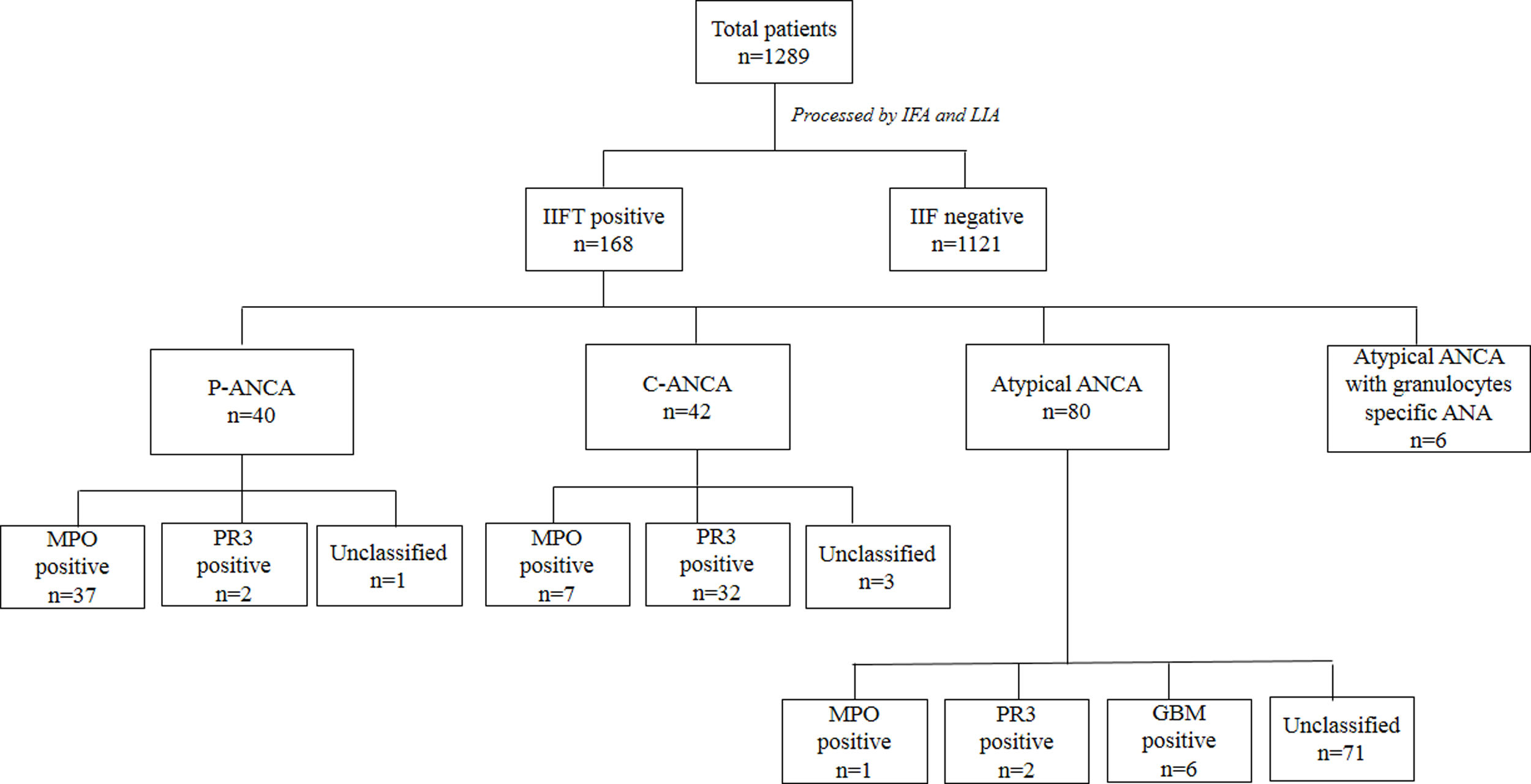
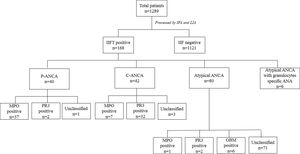
ResultsBy IIFT, ANCA was detected in 13.0% (168 out of 1289), of which 23.8% (40/168) were positive with P-ANCA pattern, 25.0% (42/168) were positive with C-ANCA and 47.6% (80/168) showed an atypical pattern. On evaluation with a line immunoassay, 6.7% (86/1289) were positive out of which 52.3% (45/86), 41.9% (36/86), 8.8% (6/86) were positive for anti-MPO, anti-PR3, and anti-GBM respectively. In eosinophilic granulomatosis with polyangiitis (EGPA) 87.5% (7/8), and microscopic polyangiitis (MPA/RLV) 91.3% (21/23), anti-MPO was the predominantly observed antibody. In granulomatosis with polyangiitis (GPA) anti-PR3 antibody was predominant in 87.5% (28/32) cases. Out of 168 IIF positive samples 8, 32, and 23 cases of EGPA, GPA, and MPA/RLV were observed respectively.
