Lupus anticoagulant-hypoprothrombinaemia syndrome (LAHPS) is a rare disorder caused by the presence of lupus anticoagulant (LA) and acquired prothrombin deficiency, which may present with severe haemorrhagic manifestations. LAHPS is usually associated with systemic lupus erythematosus (SLE), or infections and it is more frequent in the paediatric population and female gender. We describe a 42-year-old man with thrombotic antiphospholipid syndrome (APS) on chronic anticoagulation treatment with acenocoumarol who presented with spontaneous intracranial bleeding, prolongation of prothrombin time (PT), activated partial thromboplastin time (APTT) and low factor II levels (after optimal anticoagulation reversal) as a debut of SLE.
El síndrome de anticoagulante lúpico-hipoprotrombinemia (LAHPS, por sus siglas en inglés) es un trastorno raro, causado por la presencia de anticoagulante lúpico (AL) y deficiencia adquirida de protrombina, que puede cursar con manifestaciones hemorrágicas graves. El LAHPS suele asociarse a lupus eritematoso sistémico (LES) o infecciones, y es más frecuente en población pediátrica y en el género femenino. Describimos a un varón de 42 años con síndrome antifosfolípido (SAF) trombótico en tratamiento anticoagulante crónico con acenocumarol que presentó sangrado intracraneal espontáneo, prolongación tanto del tiempo de protrombina (TP) como del tiempo de tromboplastina parcial activada (TTPA) y factor bajo de nivel II (después de la reversión óptima de la anticoagulación) como inicio de LES.
The lupus anticoagulant (LA) are a heterogeneous class of immunoglobulins, antiphospholipid antibodies (aPL), that interfere with phospholipid-dependent coagulation tests in vitro.
Development of LA is associated with several conditions, such as autoimmune diseases, infections, malignancies, or drugs.1,2 LA can also be found in healthy individuals. The antibody-antigen complexes compete with clotting factors for the phospholipids necessary in clotting assays, prolonging phospholipid-dependent times.
The presence of LA can be related to venous and arterial thrombosis and pregnancy morbidity, causing antiphospholipid syndrome (APS). However, much less frequently, LA can cause bleeding when associated with hypoprothrombinemia.3 Concomitant acute acquired hypoprothrombinemia and LA is known as the lupus anticoagulant-hypoprothrombinemia syndrome (LAHPS), and is probably a consequence of anti-factor II auto-antibodies development.
We describe a case of a male adult with APS diagnosis who presented a cerebral bleeding as a SLE debut.
Case reportA 42-year-old caucasian man with a medical history of primary APS (3 episodes of deep vein thrombosis and triple positivity for antiphosphopilipid antibodies) diagnosed when he was 15 years old, on chronic anticoagulation with acenocoumarol, with an adequate target therapeutic range (TTR) (75%), presented at the emergency department complaining about a 15-days intense headache, several episodes of spontaneous self-limited mild epistaxis. He had no family history of hereditary bleeding disorders or autoimmune disease.
Neurological examination was normal, Glasgow Coma Scale 15, and his blood arterial pressure was within normal range. No pathological findings were found in the rest of the patient's physical examination.
