To assess the efficiency of secukinumab compared to adalimumab as first biologic treatment for psoriatic arthritis (PsA) from the Spanish National Health System (SNHS) perspective.
MethodsA cost-consequence analysis of the cost and clinical response of two treatment strategies was conducted over a 2-year time horizon. A hypothetical cohort of 10 patients with PsA initiated treatment with secukinumab 150mg (cohort A) or adalimumab 40mg (cohort B), respectively. Patients achieving clinical response (ACR20/50/70) at week 24 continued the initial treatment, while patients with inadequate response switched to secukinumab 300mg. Pharmacological costs were calculated based on SmPC (notified ex-factory price). The lowest cost of adalimumab biosimilar was considered. Data on clinical response were extracted from the two matching-adjusted indirect comparison (MAIC) published comparing secukinumab vs adalimumab. Results were expressed as the cost difference between the two cohorts (€, 2019) and were calculated for each clinical response criteria (ACR20/50/70) and for each MAIC. Sensitivity analysis assessed the impact of potential discounts on the cost of adalimumab while maintaining the cost of secukinumab unchanged.
ResultsDepending on the MAIC used, the cost of initiating biologic treatment for PsA with secukinumab 150mg was 18–33% lower than the one estimated for adalimumab 40mg, for ACR20, 18–28% for ACR50, and 16–23% for ACR70 response rate. Sensitivity analysis showed that it would be necessary a discount of 40–60%, 40–65% and 50–75% over the adalimumab cost to compensate for the differences in efficacy observed for ACR20/50/70, respectively, depending on the MAIC used.
ConclusionIn patients with PsA, secukinumab could be considered a more efficient first-line biologic treatment compared to adalimumab, from the SNHS perspective.
Evaluar la eficiencia de secukinumab comparado con adalimumab como primer tratamiento biológico para la artritis psoriásica desde la perspectiva del Sistema Nacional de Salud español.
MétodosSe realizó un análisis de coste-consecuencia considerando el coste y la respuesta clínica a dos estrategias de tratamiento, en un horizonte temporal de 2 años. Una cohorte hipotética de 10 pacientes con artritis psoriásica inició el tratamiento con secukinumab 150mg (cohorte A) o adalimumab 40mg (cohorte B), respectivamente. Los pacientes con respuesta clínica (ACR20/50/70) en la semana 24 continuaron el tratamiento inicial, mientras que los pacientes con respuesta inadecuada recibieron 300mg de secukinumab. Los costes farmacológicos se calcularon en base a la ficha técnica (precio notificado). Se consideró el coste más bajo de adalimumab biosimilar. Los datos de respuesta clínica se extrajeron de los dos estudios publicados de comparación indirecta ajustada entre secukinumab y adalimumab. Se calculó la diferencia de coste entre las dos cohortes (€, 2019) para cada criterio de respuesta (ACR20/50/70) y para cada estudio. El análisis de sensibilidad evaluó los resultados aplicando posibles descuentos sobre el coste de adalimumab, manteniendo constante el coste de secukinumab.
ResultadosDependiendo del estudio utilizado, el coste de iniciar el tratamiento biológico con secukinumab 150mg fue un 18-33% menor que el estimado para adalimumab 40mg, para ACR20, 18-28% para ACR50, y 16-23% para ACR70. El análisis de sensibilidad mostró que sería necesario un descuento del 40-60%, 40-65% y 50-75% sobre el coste del adalimumab para compensar las diferencias de eficacia observadas para ACR20/50/70, respectivamente.
ConclusiónEn pacientes con artritis psoriásica, la elección de secukinumab como terapia biológica inicial podría considerarse una opción más eficiente comparado con adalimumab desde la perspectiva del Sistema Nacional de Salud español.
