Sarcoidosis is a multisystemic granulomatous disease that affects the lungs in more than 90% of the patients. It is associated with a variable clinical course and considering all the different forms of disease presentation, there are an absence of reliable clinical prognostic markers that can predict the outcome at diagnosis.
ObjectiveThe aim of our study was to investigate prognostic factors at diagnosis in a population of sarcoidosis patients from Northern Portugal.
MethodsA group of 110 patients with chronic evolution was compared with 129 patients with disease resolution regarding their clinical, radiologic and laboratorial features.
ResultsWe found a positive association between the chronic forms and lung function impairment, radiologic stage II, lower lymphocyte CD4/CD8 and extrapulmonary disease. Löfgren syndrome and asthenia instead had a protective significant association to chronicity. Our final logistic regression model found a significant independent association between age (adjusted OR=1.06), extrapulmonary involvement (adjusted OR=2.68), Löfgren's syndrome (adjusted OR=0.15) with outcome toward chronicity.
ConclusionsIn this first study searching for prognostic factors at diagnosis in a Northern Portuguese population, we found clinical prognosis factors that have been described in other populations that should be considered whenever sarcoidosis is identified.
La sarcoidosis es una enfermedad granulomatosa multisistémica que afecta a los pulmones en más del 90% de los enfermos. Está asociada a un curso clínico variable y, considerando todas las formas diferentes de presentación de la enfermedad, hay una ausencia de marcadores de pronóstico clínico confiables que puedan predecir el resultado en el momento del diagnóstico.
ObjetivoEl objetivo de nuestro estudio fue investigar los factores pronósticos en el momento del diagnóstico en una población de enfermos con sarcoidosis del norte de Portugal.
MétodosSe comparó un grupo de 110 enfermos con evolución crónica con 129 enfermos con resolución de la enfermedad teniendo en cuenta sus características clínicas, radiológicas y de laboratorio.
ResultadosSe encontró una asociación positiva entre las formas crónicas y el deterioro de la función pulmonar, el estadio radiológico II, la relación CD4/CD8 más baja y la enfermedad extrapulmonar. Ya el síndrome de Löfgren y la astenia tuvieron una asociación protectora significativa con la cronicidad. Nuestro modelo de regresión logística final encontró una asociación independiente significativa entre la edad (OR ajustada=1,06), la afectación extrapulmonar (OR ajustada=2,68), el síndrome de Löfgren (OR ajustada=0,15) y el resultado hacia la cronicidad.
ConclusionesEn este primer estudio de búsqueda de factores pronósticos en el momento del diagnóstico en una población del norte de Portugal, fueron encontrados predictores clínicos, que se han descrito en otras poblaciones que se deben considerar cada vez que se hace el diagnóstico de una sarcoidosis.
Sarcoidosis is a multisystemic granulomatous disease characterized by a T-helper 1 response with accumulation of CD4+ lymphocytes and activated macrophages, resulting in granuloma formation, affecting the lungs in more than 90% of the patients.1–8 Although its pathophysiology is not entirely understood, the disease appears to be the result of an exposure to some environmental factor(s), yet to be identified, in genetically predisposed individuals.1,9 It has a worldwide distribution, despite differences in its prevalence and clinical presentation, related with different geographical origins.1,10,11 The disease is more prevalent in individuals between 25 and 45 years old and it has a female predominance.1,10,11
Most patients with sarcoidosis have a favorable evolution with spontaneous resolution occurring in nearly two thirds of them.1,5,7 However, a minority of patients have a chronic course of the disease, sometimes requiring persistent immunosuppressive therapy. It has been suggested that the immunological environment may change to a T-helper 2 response that is associated with a chronic course.3,8,12,13
At diagnosis, besides Löfgren syndrome (LS), we still do not have any reliable and recognized clinical or serum markers that can predict the outcome of a particular patient. It was our main purpose to search retrospectively, in a population of sarcoidosis patients with pulmonary involvement, any prognosis marker that could be considered at presentation.
MethodsOur study incorporates patients with sarcoidosis followed at Centro Hospitalar São João (CHSJ), a tertiary hospital in the Northern region of Portugal. We included 239 patients with pulmonary stage I and II involvement, successively diagnosed between 1990 and 2014.