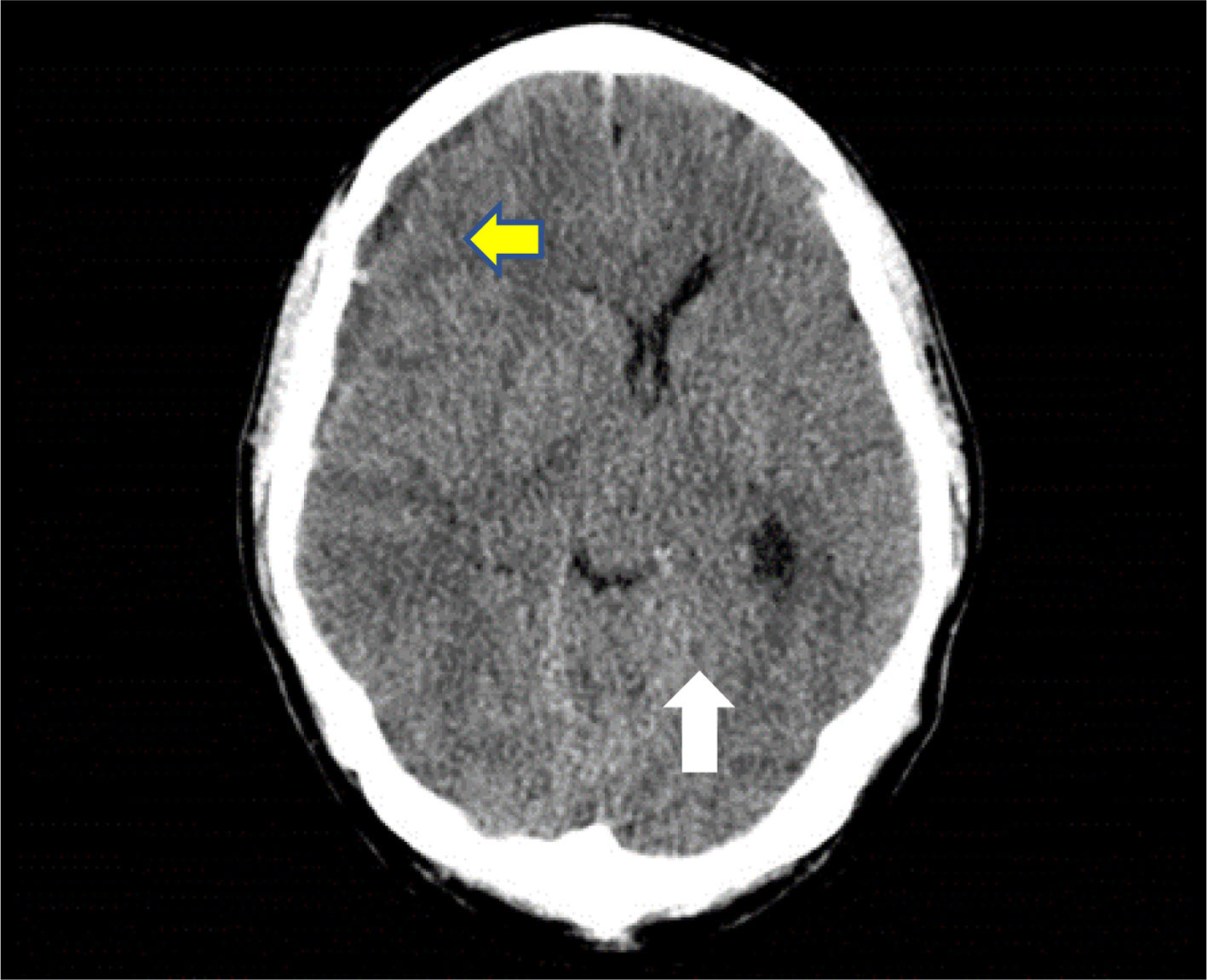
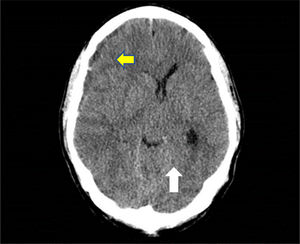
A skull CT scan was performed and revealed a hypodense collection with hyperdense areas at the right parietal subdural level. This was compatible with chronic subdural hematoma with rebleeding areas and large mass effect with ipsilateral ventricular compression and 9mm midline deviation (Fig. 1). With these findings and neurosurgical assessment, treatment started with intravenous dexamethasone 40mg daily was iniciated.
His complete blood test revealed a white blood cell count (WBC) and platelet count within normal range, whereas hemoglobin was slightly low (11.8g/l). Coagulation tests showed prolonged prothrombin time (PT) [28.7seconds (s)], INR 2.5, prolonged activated partial thromboplastin time (APTT) (82.5s) and normal derived fibrinogen. Prolongation of APTT was not corrected when mixed with normal pooled plasma (1:1 ratio) (77.3s) (Table 1).