ConclusionsThe primary aim of the study was to provide single-centre data to determine the laboratory diagnosis of AAV. A combination of IIFT and LIA was found to be an optimum testing strategy for the laboratory diagnosis of AAV.
La vasculitis asociada a anticuerpos anticitoplasma de neutrófilos –ANCA– (VAA) es una vasculitis de pequeños vasos con cálculos epidemiológicos insuficientes en India. Nuestro objetivo fue determinar las características demográficas y clínicas, y los diagnósticos de laboratorio de los pacientes con VAA que se presentaron en un gran centro de cuidados terciarios en India.
Material y métodosSe realizó un cribado de ANCA en 1.289 pacientes mediante test de inmunofluorescencia directa (IIFT), realizándose la confirmación de los antígenos diana de ANCA mediante inmunoensayo lineal. La asociación entre IIFT y LIA fue determinada en VAA.
ResultadosMediante IIFT, se detectó ANCA en el 13% de los pacientes (168 de 1.289), de los cuales, el 23,8% (40/168) fue positivo con el patrón P-ANCA, el 25% (42/168) fue positivo con C-ANCA y el 47,6% (80/168) reflejó un patrón atípico. En la evaluación con inmunoensayo lineal, el 6,7% (86/1.289) fue positivo, de los cuales, el 52,3% (45/86), 41,9% (36/86) y 8,8% (6/86) fueron positivos para anti-MPO, anti-PR3 y anti-GBM, respectivamente. En la granulomatosis eosinofílica con poliangitis (EGPA), en el 87,5% (7/8) de los casos, y en poliangitis microscópica (MPA/RLV) en el 91,3% (21/23), anti-MPO fue el anticuerpo predominantemente observado. En granulomatosis con poliangitis (GPA), el anticuerpo anti-PR3 fue predominante en el 87,5% (28/32) de los casos. De entre 168 muestras positivas de IIF, se observaron 8, 32, y 23 casos de EGPA, GPA y MPA/RLV, respectivamente.
ConclusionesEl objetivo primario del estudio fue aportar los datos de un único centro para determinar el diagnóstico de laboratorio de VAA. El resultado fue que la combinación de IIFT y LIA es una estrategia óptima para el diagnóstico de laboratorio de VAA.
Anti-neutrophil cytoplasmic antibodies (ANCA), mainly directed towards proteinase 3 (PR3) and myeloperoxidase (MPO), are associated with primary systemic small-vessel vasculitis known as ANCA-associated vasculitis (AAV), a term which includes microscopic polyangiitis (MPA)/renal limited vasculitis (RLV), granulomatosis with polyangiitis (GPA or Wegener's granulomatosis), and eosinophilic granulomatosis with polyangiitis (EGPA or Churg Strauss syndrome).1,2 AAV with multi-organ secondary inflammation and necrosis within small blood vessels causes an increase in morbidity and mortality. Screening for the presence of ANCAs is a commonly used diagnostic assay for AAV. A variety of diagnostic assays with different sensitivity and specificity are being used for the screening and confirmation of the specific ANCA antigens viz. MPO and PR3. The assays include but not limited to Immunofluorescence test (IIFT), Line immunoassay (LIA), Enzyme-linked immunosorbent assay (ELISA), and its variants, Immunoblots, and solid-phase bead-based assays.