The diagnosis was based on the ATS/ERS/WASOG statement.7 All patients had pulmonary sarcoidosis, defined by symptoms, radiology and pulmonary function tests, which were supported by the evidence of non-caseating epithelioid cell granulomas in biopsy of 63.4% of them. In patients without histologic evidence of granuloma, sarcoidosis diagnosis was defined by the ERS/ATS/WASOG statement criteria: compatible clinical and radiographic features, a lymphocyte ratio CD4/CD8>3.5 in bronchoalveolar lavage (BAL), and an observation period of 2 years to exclude other medical conditions.7 LS was defined as a bilateral hilar lymphadenopathy, ankle arthralgia and erythema nodosum with or without fever.14,15 Thoracic involvement was described, at diagnosis, according with Scadding criteria: stage 0 – no thoracic involvement; stage I – adenopathies without lung involvement; stage II – adenopathies and lung involvement, stage III – only lung involvement and stage IV – lung fibrosis.13 We considered disease resolution when disappearance of the symptoms, normalization of the thoracic X-ray and the pulmonary function tests occurred within 2 years after diagnosis. On the contrary, patients with evidence of disease after 2 years were considered to have chronic sarcoidosis. All patients whose radiographic stage was unknown or were diagnosed at stage 0, III or IV were excluded. BAL lymphocytosis was considered when the percentage of lymphocytes was above 15% and a higher CD4/CD8 when the ratio was beyond 3.5. Patients whose clinical evolution or clinical data at diagnosis were unknown were excluded. All data was obtained from patients’ clinical files and the study protocol was approved by the Ethics Committee of this hospital.
Finally, in this retrospective study, 110 chronic patients were compared against 129 patients with disease resolution, concerning their demographic, clinical, radiologic and laboratorial characters. We analyzed patients’ age, gender, smoking status, symptoms, pulmonary function tests, radiographic stage and BAL features at time of diagnosis. The results are given as frequencies, mean±standard deviation and median and Interquartile range (IQR). In the univariate analysis, we used the chi-squared test to compare differences in proportions and Mann–Whitney U tests to compare continuous variables. Variables with a p-value less than 0.1 were used to calculate adjusted odds ratios (OR) and 95% confidence intervals (CI) in multivariate logistic regressions models. For the final logistic regression model coefficients were selected using backwards variable elimination. The goodness-of-fit of the final model was assessed with the Homer–Lemeshow test. We considered a statistical significance level of 5%.
All statistical analysis and graphic representations were performed on RStudio an environment for R programming language.
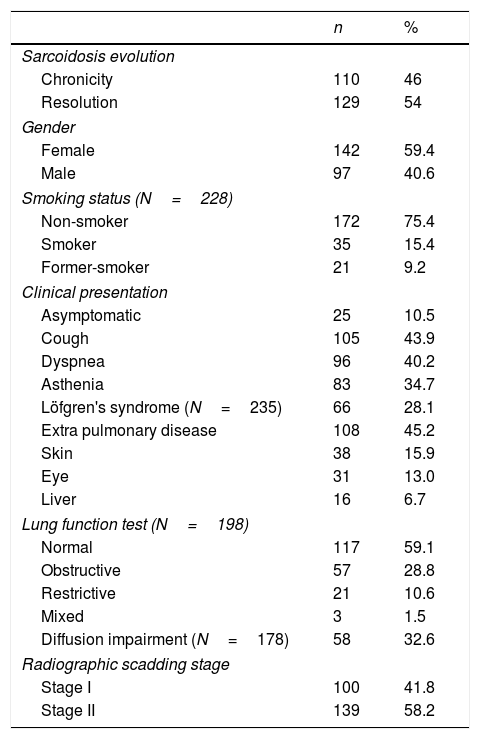