Results of the coagulation studies on admission.
| Patient | Normal range | |
|---|---|---|
| Platelet/L | 152×109 | 150–450×109 |
| Hb (g/L) | 11.8 | 13–17 |
| PT (s) | 28.7 | 10–14 |
| PT after mixing 15 | ||
| APTT (s) | 82.5 | 26–36 |
| APTT after mixing 75 | ||
| Fibrinogen (mg/dl) | 318 | 200–400 |
| Factor II (%) | 12 | 60–120 |
| Factor V (%) | 70 | |
| Factor VII (%) | 60 | |
| Factor X (%) | 78 | |
| Factor VIII (%) | 40 | |
| Factor IX (%) | 20 | |
| Factor XI (%) | 12 | |
| Factor XII (%) | 16 |
APTT, activates partial thromboplastin time; PT, prothrombin time; Hb, hemoglobin.
The anticoagulation was reversed with 10mg every 12/24h endovenous vitamin K. Given the life-threatening bleeding suffered by the patient and before a possible emergent surgery a prothrombin complex concentrate (CCP) of 4 factors at a dose (10–30IU/kg) and plasma (15–30mL/kg body weight) achieving an INR of 1.75.
Since the patient remained clinically stable through the next hours, urgent surgery was dismissed, unless neurological worsening.
The same results were observed in a repeated basic coagulation study; thus an extended coagulation profile was performed in the next days.
Given the suspicion of the existence of an inhibitor of the coagulation factors, corticotherapy was continued with prednisone a dose of 1mg/kg-weight per day.
A strong positive LA was detected, with diluted Russell Viper Venom Time (dRVVT) and Silica Clotting Time (SCT) normalized ratios of 3.3 and 2.8 respectively. Positivity for anti-Cardiolipin (Ig G 164 GPL; Ig M 143 MPL), anti-β2-Glycoprotein I (anti-β2GPI) (Ig G: 176.77U/ml; Ig M: 130U/ml) and anti-Prothrombin (anti-PT) was also detected.
Low levels of factor II (12%) were observed. Increasing dilutions with normal pooled plasma showed persistently decreased factor II levels, even at a 1/100 dilution. Decreased levels of factor VIII (40%), factor IX (20%), factor XI (12%) and factor XII (16%) were observed, with normalization of their levels when patient plasma was tested in higher dilutions. These findings were compatible with an in vitro non-specific inhibition effect of LA against intrinsic pathway factors. The intensity of ACL did not vary in the successive determinations, and neither did its inhibitory effect against intrinsic pathway factors.
The dilutions were made on several occasions at the beginning of the episode. Subsequently, after starting treatment, with the progressive increase to the normalization of the factor and the good clinical evolution, no additional study of factor II dilutions were made.
Positivity for antinuclear antibodies (1/80 without other specificity) and hypocomplementemia [C3: 70mg/dl (normal range 90–170); C4: 3mg/dl (normal range 12–36) were also observed. Direct Coombs was positive.
In addition to headache, during hospitalization in the next weeks, he referred generalized arthralgias and a striking malar erythema was observed for the first time. With the results obtained from the complementary tests and with the suspicion of autoimmune disease, an interconsultation requests from the rheumatology department, who, after adequate anamnesis of the patient, the diagnosis of SLE was concluded.
LAHPS, debut of SLE and thrombotic APS were the final diagnosis. Treatment was initiated with prednisone at a dose of 1mg/kg once daily, hydroxicloroquine 200mg every 12hours, non-specific human immunoglobulins at a dose of 0.4g/kg for 5 days and Anti-CD20 at a dose of 1g every 15 days (2 doses).