Psoriatic arthritis (PsA) is a chronic, progressive inflammatory disease characterized by skin and musculoskeletal manifestations.1 PsA can cause permanent joint damage, which can be very debilitating for patients, and is associated with a significant economic burden.2,3
The main treatment goal for patients with PsA is to maximize health-related quality of life through control of inflammation, symptoms, prevention of structural damage, normalization of physical function and social participation.4–7 The European League Against Rheumatism (EULAR)4,5 recommends conventional disease-modifying drugs (DMARDs) (methotrexate, leflunomide, sulfasalazine) as first-line treatment for PsA. For patients with an inadequate response or those in whom conventional treatment has failed, biologic treatments that act as tumour necrosis factor inhibitors (TNFi), such as adalimumab, certolizumab pegol, etanercept, golimumab and infliximab, are recommended, to be followed by inhibitors of interleukin (IL) 12, 23 and 17, such as ustekinumab (IL12/23i) and secukinumab (IL17i). For its part, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)6,7 recommends phosphodiesterase 4 inhibitor (PDE4i) apremilast or biologic treatments (TNFi, IL12/23i, IL17i) in peripheral PsA patients in whom DMARD treatment has been unsuccessful. Regarding the Spanish Society of Rheumatology (SER)8 recommends the use of biologic treatments for patients with peripheral PsA refractory to at least one DMARD without prioritization among biologic treatments, leaving the choice to the physician. Furthermore, it recommends that patients who fail to TNFi switch to another biologic treatment (TNFi) or a different mechanism of action, such as IL12/23i, IL17i or targeted DMARD (PDE4i) when this reveals most appropriate than a biologic treatment due to the patient profile (Grade of recommendation B).8 According to the SER, the choice of any available biologic treatment would be a valid option in most cases. Nevertheless, up to now, only one head-to-head study comparing biologics used to treat PsA has been published,9 which makes comparisons difficult and, by extension, decision-making in clinical practice. The results of a recently published network meta-analysis found that secukinumab was the most efficacious and safest treatment for PsA among all IL-inhibitor biologics.10 However, there are two recent matching adjusted indirect comparison studies (MAIC) analysing differences between secukinumab and adalimumab for treating PsA11,12 and reporting discordant results. When selecting a given drug to treat PsA, consideration should be given both to the features of the disease (clinical phenotype, severity, poor prognostic factors) as well as to those of the molecule itself (evidence, experience, efficacy, safety, optimization) and, in cases where it is not possible to establish differences based on the available scientific evidence, the economic data, as they provide information for decision making.8
The choice between biologic treatment with adalimumab or with secukinumab is of special interest because of the number of elements that inform decision-making from the clinical as well as the economic point of view that are present in both. Based on current available scientific evidence13 and existing guidelines,4–8 the options of initiating biologic treatment with secukinumab and, in the event of inadequate response, to intensify the dose, or initiating treatment with adalimumab and, in the event of treatment failure, to switch to secukinumab are both equally viable. Moreover, when it comes to determining which of the two options would be most efficient, the recent appearance on the market of drugs that are biologically similar to adalimumab at lower prices than the original biologic drug plays an important role, as it is a factor that may add additional uncertainty to decision-making.
In this context, we sought to conduct a cost-consequence study to help make decision-making about treatment for patients with PsA easier, through an analysis of the efficiency of secukinumab and adalimumab as first biologic treatments.
Material and methodsA cost-consequence analysis was conducted using an Excel spreadsheet to assess the cost of pharmacological treatment with respect to clinical response of two possible treatment strategies for PsA, with a time horizon of 2 years, from the perspective of the Spanish National Health System (SNHS).
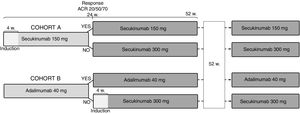
The analysis was based on a hypothetical cohort of 10 patients with PsA who initiated biologic treatment with secukinumab 150mg (cohort A) or adalimumab 40mg (cohort B) (Fig. 1). Patients in both cohorts who achieved clinical responses defined as the proportion of patients achieving improvement equal to or greater than 20%, 50% and 70% in the American College of Rheumatology (ACR 20/50/70) response criteria at week 24 of treatment would continue the initial treatment with secukinumab 150mg or adalimumab 40mg, respectively, while patients with inadequate response would receive secukinumab 300mg. In order to simplify the analysis, it has been assumed that all patients respond after week 24, without regard to other potential subsequent treatment failures.