ResultsA total of 239 patients were selected and analyzed. The demographics and clinical features of the population are presented in Table 1. The mean age at diagnosis was 37.9 years (±11.7) and 59.4% were female. Regarding smoking status, 75.4% of the patients were non-smokers (n=172), 15.4% smokers (n=35) and 9.2% former-smokers (n=21) at time of diagnosis. The most prevalent symptoms at presentation were cough (43.9%; n=105), dyspnea (40.2%; n=96) and asthenia (34.7%; n=83). LS was the clinical presentation of 28.1% of the patients (n=66). At diagnosis, 10.5% were asymptomatic (n=25). From the patients which respiratory function tests were available at diagnosis (n=198), 59.1% had a normal lung function (n=117), 28.8% an obstructive pattern (n=57), 10.6% a restrictive pattern (n=21) and only 1.5% a mixed pattern (n=3). Regarding diffusion capacity, the mean DLCO was 83.1%±18.3 (n=178) and it was decreased in 32.6% of the patients (n=58), although 79.8% showed only a mild impairment. At time of diagnosis, 41.8% of the patients were classified as Scadding radiologic stage I (n=100) and 58.2% as stage II (n=139). BAL was performed in 145 patients. Lymphocytes median value was 40.9 (IQR 24.3–57.8), neutrophils 1.4 (IQR 0.4–2.4) and eosinophils 0.4 (0.0–1.0). Median lymphocyte CD4/CD8 ratio was 4.2 (IQR 2.3–7.6) and 78 (57.4%) of the patients presented a CD4/CD8 ratio >3.5. Regarding the extrapulmonary manifestations, 45.2% of the patients (n=108) have extrapulmonary disease, 26.8% have only 1 extrapulmonary manifestation (n=64), 9.2% have 2 extrapulmonary manifestations (n=22) and 9.2% of the patients have 3 or more (n=22). The skin (15.9%; n=38), the eye (13%; n=31) and the liver (6.7%; n=16) are the most prevalent locations for extrapulmonary sarcoidosis. In our population, 55.2% of the patients didn’t require any treatment (n=132) and the remaining 44.7% needed systemic treatment (n=107).
Demographics and clinical features of Sarcoidosis patients (N=239).
| n | % | |
|---|---|---|
| Sarcoidosis evolution | ||
| Chronicity | 110 | 46 |
| Resolution | 129 | 54 |
| Gender | ||
| Female | 142 | 59.4 |
| Male | 97 | 40.6 |
| Smoking status (N=228) | ||
| Non-smoker | 172 | 75.4 |
| Smoker | 35 | 15.4 |
| Former-smoker | 21 | 9.2 |
| Clinical presentation | ||
| Asymptomatic | 25 | 10.5 |
| Cough | 105 | 43.9 |
| Dyspnea | 96 | 40.2 |
| Asthenia | 83 | 34.7 |
| Löfgren's syndrome (N=235) | 66 | 28.1 |
| Extra pulmonary disease | 108 | 45.2 |
| Skin | 38 | 15.9 |
| Eye | 31 | 13.0 |
| Liver | 16 | 6.7 |
| Lung function test (N=198) | ||
| Normal | 117 | 59.1 |
| Obstructive | 57 | 28.8 |
| Restrictive | 21 | 10.6 |
| Mixed | 3 | 1.5 |
| Diffusion impairment (N=178) | 58 | 32.6 |
| Radiographic scadding stage | ||
| Stage I | 100 | 41.8 |
| Stage II | 139 | 58.2 |
N: overall number of patients with information; n: number of observations.
Concerning the outcome, 54% of the patients (n=129) had disease resolution and 46% had a chronic course (n=110). From the group of patients with disease resolution, 67.4% had a spontaneous resolution (n=87) and 32.5% required treatment (n=42).