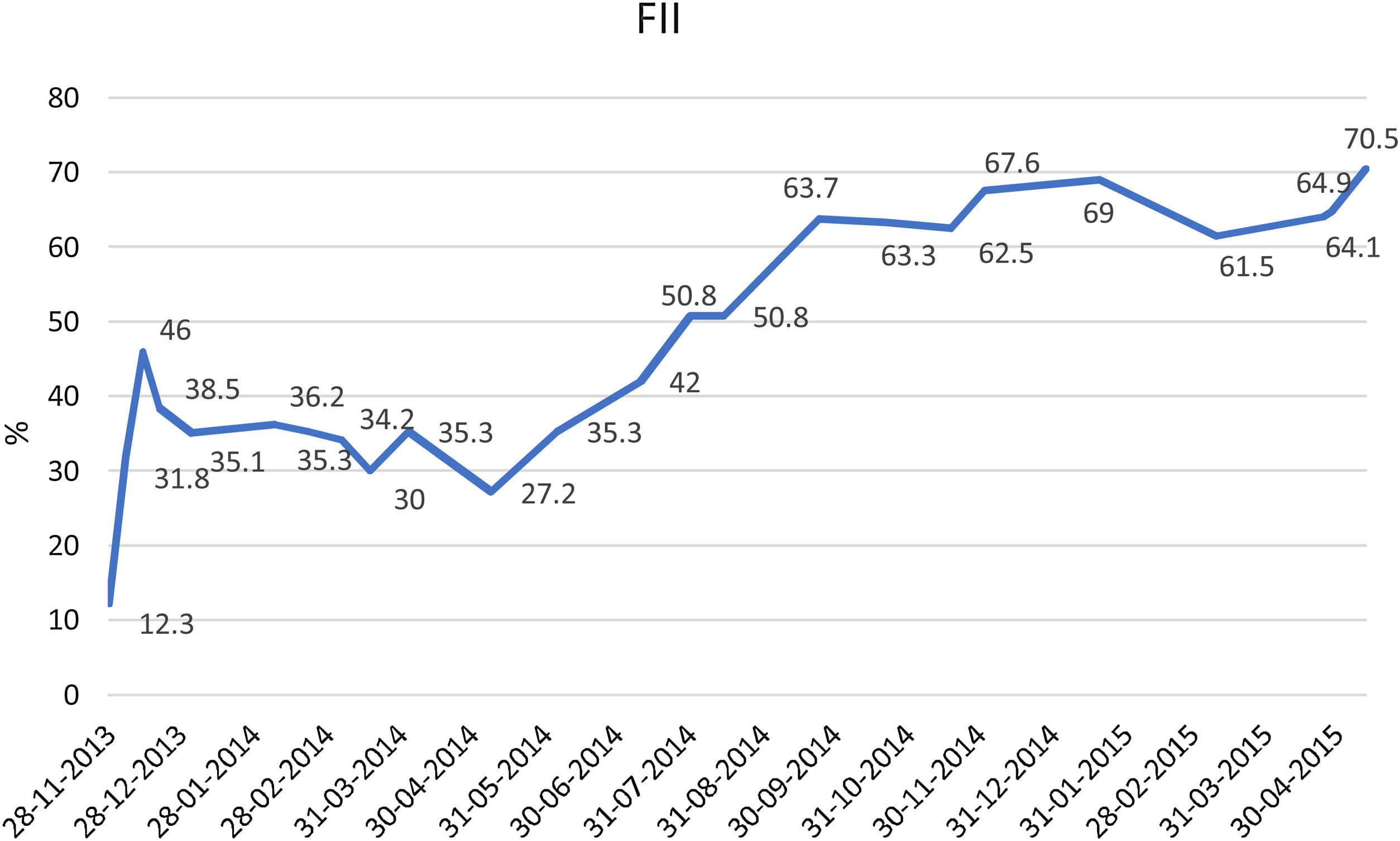
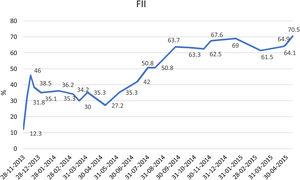
He remained asymptomatic from the neurological point of view, with progressive hematoma resolution and factor II levels improvement (Table 2). He received appropriate antithrombotic therapy with low molecular weight heparin (LMWH) at prophylactic dose (enoxaparin 40mg once daily), with progressive dose increase as the hematoma and the factor II levels improved (Fig. 2).