Efficacy of the treatmentsData on clinical response to treatment at week 24 (ACR 20, ACR 50 and ACR 70) were extracted from the two matching-adjusted indirect comparison studies of secukinumab and adalimumab11,12 (Table 1). Although both publications compare the same treatments, different methodologies were used and the data used to determine effectiveness were from different clinical trials, so a priori, discordant results are to be expected. While Nash P. et al.11 found that response rates were numerically and statistically higher for secukinumab compared to adalimumab, Strand V. et al.12 found that, although response rates were numerically higher for adalimumab compared to secukinumab, these differences were not statistically significant. Given the discrepancies between both sources with respect to their methodologies and, consequently, their results, the data from the two available articles was analyzed separately. This way, it was possible to assess and compare the results from both perspectives and there was no loss of information that could be useful for decision-making, since these are the only comparative studies between both treatments for PsA.
Clinical efficacy data (at 24 weeks) used in the analysis: matching-adjusted indirect comparisons of secukinumab and adalimumab.
The main difference in methodology between the two articles is centred on the clinical trials selected for the comparisons. Whereas Nash P. et al.11 looked at the pivotal FUTURE 2 (secukinumab 150mg versus placebo) and ADEPT (adalimumab 40mg versus placebo) trials, Strand V. et al.12 also included the pivotal FUTURE 1 trial (secukinumab 150mg versus placebo). The reason Nash P. et al.11 gave for excluding the FUTURE 1 study was the fact that the dose of secukinumab that was administered was not the one approved on the summary of product characteristics (SmPC)13 and that the induction phase consisted of a 10mg/kg intravenous dose at weeks 0, 2 and 4. Strand V. et al.12 were aware of this limitation, so they performed a sensitivity analysis that did not include the FUTURE 1 study. Although they indicate that the efficacy of ACR 20 and ACR 50 responses of adalimumab compared to secukinumab were 6.4% lower and 5.9% lower, respectively, while the ACR 70 response was 0.6% higher (no statistically significant differences) authors did not provide detailed results thus these data has not been included in our analysis.
This data was used to determine the number of patients in each cohort with a clinical response (ACR 20/50/70) at 24 weeks. For example (Fig. 2), using the ACR 20 results reported in the Nash P. et al.11 analysis, we can see how 10 patients in cohort A start out by receiving treatment with secukinumab at a dose of 150mg and that at 24 weeks, 8 (81.0%)11 continue receiving the same treatment until the end of the time horizon while the dose administered to the other 2 (19%) would be increased (secukinumab 300mg). With respect to cohort B, of the initial 10 patients, 6 would continue to receive adalimumab 40mg until the end of the time horizon while the other 4 would switch to secukinumab 300mg. We proceeded in the same way with regard to the study conducted by Strand V. et al.12 (Fig. 2).
Cost of the treatmentOnly the notified ex-factory price14 has been taken into account, after the 7.5% discount was applied as per the deductions under Royal Decree-Law 8/201015 (Table 2). In the case of adalimumab, the biosimilar drug with the lowest price has been considered.
Dosage and unit cost of the treatments included in the analysis.13,14
| Treatment | Presentation | Dosage | Cost (notified ex-factory price)(€, 2019) |
|---|---|---|---|
| Adalimumab 40mg | 40mg – Two 0.8ml pre-filled pens | 40mg every other week | 874.05 € |
| Secukinumab 150mg | 150mg – One 1ml pre-filled pen | Inadequate responders (IR) to anti-TNFsInduction: 300mg on weeks 0, 1, 2, 3 and 4Maintenance: 300mg per month | 571.56 € |
| Secukinumab 300mg | 150mg – Two 1ml pre-filled pen | Anti-TNF-naïveInduction: 150mg on weeks 0, 1, 2, 3 and 4Maintenance: 150mg per month | 1143.11 € |
We calculated the cost of treatment by multiplying the notified ex-factory price per dose of each presentation by the number of doses required over the time horizon analyzed (Table 3). For secukinumab, we took into account the induction and maintenance phases as recommended in the SmPC,12 applicable to secukinumab 150mg for TNFi-naive patients and to secukinumab 300mg for inadequate responders to TNFi, along with the option of increasing the dose from 150mg to 300mg according to clinical response.12 Hence, when calculating the cost of the treatment for the cohort of patients who receive adalimumab 40mg and who switch to secukinumab 300mg after 24 weeks, the induction and maintenance phases are imputed for secukinumab. This is not the case for patients who start treatment with secukinumab 150mg and whose dose is increased to secukinumab 300mg after 24 weeks, since only the dose is increased (Table 3).