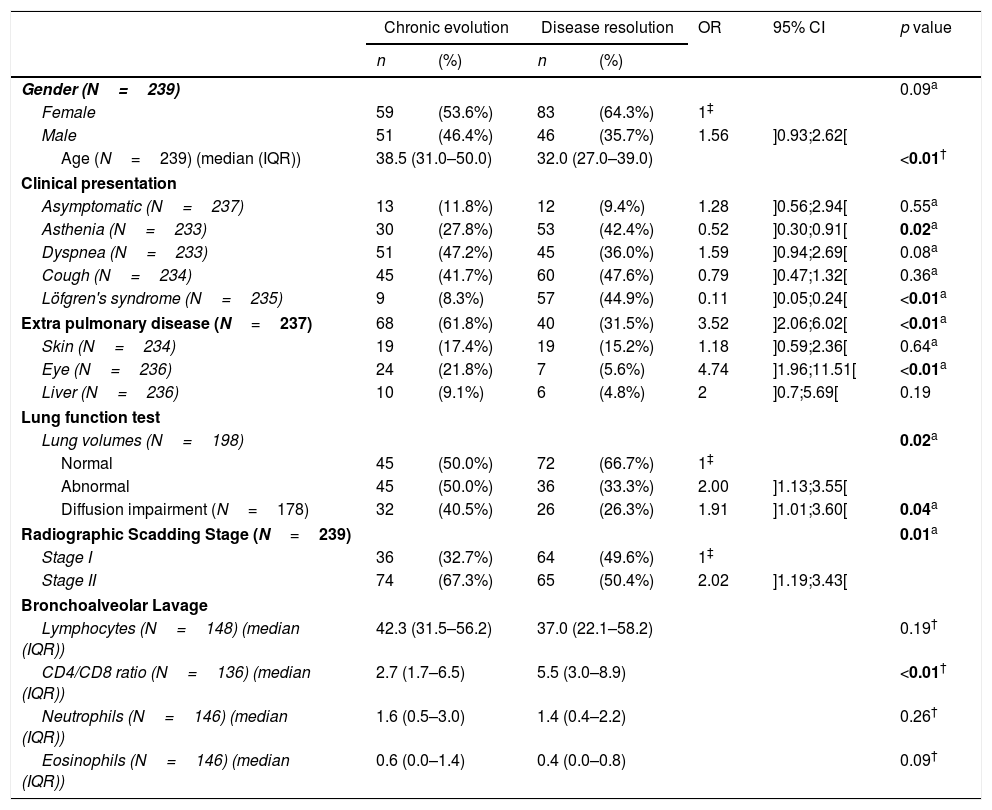
Although females are more frequent among patients with disease resolution (64.3% versus 53.6%), we did not find a significant difference between the two groups regarding gender, but an older age seems to be associated with a worse outcome (38.5 years, IQR: 31.0–50.0 in chronic course versus 32 years, IQR 27–39 in disease resolution, p<0.01). Regarding clinical presentation, patients with disease resolution presented, in the univariate analysis (Table 2), higher frequencies of LS (44.9% versus 8.3%, p<0.01) and asthenia (42.4% versus 27.8%, p=0.02). When lung function tests were analyzed, those patients with lung volumes abnormalities have a clear tendency to chronicity (50.0% versus 33.3%, p=0.02). Additionally, those with diffusion capacity impairment had also a significant association with chronic outcome (40.5% versus 26.3%, p=0.04). The radiologic stage was also associated with the outcome of the disease. Patients at stage II are more frequent within the group with chronicity than within those with disease resolution (67.3% vs 50.4%, p=0.01). We found no statistical differences in the BAL differential cell count between the two groups, but the mean lymphocyte CD4/CD8 ratio is significantly lower in the patients with chronic evolution (2.7, IQR 1.7–6.5 versus 5.5, IQR 3–8.9). The manifestation of extrapulmonary disease seems to have also an influence on the prognosis, since 61.8% of the patients had chronic course comparing with only 31.5% with disease resolution (p<0.01).
Clinical characteristics of sarcoidosis patients classified according to disease evolution.