Coagulation studies at admission and follow-up in patient with lupus anticoagulant hypoprothrombinemia syndrome.
| Laboratory | Normal range | On admission | 2 months after | 12 months after |
|---|---|---|---|---|
| PT (s) | 10–14 | 28.7 | 14.1 | 11 |
| T. mixes PT | 15.5 | |||
| aPTT (s) | 26–36 | 82.5 | 67.4 | 51.5 |
| aPTT (s)a | 77.3 | 47.30 | 44.10 | |
| Factor II assay (%) | 60–120 | 12 | 36.20 | 67.68 |
| Lupus anticoagulant | Positive | |||
| drVVT normalized ratio | 3.3 | 3.03 | ||
| SCT normalized ratio | 2.8 | 1.7 | ||
| Anticardiolipin antibody (GPL) | Positive | |||
| IgG | 164 | 174 | ||
| IgM | 143 | 47.12 | ||
| Anti β2 GPI antibody (U/ml) | ||||
| IgG | 176 | 184.48 | 156.03 | |
| IgM | 130 | 42.77 | 32.67 | |
| ACs anti-PT IgG | Positive | No tested | No tested | |
| ACs anti-PT IgM | Positive | |||
Prothrombin time (PT); activated partial thromboplastin time (APTT); antibody to prothrombin (ACs anti-PT).
After 2 weeks hospitalizated, the patient initiated outpatient follow-up in rheumatology and hematology. He has presented several SLE outbreaks consisting on arthralgia, asthenia and headache, but no bleeding episode has occurred and his factor II levels have remained normal. As an antithrombotic treatment, secondary prophylaxis with enoxaparin at a dose of 1mg/kg once daily during the first three months was prescribed with adequate compliance, increasing the dose until therapeutic level after the brain hemorrhage was completely resolved.
DiscussionClotting factor II, or prothrombin, is a vitamin K-dependent coagulation cofactor that is cleaved by factor Xa to form thrombin, therefore inducing platelet aggregation and coagulation cascade activation.
Inherited factor II deficiency is uncommon, whereas acquired deficiency is a frequent finding in severe liver disease, vitamin K deficiency, or vitamin K antagonist treatment. Commonly, in these situations other coagulation factors are also decreased, such as factors VII and X, and factor V in some cases.
The isolated acquired factor II deficiency can occasionally be observed in patients with LA, a laboratory interference produced by antiphospholipid antibodies. This association is known as LAHPS, a rare syndrome that normally has been reported to be related to certain situations such as primary APS, infections, and occasionally drugs, but mostly to LES.4
The existence of Anti-prothrombin antibodies has been proven. Anti-prothrombin are non-neutralizing antibodies directed against the inactive sites of prothrombin (i.e., prothrombin I and α-thrombin) and therefore do not cause immediate inhibition. Different published studies suggest an increased clearance of Factor II from circulation, leading to its deficiency.5
The incidence of acquired hypoprothrombinemia associated with LA appears to be higher in the paediatric age group, since 55% of reported cases (49 of 89) were diagnosed in patients under the age of 16, with a median age at disease onset of 13 years (range 1–86 years).1,2,6–8 This may be explained by the naturally faster hepatic clearance of the prothrombin/prothrombin antibody complex in children compared to adults.6 Women are more affected than men, with an overall female: male ratio of 1.5:1 (54 women and 35 men).1,2
An underlying pathology can be identified, such as an autoimmune disease (most likely SLE), viral o bacterial infection, the most common underlying disease in paediatric population. There are few reported cases associated with malignancy (e.g. lymphoma and multiple myeloma) almost exclusively in adults or drugs (phenytoin or quinidine). In around 10% of cases, no underlying illness is evident.2,4,9,10
In most cases the initial symptom is bleeding, which can vary between minor bleeding, like epistaxis, ecchymoses, gingival bleeding, petechiae, to severe haemorrhagic symptoms such as gynecologic bleeding, macroscopic hematuria, digestive tract hemorrhage, intracerebral hematoma, and/or intramuscular hematoma. Bleeding can appear spontaneously or after trauma or surgery.2,4 On the other hand, arterial or venous thromboses as well as pregnancy morbidity are frequent in patients who have LA associated to SLE or APS. These thrombotic events are described in patients with LAHPS and autoimmune disease, but not with infections.2,9