Cost of treatment for each treatment sequence considered in the analysis according to dosage.
| No. of dosesWeek 0–23 | No. of dosesWeek 24–51 | Cost of the treatment(€, 2019) | |||
|---|---|---|---|---|---|
| Treatment | Induction | Maintenance | Induction | Maintenance | |
| Year 1 | |||||
| Secukinumab 150mg→secukinumab 150mg | 4 | 5 | – | 6 | 7930 € |
| Secukinumab 150mg→secukinumab 300mga | 4 | 5 | – | 12c | 11,103 € |
| Adalimumab 40mg→secukinumab 300mgb | – | 12 | 4 | 6c | 15,425 € |
| Adalimumab 40mg→adalimumab 40mg | – | 12 | – | 14 | 10,510 € |
| Year 2 | |||||
| Secukinumab 150mg | 12 | 6344 € | |||
| Secukinumab 300mg | 12 | 12,689 € | |||
| Adalimumab 40mg | 26 | 10,510 € | |||
We used the data on efficacy and cost of treatment to calculate the costs for cohort A and cohort B and the difference between the two. The results are shown for the first year, the second year and in total, as well as for each of the clinical response criteria (ACR 20, 50 and 70). Given that there are discrepancies in the results of the two articles that have been published so far on the clinical efficacy of secukinumab versus adalimumab11,12 as assessed by matching-adjusted indirect comparison, the results are shown separately for each.
We also performed a sensitivity analysis to assess the impact on the results of a modification in the notified ex-factory price of adalimumab, while maintaining the cost of secukinumab unchanged, as we considered a probable scenario in which the available evidence points to lower efficacy of adalimumab versus secukinumab. For this, results were calculated using discount rates ranging between 0 and 100% (in increments of 5%) on the notified ex-factory price for adalimumab. The results of the sensitivity analysis are shown for the total costs (first and second years) and for each of the clinical response.
ResultsBase caseThe results obtained show greater efficiency when secukinumab 150mg is used as the first biologic treatment for patients with PsA and the dose is subsequently increased in cases where clinical response is not achieved, compared to first biologic treatment with adalimumab 40mg and, in the event of inadequate response, switching to secukinumab 300mg. These results were consistent across the different periods analyzed, i.e., during the first and second years (and in total), at all levels of clinical response (ACR 20, 50 and 70) and using the efficacy data from the reports published by Nash P. et al.10 and Strand V. et al.11 (Table 4 and Table 5, respectively).
Cost of treatment for each hypothetical cohort of 10 patients considering the clinical response (ACR) extracted from Nash P. et al.10
| Cohort | Sequence | Year 1 | Year 2 | Total cohort | Difference between cohortsa | |
|---|---|---|---|---|---|---|
| Week 0–23 | Week 24–52 | |||||
| ACR 20 | ||||||
| Cohort A | Secukinumab 150mg | 47,582 € | 25,694 € | 51,389 € | 160,828 € | −79,878 € |
| Secukinumab 150mg→Secukinumab 300mg | 12,054 € | 24,108 € | ||||
| Cohort B | Adalimumab 40mg | 48,510 € | 32,259 € | 59,910 € | 240,706 € | |
| Adalimumab 40mg→Secukinumab 300mg | 45,467 € | 54,561 € | ||||
| ACR 50 | ||||||
| Cohort A | Secukinumab 150mg | 47,392 € | 18,589 € | 37,178 € | 181,193 € | −72,208 € |
| Secukinumab 150mg→Secukinumab 300mg | 16,998 € | 52,023 € | ||||
| Cohort B | Adalimumab 40mg | 48,510 € | 22,129 € | 41,096 € | 253,402 € | |
| Adalimumab 40mg→Secukinumab 300mg | 64,394 € | 77,273 € | ||||