| Chronic evolution | Disease resolution | OR | 95% CI | p value | |||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | ||||
| Gender (N=239) | 0.09a | ||||||
| Female | 59 | (53.6%) | 83 | (64.3%) | 1‡ | ||
| Male | 51 | (46.4%) | 46 | (35.7%) | 1.56 | ]0.93;2.62[ | |
| Age (N=239) (median (IQR)) | 38.5 (31.0–50.0) | 32.0 (27.0–39.0) | <0.01† | ||||
| Clinical presentation | |||||||
| Asymptomatic (N=237) | 13 | (11.8%) | 12 | (9.4%) | 1.28 | ]0.56;2.94[ | 0.55a |
| Asthenia (N=233) | 30 | (27.8%) | 53 | (42.4%) | 0.52 | ]0.30;0.91[ | 0.02a |
| Dyspnea (N=233) | 51 | (47.2%) | 45 | (36.0%) | 1.59 | ]0.94;2.69[ | 0.08a |
| Cough (N=234) | 45 | (41.7%) | 60 | (47.6%) | 0.79 | ]0.47;1.32[ | 0.36a |
| Löfgren's syndrome (N=235) | 9 | (8.3%) | 57 | (44.9%) | 0.11 | ]0.05;0.24[ | <0.01a |
| Extra pulmonary disease (N=237) | 68 | (61.8%) | 40 | (31.5%) | 3.52 | ]2.06;6.02[ | <0.01a |
| Skin (N=234) | 19 | (17.4%) | 19 | (15.2%) | 1.18 | ]0.59;2.36[ | 0.64a |
| Eye (N=236) | 24 | (21.8%) | 7 | (5.6%) | 4.74 | ]1.96;11.51[ | <0.01a |
| Liver (N=236) | 10 | (9.1%) | 6 | (4.8%) | 2 | ]0.7;5.69[ | 0.19 |
| Lung function test | |||||||
| Lung volumes (N=198) | 0.02a | ||||||
| Normal | 45 | (50.0%) | 72 | (66.7%) | 1‡ | ||
| Abnormal | 45 | (50.0%) | 36 | (33.3%) | 2.00 | ]1.13;3.55[ | |
| Diffusion impairment (N=178) | 32 | (40.5%) | 26 | (26.3%) | 1.91 | ]1.01;3.60[ | 0.04a |
| Radiographic Scadding Stage (N=239) | 0.01a | ||||||
| Stage I | 36 | (32.7%) | 64 | (49.6%) | 1‡ | ||
| Stage II | 74 | (67.3%) | 65 | (50.4%) | 2.02 | ]1.19;3.43[ | |
| Bronchoalveolar Lavage | |||||||
| Lymphocytes (N=148) (median (IQR)) | 42.3 (31.5–56.2) | 37.0 (22.1–58.2) | 0.19† | ||||
| CD4/CD8 ratio (N=136) (median (IQR)) | 2.7 (1.7–6.5) | 5.5 (3.0–8.9) | <0.01† | ||||
| Neutrophils (N=146) (median (IQR)) | 1.6 (0.5–3.0) | 1.4 (0.4–2.2) | 0.26† | ||||
| Eosinophils (N=146) (median (IQR)) | 0.6 (0.0–1.4) | 0.4 (0.0–0.8) | 0.09† | ||||
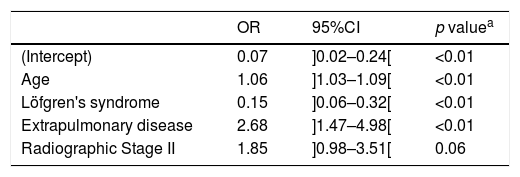
From our final logistic regression model (Table 3) we can conclude that age, LS and extra-pulmonary disease are statistically significant factors independently associated with sarcoidosis evolution toward chronicity. While age and extrapulmonary disease are risk factors (adjusted OR=1.06 and adjusted OR=2.68, respectively) for chronicity, LS is a protective factor (adjusted OR=0.15). Scadding stage II is also a risk factor to chronicity (adjusted OR=1.85), although it does not reach statistical significance (p=0.06). Goodness-of-fit test yielded a p-value of 0.38, indicating no evidence of poor fit (Table 3). Considering the potential influence of the therapeutic in the disease evolution, a comparison between the subgroup of patients with spontaneous resolution and the patients with a chronic course was analyzed and we found exactly the same significant associations with resolution and chronicity reported previously.
Adjusted coefficients from a logistic regression model comparing sarcoidosis chronic evolution against disease resolution.
| OR | 95%CI | p valuea | |
|---|---|---|---|
| (Intercept) | 0.07 | ]0.02–0.24[ | <0.01 |
| Age | 1.06 | ]1.03–1.09[ | <0.01 |
| Löfgren's syndrome | 0.15 | ]0.06–0.32[ | <0.01 |
| Extrapulmonary disease | 2.68 | ]1.47–4.98[ | <0.01 |
| Radiographic Stage II | 1.85 | ]0.98–3.51[ | 0.06 |
Our analysis of sarcoidosis patients with pulmonary stages I and II at time of diagnosis found that an older age, lung volumes and diffusion capacity impairment, a higher radiological extension and extrapulmonary disease had a risk association with a chronic course. Löfgren syndrome or asthenia had instead a protective association with evolution to chronicity. The final logistic regression model found a statistically significant independent association between age, extrapulmonary involvement and Löfgren's syndrome with disease evolution toward chronicity.