As per recommendations of 1999 International Consensus Statement on Testing and Reporting ANCA,3 the combination of both techniques (IIFT and antigen-specific test like LIA/ELISA were used for the detection of ANCA in patients under investigation for ANCA-associated vasculitis. We aimed to determine demographic, clinical features, and laboratory diagnosis of AAV patients presenting to a large tertiary care center in India.
Material and methodsStudy population and specimenThis retrospective study includes 1289 patients who were tested for ANCA serology at Molecular Genetics laboratory in a large tertiary care center in the National Capital Region (NCR), India during 2011–2016. The study protocol conformed to the provisions of the 1975 Declaration of Helsinki (as revised in Seoul, Korea, October 2008). Samples were mainly received from patients undergoing secondary or tertiary referral to specialists, i.e., nephrologists, pulmonologists, rheumatologists, gastroenterologists, gynecologists, and general physicians. Whole blood was collected from the patients by venipuncture into plain vacuumed tubes, and separated serum was stored at −80°C until testing. However, to prevent repeated freezing and thawing, serum aliquots were thawed only once and immediately processed after thawing to prevent discrepancies in results. Based on standardized assays used in our laboratory, screening of ANCA was performed by IIFT and specific antigens [MPO, PR3, and (glomerular basement membrane) GBM] were identified using LIA.
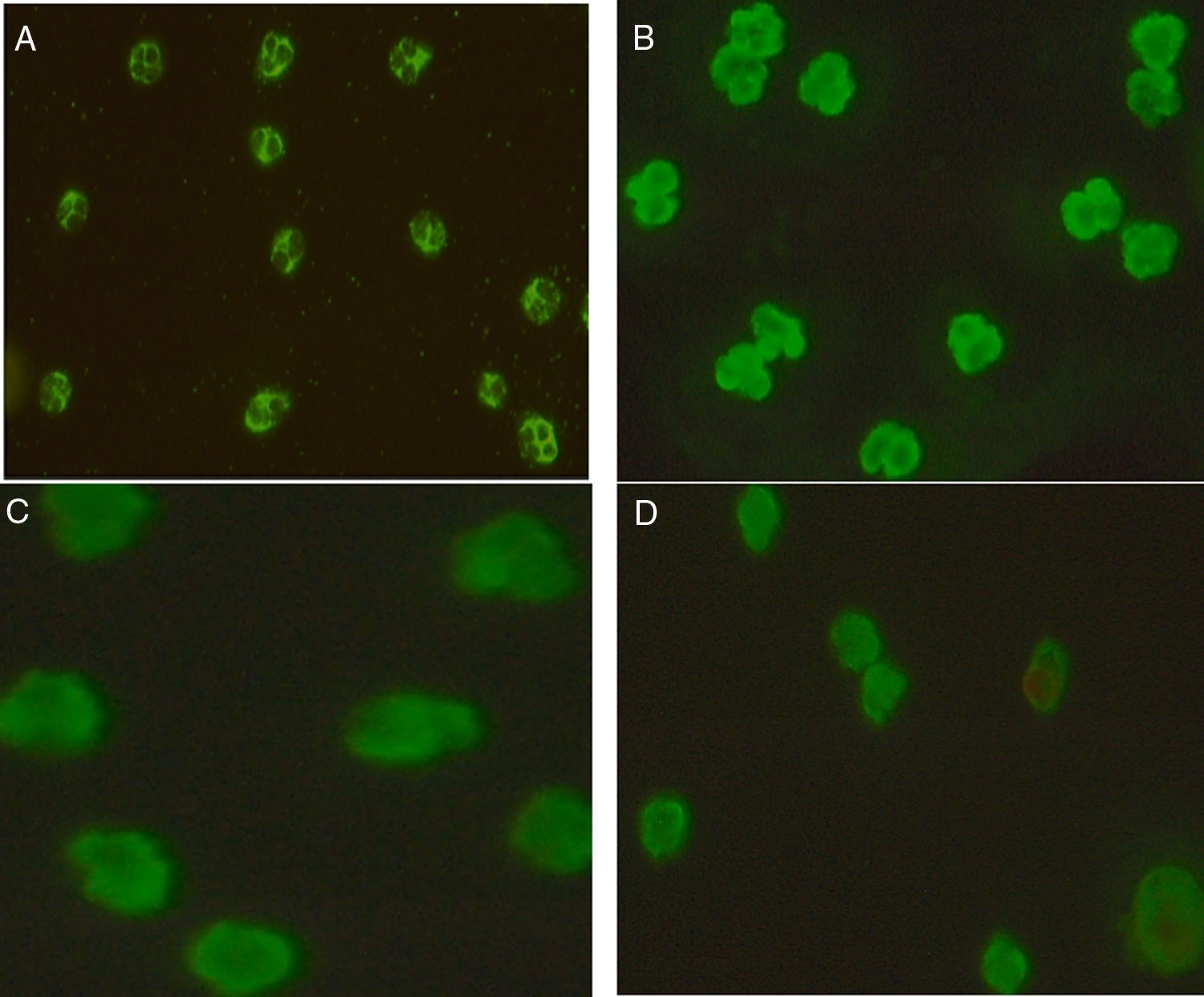
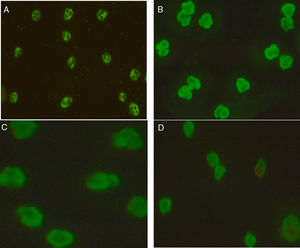
Technology, technique, and equipmentIndirect Immunofluorescence test (IIFT): Screening of ANCAs was performed using ethanol fixed neutrophils from Immunoconcepts (Cat no: 10070-11). Serum diluted (1:20) in phosphate-buffered saline was incubated with ethanol fixed neutrophils for 30min at room temperature. Slides were washed twice for five minutes with PBS, incubated for an additional 30min with fluorescent-labeled conjugated antihuman IgG. Subsequently, a cover-slip was placed over the slide and analyzed using a fluorescence microscope at 