This study aimed to assess the agreement between cardiovascular risk scores in patients with rheumatoid arthritis (RA).
MethodsWe conducted a cross-sectional study of adult patients with RA at the Hospital Nacional Adolfo Guevara Velasco in Cusco-Peru in 2024. The 2019 World Health Organization cardiovascular risk score (2019-WHO-CRS), Framingham risk score (FRS), and Expanded cardiovascular Risk prediction Score for Rheumatoid Arthritis (ERS-RA) were used to estimate the 10-year risk of cardiovascular disease. Agreement was assessed through Bland–Altman plots and Kappa statistics.
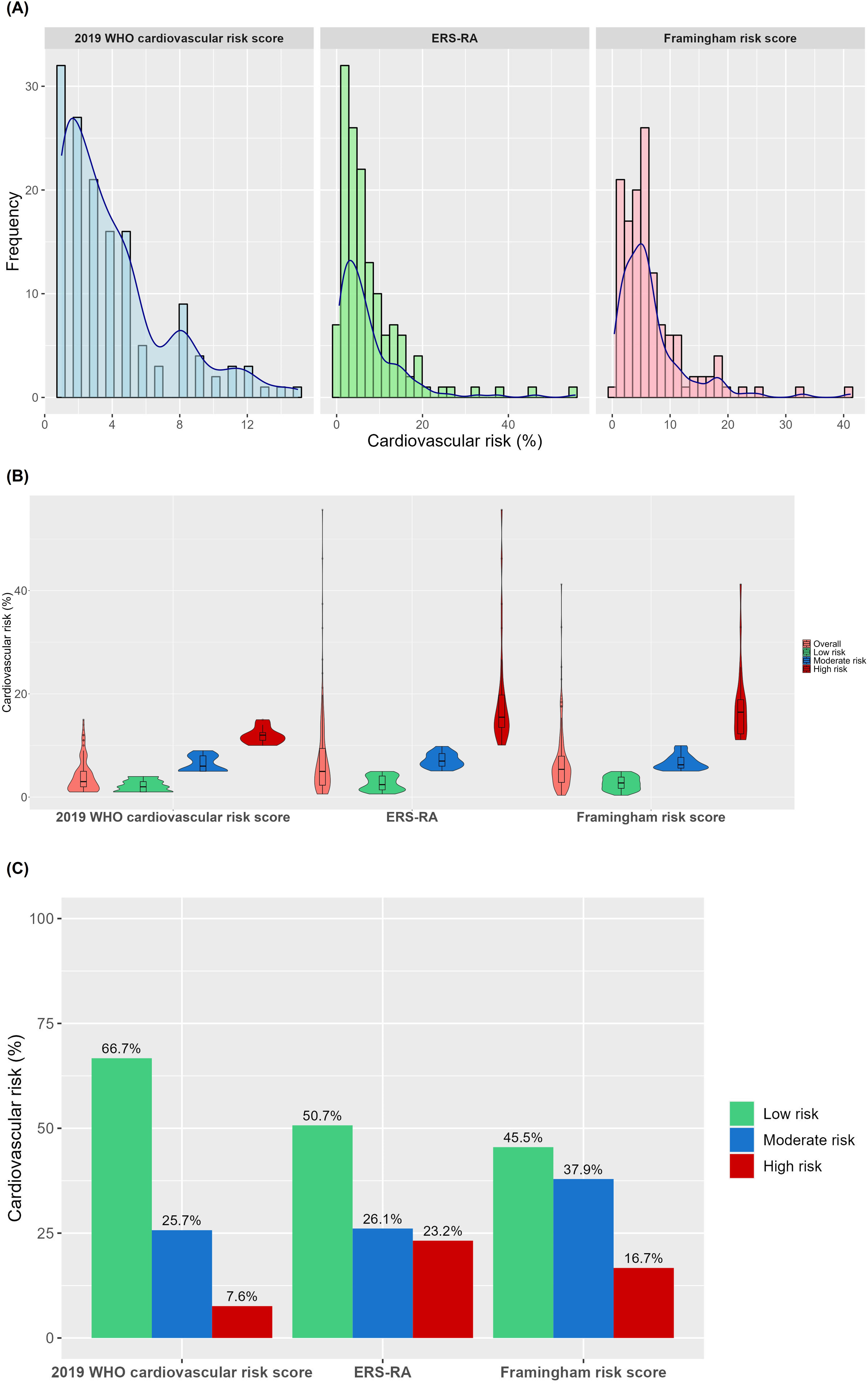
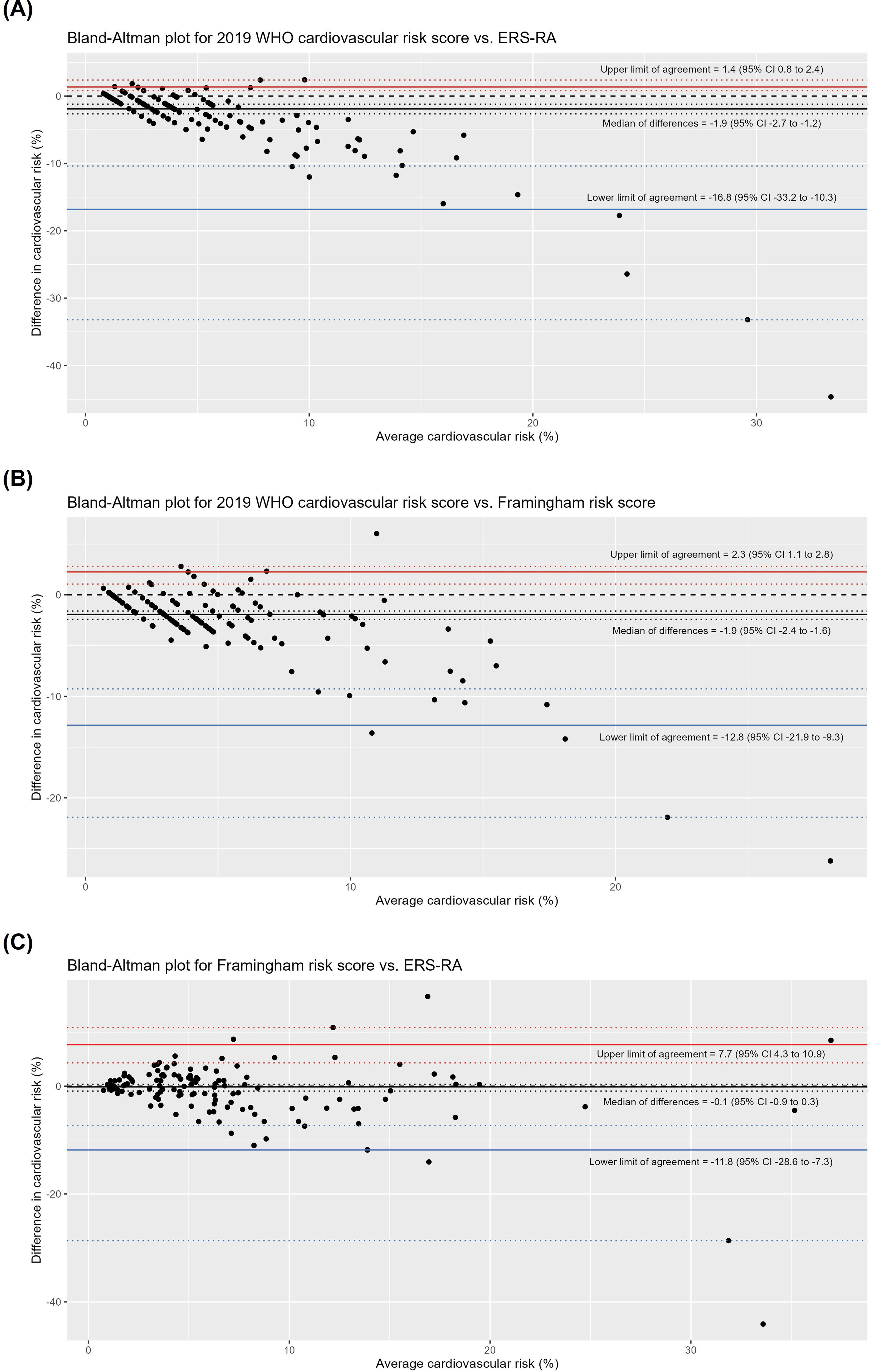
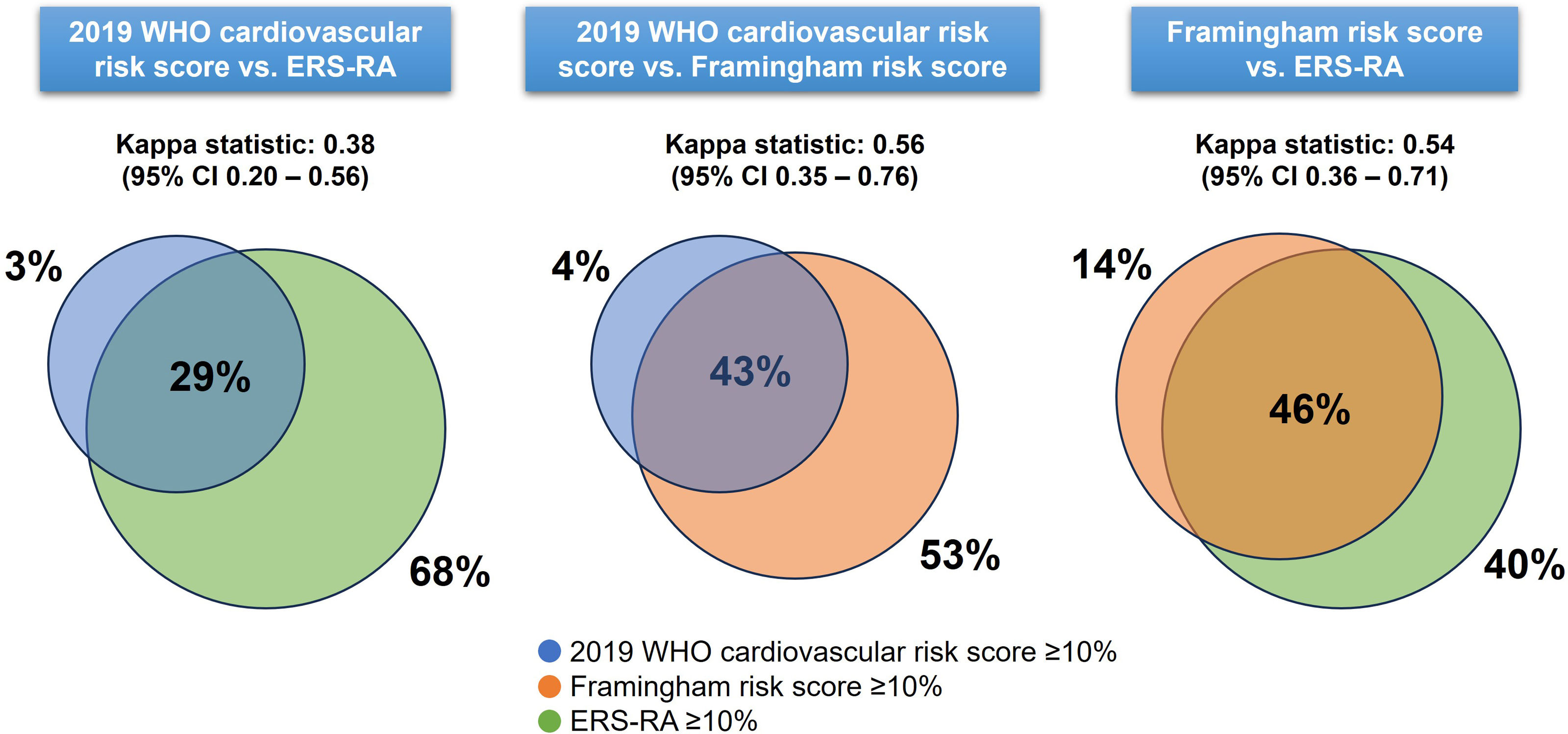
ResultsA total of 145 patients were included. The median age was 56 years (47–65) and 92% were female. The median scores using the 2019-WHO-CRS was 3% (2–5), FRS was 5.4% (2.8–7.9), and ERS-RA was 5% (2.3–9.4). Using a cut-off point >10%, the proportion of patients with high cardiovascular risk was 7.6%, 16.7%, and 23.2% for 2019-WHO-CRS, FRS, and ERS-RA, respectively. In the Bland–Altman plots, the limits of agreement were wide between risk scores (−16.8% to 1.4% for 2019-WHO-CRS vs. ERS-RA, −12.8% to 2.3% for 2019-WHO-CRS vs. FRS, and −11.8% to 7.7% for FRS vs. ERS-RA). The highest agreement (Kappa statistic: 0.56) in predicting high risk was between 2019-WHO-CRS and FRS scores. Our results suggest that there was disagreement between the 2019-WHO-CRS, FRS, and ERS-RA cardiovascular risk scores in an Andean population with RA.