Although the majority of sarcoidosis patients will have a disease resolution, there is however a significant amount of them that will have a chronic disease, sometimes even with a persistent therapeutic intervention.1,5 At diagnosis, excluding LS, a specific sarcoidosis clinical presentation associated with a good prognosis,15,16 the clinicians haven’t found real, consistent and validated clinical markers that can predict the clinical evolution of a particular patient. Some genetic polymorphisms associated with clinical evolution have been described, even in the population included in this study.17–19 However, none of these potential genetic markers have been validated either as worldwide established prognostic markers.
We included only stage I and II pulmonary sarcoidosis, because they are similar and rather different specially from stage IV, whose patients have eventually always chronic forms of the disease.1,5,7 Pulmonary stage III is rare and have a substantial proportion of patients that progress to chronic forms.1,5,7 Moreover, in stage I and II about 2/3 will have a disease resolution, usually spontaneous.1,5,7 Therefore, it would be very useful to have prognosis factors that could predict the chronic course in these two stages of pulmonary involvement.
In our population, higher age at diagnosis was clearly associated with a disease chronic course, which are consistent with previous reports.1,7,20,21
Nonspecific constitutional symptoms such as fever, fatigue, asthenia and weight loss may occur in about one/third of patients with sarcoidosis.7 Although some confounding concepts by patients and physician's related with asthenia, malaise or fatigue that can lead to an inappropriate interpretation of patients complains, seems to be obvious that asthenia is the right definition regarding what was reported by patients at time of diagnosis. Moreover, its protective association for chronicity clearly suggests the context of an acute clinical presentation, with an initial systemic inflammatory response, but usually with a good prognosis associated with spontaneous remissions as for instance LS. Fatigue is frequently associated with sarcoidosis in a multifactorial background, although its definition is not consensual and it has usually a risk association with a chronic course.22
The ongoing inflammation and interstitial fibrosis, characteristic of the chronic phase of the disease, leads to lung function and diffusion capacity impairment,23 that when present in an early phase of the disease may characterize a more serious form and consequently a tendency to chronicity.
The reason for a higher amount of stage II disease (58.2%) in this cohort comes from the fact that the data is related with high resolution computer tomography scan features. Within chronic patients only 32.7% had stage I while approximately half of the patients with disease resolution had that stage. This is in line with previous data.6,7,14,16,24,25
BAL persists as an important tool in sarcoidosis diagnostic approach.1,2,26–28 In this group of patients, the chronic course has a significant association with lower lymphocyte CD4/CD8 ratio and no significant difference was found in the lymphocyte count. This difference has already been observed in previous studies and may be explained by the shift to a Th2 environment found in patients who progress to chronic sarcoidosis with pulmonary fibrosis.3,6 Although some studies identified a relationship between higher percentage of BAL neutrophils and a chronic course,9,23 no statistically significant difference was found in our study.
The manifestation of the extrapulmonary involvement clearly influenced the outcome. Among the most prevalent organs, ocular involvement showed a significant association with chronicity in this cohort, which, as far as we know, was not described previously.4,7,20,21
Regarding the final logistic regression model, LS and age were already described as prognostic factors in cohorts from other regions and populations.1,7,21,29 The presence of extrapulmonary disease not only represents a widespread disease but could also suggest an underlying persistent inflammation with tendency to progression.
The epidemiological, genetic and environmental origin of the patients that were included in this cohort are similar, since all of them are Caucasian and from a specific region of Portugal-Oporto district. The fact that this study is retrospective and these patient data were obtained from the patients’ clinical files is a pitfall and prevents the impossibility of canceling the inter-observed variability, a potential cause of bias. Nevertheless, several statistically significant results were obtained in this first study searching for clinical prognostic factors at diagnosis in this particular population, which is relevant given the racial and geographic variability in disease prevalence, clinical presentation and outcome. Probably only a network of genetic polymorphisms and/or a serum marker related with the granuloma burden will give us in the future more precise prognostic markers.
Conflict of interestThe authors declare that they have no conflicts of interest.