40× magnification. The fluorescence of each sample was compared with the negative control and the pattern of fluorescence was determined and recorded. While reporting, the results were interpreted as (a) C-ANCA positive: diffuse granular cytoplasmic staining with interlobular accentuation (b) P-ANCA positive: peri-nuclear fluorescence with the nuclear extension (c) Atypical ANCA: peri-nuclear staining without nuclear extension, or diffuse flat cytoplasmic staining, or the combination of both cytoplasmic and nuclear/peri-nuclear staining on neutrophils (d) Atypical ANCA with granulocytes specific ANA: showing atypical staining with ANA interference (e) ANCA negative: shows no fluorescence.3–5
Line Immunoassay (LIA): Imtech ANCA LIA MAXX (Cat No: ITC92005) was used for the qualitative detection of three antigen panels (PR3, MPO, and GBM) according to manufacturer's instructions. Three antigens, cut-off control, and positive control are applied as lines on a nitrocellulose membrane. The nitrocellulose membrane was blocked by adding a blocking buffer for 5min to prevent non-specific reactions. Incubation of a strip with diluted patient samples was done for 30min to allow binding of the antigens on the strip with autoantibodies present in the sample. For the detection of the bound antibodies, an alkaline phosphatase labeled anti-human IgG antibody was added to strips for 30min. After the addition of the substrate solution for 30min, the appearance of brown lines indicated the existence of (auto) antibodies against the respective antigens. The intensity of brown lines was measured using Huma-Scan software. The test result is negative, if no band is to be recognized or if the band exhibits a smaller intensity in comparison to the cut-off control. The test is equivocal if the intensity of the band and the intensity of the cut-off control do not significantly differ. The test result is positive if a band exhibits stronger staining in comparison to the cut-off control.
Statistical analysisTo summarize the data, counts and percentages were used for categorical variables. Percentages were calculated using Microsoft Office Standard 2013.