| ACR 70 | ||||||
| Cohort A | Secukinumab 150mg | 47,582 € | 11,737 € | 23,474 € | 202,700 € | −61,978 € |
| Secukinumab 150mg→Secukinumab 300mg | 26,118 € | 79,938 € | ||||
| Cohort B | Adalimumab 40mg | 48,510 € | 13,130 € | 24,384 € | 264,678 € | |
| Adalimumab 40mg→Secukinumab 300mg | 81,207 € | 97,448 € | ||||
Cost of treatment for each hypothetical cohort of 10 patients considering the clinical response (ACR) extracted from Strand V. et al.11
| Cohort | Sequence | Year 1 | Year 2 | Total cohort | Difference between cohortsa | |
|---|---|---|---|---|---|---|
| Week 0–23 | Week 24–52 | |||||
| ACR 20 | ||||||
| Cohort A | Secukinumab 150mg | 47,582 € | 10,690 € | 21,380 € | 205,841 € | −44,653 € |
| Secukinumab 150mg→Secukinumab 300mg | 27,486 € | 84,125 € | ||||
| Cohort B | Adalimumab 40mg | 48,510 € | 24,449 € | 45,405 € | 250,494 € | |
| Adalimumab 40mg→Secukinumab 300mg | 60,059 € | 72,071 € | ||||
| ACR 50 | ||||||
| Cohort A | Secukinumab 150mg | 47,582 € | 8723 € | 17,447 € | 211,741 € | −47,760 € |
| Secukinumab 150mg→Secukinumab 300mg | 30,057 € | 91,992 € | ||||
| Cohort B | Adalimumab 40mg | 48,510 € | 17,261 € | 32,057 € | 259,501 € | |
| Adalimumab 40mg→Secukinumab 300mg | 73,488 € | 88,185 € | ||||
| ACR 70 | ||||||
| Cohort A | Secukinumab 150mg | 47,582 € | 5678 € | 11,356 € | 220,876 € | −43,305 € |
| Secukinumab 150mg→Secukinumab 300mg | 34,037 € | 104,173 € | ||||
| Cohort B | Adalimumab 40mg | 48,510 € | 13,526 € | 25,120 € | 264,182 € | |
| Adalimumab 40mg→Secukinumab 300mg | 80,466 € | 96,560 € | ||||
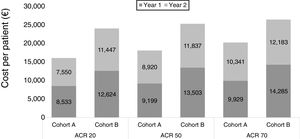
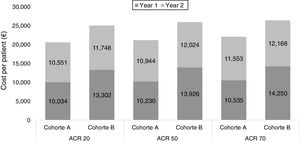
Specifically, using the study by Nash P. et al.10 as a source of efficacy data, we estimated the costs for cohort A were 33%, 28% and 23% lower than those for cohort B, for ACR 20, 50 and 70 response rates, respectively (Table 4, Fig. 3). When using the study by Strand V. et al.12 data, the cost of treatment for cohort A was 18%, 18% and 16% lower than that for cohort B, for ACR 20, 50 and 70 response rates, respectively (Table 5, Fig. 4).
Cost per patient in each cohort according to clinical response (ACR) extracted from Nash P. et al.10
Cost per patient in each cohort according to clinical response (ACR) extracted from Strand V. et al.11
The results of the sensitivity analysis show that it would be necessary to apply a discount of at least 60%, 65% or 75% to the notified ex-factory price of an adalimumab biosimilar (for ACR 20, 50 or 70, respectively) to compensate for the differences in efficacy observed in the study by Nash P. et al.10 (Supplementary material, Figure S1). When the report by Strand V. et al.11 was used, it was found that the discount should be equal to or greater than 40%, 40% or 50% (for ACR 20, 50 or 70, respectively) (Supplementary material, Figure S2). In both cases it should be assumed that the notified ex-factory price of secukinumab remains unchanged.
DiscussionPharmacoeconomics allows treatment options to be prioritized by taking into account both the health benefits and the economic impact of their use. This paper provides additional information to help decision-making before initiating biologic treatment for patients with PsA, showing the clinical and economic importance of making the appropriate choice with regard to the first line of therapy for a chronic disease that requires prolonged treatment and may result in a gradual ‘exhaustion’ of therapeutic alternatives.