In our patient with an intracraneal bleeding, the initial suspicious was cause of the oral anticoagulant (OAC). Cerebral hemorrhage is the most serious complication of anticoagulant therapy. Of all intracranial hemorrhages, between 10 and 25% occur in patients receiving oral anticoagulant therapy with vitamin K antagonists.11
Hemorrhagic strokes are rare in young adults and most often attributable to vascular anomalies or bleeding diathesis. While ischemic infarcts are a more typical complication of antiphospholipid antibody syndrome, bleeding can be associated with thrombocytopenia, acquired factor VIII inhibitors, or hypothrombinemia through the presence of anti-prothrombin antibodies. Antibodies to prothrombin most likely cause bleeding by accelerating the clearance of antibody—antigen complexes from the circulation.12
We had to suspect LAHPS when we found a prolongation of PT and APTT in a patient with LA, in the absence of sepsis or disseminated intravascular coagulation (DIC), facing the failed reversal of coagulation with the appropriate measures.
The other laboratory findings are a normal TT, fibrinogen and normal platelet count. A mixing study of patient plasma with normal pooled plasma in a ratio of 1:1, can distinguish between a factor deficiency (normalized APTT and PT) in contrast to LAHPS (only PT will get normalized after mixing).2 A factor II dosage and anti-prothrombin antibodies complete the diagnostic studies. Apart from the vit K dependent factors, the factors of the intrinsic pathway are decreased by the effect of the lupus anticoagulant, demonstrating normalization with a dilution/parallelism study, so that specific inhibitors directed against these factors were not suspected. When LAHPS patients occasionally show reduced FVIII activity and bleeding tendency Is important to differentiate from acquired hemophilia A (AHA) because these are false findings caused by the influence of LA on clotting time. For differential diagnosis, a reported method for measuring factor VIII activity using diluted patient plasma is relatively effective. Dilution of the sample attenuates the influence of LA on in vitro clotting time.13
As first line treatment, corticosteroids at a dose of 1mg/kg once daily are recommended, alone or in combination with other immunosuppressant agents (azathioprine, cyclophosphamide, mycophenolate mofetil, immunoglobulins or rituximab).
In case of severe bleeding, supportive care with plasma, vitamin K, prothrombin complex concentrate or recombinant factor VII can be used, as well as red blood cells and platelet transfusions.
Special attention should be paid to thrombosis, which can be present when factor II levels normalize. Thus, in these cases, anticoagulation or an antiplatelet agent after initial stabilization is recommended.4,9
ConclusionThis case highlights the importance of taking into account the possibility of LAHPS in any patient with LA, regardless of his/her age, with prolonged TP and APTT and bleeding history. Although in our patient the hypothesis about the origin of bleeding was more in favor of an adverse effect of oral anticoagulation, an early diagnosis of this clinical entity and a quick initiation of immunosuppressive treatment, as well as a diagnosis of the underlying disease determines patient's clinical development. The therapeutic challenge was to delay the urgent surgical option since it could have entailed a high risk of bleeding and therefore a fatal outcome for the patient.
FundingThe authors did not receive support from any organization for the submitted work.
Consent to publicationFor the presentation of this case report, informed consent was obtained from the patient.
Conflicts of interestThe authors have no conflicts of interest to declare.