Ethical approvalPatient consent was obtained for diagnosis and treatment at the hospital. This was an observational study; no personal identifiers like name, addresses were used and no additional sample was drawn for this study. All investigation, treatment, and monitoring were according to the current ‘standard-of-care’.
ResultsThe incidence of serum ANCA and specific autoantibodiesUpon IIF investigation, ANCA was detected in 13.0% (168 out of 1289) at 1:20 dilution, of which 23.8% (40/168), 25.0% (42/168, and 47.6% (80/168) were positive with P-ANCA, C-ANCA and atypical pattern respectively. Atypical ANCA with granulocytes specific ANA was observed in 3.6% (6/168) cases (Fig. 1). On evaluation with a line immunoassay, 6.7% (86/1289) were positive out of which 52.3% (45/86), 41.9% (36/86), 8.8% (6/86) were positive for anti-MPO, anti-PR3, and anti-GBM respectively.
Association of ANCA patterns with specific autoantibodiesThe association of a specific autoantibody with specific immunofluorescence (IIF) pattern was observed. Out of 40 P-ANCA positive cases, 92.5% (37/40) were positive for anti-MPO and 5.0% (2/40) were positive for anti-PR3. Among 42 C-ANCA cases, 76.2% (32/42) and 16.7% (7/42) were positive for anti-PR3 and anti-MPO respectively (Fig. 1). Images of various ANCA IIF patterns are shown in Fig. 2.
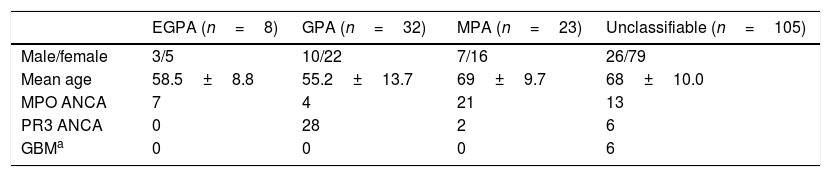
Incidentally, while studying the association of IIF patterns and specific autoantibodies, we found the distribution of anti-MPO and anti-PR3 in AAV (shown in Table 1). Anti-MPO antibody was predominant in 87.5% (7/8) of EGPA and 91.3% (21/23) cases of MPA/RLV. The anti-PR3 antibody was predominant in 87.5% (28/32) cases of GPA.
Comparison of demographics among AAV diseases in IIFT positive patients (n=168).
| EGPA (n=8) | GPA (n=32) | MPA (n=23) | Unclassifiable (n=105) | |
|---|---|---|---|---|
| Male/female | 3/5 | 10/22 | 7/16 | 26/79 |
| Mean age | 58.5±8.8 | 55.2±13.7 | 69±9.7 | 68±10.0 |
| MPO ANCA | 7 | 4 | 21 | 13 |
| PR3 ANCA | 0 | 28 | 2 | 6 |
| GBMa | 0 | 0 | 0 | 6 |
EGPA, eosinophillic granulomatosis with polyangitis; GPA, granulomatosis with polyangitis; MPA, microscopic polyangitis; MPO, myeloperoxidase; PR3, proteinase-3.
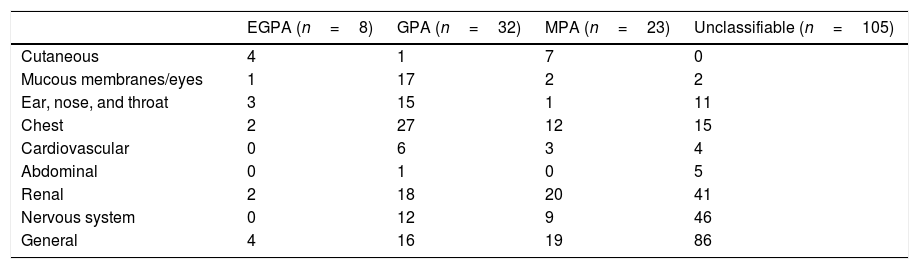
Out of 168 IIF positive samples 8, 32, and 23 cases were observed as EGPA, GPA, and MPA/RLV respectively. Six cases of rapidly progressive glomerulonephritis (RPGN) were identified as positive for GBM. 105 cases remained unclassifiable. A comparison of demographics among AAV diseases were shown in Table 1. Kidney and chest involvement was observed in most of the diseases. Table 2 indicates the involvement of different organs in AAV diseases.