The main results show that secukinumab is more efficient as first-line biologic treatment for patients with PsA compared to initiating treatment with adalimumab. The results obtained are sustained at every level of clinical response (ACR 20, 50 and 70) that was analyzed and by the two matching-adjusted indirect comparison studies of secukinumab and adalimumab that are published at present and were used as sources of scientific evidence.10,11 In particular, with regard to the results obtained for the efficacy outcomes ACR 50/70, which are those most often considered when assessing clinical response in routine clinical practice, the cost for cohort A was 28%/23% (Nash P. et al.10) and 18%/16% (Strand V. et al.11) lower than for cohort B.
Also, the sensitivity analysis performed allowed us to establish the threshold of the discount rate on the notified ex-factory price of adalimumab that would cause results to vary and make secukinumab less efficient than adalimumab. In this regard, we found that the notified ex-factory price of adalimumab would have to be at least 65%/75% lower than the current price for the least expensive biosimilar drug when we used the ACR 50/70 outcomes reported by Nash P. et al.10 and at least 40%/50% lower when we used the ACR 50/70 outcomes reported by Strand V. et al.,11 reflecting that the efficacy of secukinumab for the treatment of PsA weighs more heavily than the cost when comparing its efficiency to that of adalimumab. If the cost of secukinumab were to decrease, these percentages would no longer be valid and would have to be recalculated, since the discount on the price of adalimumab would have to be even greater.
These findings are based on the available evidence and are consistent with recent recommendations by the SER.8 On the one hand, the available evidence suggests that the response to treatment of biologic-naive patients will be superior to that of patients who have previously failed an TNFi, so the hoped-for efficacy will always be better the sooner a biologic drug is used, regardless of which one. On another hand, we have demonstrated that initiating treatment with secukinumab 150mg and increasing the dose in the event of inadequate response is less costly than initiating treatment with adalimumab 40mg and then switching to secukinumab after failure. The flexibility in the choice of the dose provided by secukinumab, by starting at 150mg in treatment-naive patients with the possibility of increasing to a dose of 300mg if there is a lack of primary response, is considered a big advantage when it comes to achieving efficiency in treatment. Since a priori higher costs are expected, given the induction phase that is required when secukinumab is initiated, whether for biologic-naive patients receiving secukinumab 150mg or for patients who have had an inadequate response to an TNFi and switch to secukinumab 300mg, using it sooner in naïve patients provides benefits in efficiency in terms of cost of treatment and in efficacy in terms of clinical response, and therefore costs avoided with respect to using it after adalimumab failure.
This analysis has some limitations. Firstly, since no published clinical trial at the time of the analysis has directly compared secukinumab and adalimumab in PsA patients, this study relied on two available matching-adjusted indirect comparison studies. With regard to the specific sequences that were analyzed, although recent recommendations for the treatment of PsA8 include switching to another biologic treatment (IL12/23i or IL17i or a targeted DMARD (apremilast)) after TNFi failure, the only indirect comparisons that have been published are for the two drugs we analyze here. Also, we did not consider switching from secukinumab to adalimumab, as there is no evidence to support its use after the failure of IL17i. Secondly, we did not analyze the clinical response of patients whose dose was increased or who switched treatments at week 24. Nevertheless, the analysis structure responds to the study's objective of assessing the efficiency of two possible options for initiating biologic treatment and this allowed us to simplify the analysis and obtain results for that change, whereas if we had included possible benefits of the subsequent therapy, it could have masked the results that were the objective of the study. Finally, a potential limitation may be the use of the notified ex-factory prices in the analysis, as it is usually somewhat higher than the final cost of drugs incurred by SNHS hospitals.
Despite the limitations identified above, the results of this cost-consequence analysis offer valuable information for decision-making and highlight the importance of making an appropriate choice of the first biologic treatment option for patients with PsA, since it may prove decisive both for clinical outcomes as well as for potential subsequent alternatives and economic impact on the health system. In any event, this analysis seeks to support health professionals in real-life situations who must make decisions about the choice of certain treatments based on the available scientific evidence and incorporating efficiency as another criterion. Future research may shed more light and provide for further analysis. Decision-making should be based on evidence and updated as knowledge increases. In this sense, this cost-consequences analysis based on matching-adjusted indirect comparison studies shows that the choice of secukinumab as first biologic treatment for patients with PsA may be considered a more efficient option with respect to adalimumab from the perspective of the SNHS.
FundingThis work was supported by Novartis Farmacéutica, S.A.