Comparison of organ involvement among AAV diseases in ANCA IIFT positive patients (n=168).
| EGPA (n=8) | GPA (n=32) | MPA (n=23) | Unclassifiable (n=105) | |
|---|---|---|---|---|
| Cutaneous | 4 | 1 | 7 | 0 |
| Mucous membranes/eyes | 1 | 17 | 2 | 2 |
| Ear, nose, and throat | 3 | 15 | 1 | 11 |
| Chest | 2 | 27 | 12 | 15 |
| Cardiovascular | 0 | 6 | 3 | 4 |
| Abdominal | 0 | 1 | 0 | 5 |
| Renal | 2 | 18 | 20 | 41 |
| Nervous system | 0 | 12 | 9 | 46 |
| General | 4 | 16 | 19 | 86 |
ANCAs are important biomarkers to diagnose GPA, MPA/RLV, EGPA, and their limited forms such as pauci immune glomerulonephritis and pulmonary capillaritis and can also be useful in monitoring disease activity. ANCA screening is performed by IIFT on neutrophils to detect predominantly central (C-ANCA) or cytoplasmic (P-ANCA) staining. Antibodies against MPO and PR3 antigens expressed on the surface of neutrophils and monocytes are recognized as the main ANCA targets.6,7 The antigen specific testing for anti-MPO and anti-PR3, in addition to ANCA IIFT, increases the sensitivity and specificity for the diagnosis of AAV.8
In the present study, the PR3 antigen was positive with C-ANCA in 88% of patients with GPA. While P-ANCA and MPO antibodies were positive in 91% patients with MPA/RLV which were compatible with results reported earlier in the literature showing PR3-C-ANCA pairing, is seen in 90% patients with GPA,9,10 while P-ANCA and MPO antibodies result positive in 60–80% of patients with MPA/RLV.11 GBM positivity was observed in six patients with RPGN.
The other category of atypical ANCA is uncommon, found in 80 cases in the present study, and usually caused by a combination of both cytoplasmic and nuclear/peri-nuclear fluorescence. These sera have multiple antigenic specificities including PR3, MPO, bactericidal permeability-increasing protein, cathepsin G, elastase, lactoferrin, and lysozyme.3,12 Atypical ANCA occurs in inflammatory bowel disease, some autoimmune forms of arthritis, and drug-induced vasculitides. Out of 80 cases of atypical ANCA pattern, only one was positive for anti-MPO and 2 were positive for the anti-PR3 antibody. However, these samples were not tested for antigen specificity (other than MPO, PR3, and GBM) in the present study.
AAVs are small-vessel vasculitides with a broad spectrum of manifestations since almost all organs can be affected. Miscellaneous organ manifestations including the involvement of the cardiovascular and musculoskeletal system, gastrointestinal and urogenital tract, occur in only a minority of patients but may have a profound impact on morbidity and mortality.13 Here, in this study, the organ involvement was observed in 168 ANCA IIF positive cases in which kidney and chest were the major organs involved along with general manifestations.
The primary aim of the study is to provide a single-center report to determine the laboratory diagnosis of AAV. A combination of IIFT and LIA was found to be an optimum testing strategy for the laboratory diagnosis of AAV.
Limitation of the study: This study followed the recommendations of the 1999 International Consensus Statement. However, The international guidelines were updated in 201714 which mentions that antigen-specific tests like LIA and ELISA15 should be used as a first-line test for diagnosis as monitoring is an additional advantage of using antigen-specific assays.
Funding sourceNone.
Conflict of interestAll the authors of the manuscript hereby declare that we have no conflict of interest.
We would like to thank the team members of Molecular and Transplant Immunology laboratory of Medanta-The Medicity and Amity University for their support.