ConclusionThe identification of patients at high cardiovascular risk varied considerably among the scores, with the ERS-AR yielding the highest values. Further prospective studies evaluating the prognostic performance of these scores are needed.
Este estudio tuvo como objetivo evaluar la concordancia entre las puntuaciones de riesgo cardiovascular en pacientes con artritis reumatoide (AR).
MétodoSe realizó un estudio transversal de pacientes adultos con AR en el Hospital Nacional Adolfo Guevara Velasco de Cusco-Perú en el año 2024. Se utilizó el puntaje de riesgo cardiovascular de la Organización Mundial de la Salud 2019 (2019-WHO-CRS), el puntaje de riesgo de Framingham (FRS) y el puntaje de predicción de riesgo cardiovascular expandido para la artritis reumatoide (ERS-RA) para estimar el riesgo de enfermedad cardiovascular a 10años. La concordancia se evaluó mediante gráficos de Bland-Altman y el estadístico Kappa.
ResultadosSe incluyó a un total de 145 pacientes. La mediana de edad era de 56años (47-65) y el 92% eran mujeres. La mediana de las puntuaciones utilizando el 2019-WHO-CRS fue del 3% (2-5), el FRS fue del 5,4% (2,8-7,9) y el ERS-RA fue del 5% (2,3-9,4). Utilizando un punto de corte >10%, la proporción de pacientes con alto riesgo cardiovascular fue del 7,6%, del 16,7% y del 23,2% para 2019-WHO-CRS, FRS y ERS-RA, respectivamente. En los gráficos de Bland-Altman, los límites de concordancia fueron amplios entre las puntuaciones de riesgo (−16,8% a 1,4% para 2019-WHO-CRS frente a ERS-RA, −12,8% a 2,3% para 2019-WHO-CRS frente a FRS, y −11,8% a 7,7% para FRS frente a ERS-RA). El mayor acuerdo (estadística Kappa: 0,56) en la predicción de alto riesgo se produjo entre las puntuaciones 2019-WHO-CRS y FRS. Nuestros resultados sugieren que hubo desacuerdo entre los puntajes de riesgo cardiovascular 2019-WHO-CRS, FRS y ERS-RA en una población andina con AR.
ConclusionesLa identificación de pacientes con alto riesgo cardiovascular varió considerablemente entre los scores, siendo el ERS-AR el que arrojó los valores más altos. Se necesitan más estudios prospectivos que evalúen el rendimiento pronóstico de estas puntuaciones.
Rheumatoid arthritis (RA) is a chronic inflammatory condition that not only affects joints but also increases the risk of cardiovascular diseases.1,2 Patients with RA are at higher cardiovascular risk likely due to a combination of traditional and non-traditional risk factors.3 This has prompted a growing interest in improving cardiovascular risk prediction tools specified for RA, as traditional models may underestimate the risk in these patients.3,4
Existing cardiovascular risk prediction scores were primarily developed and validated for the general population.5 However, these models perform suboptimally in patients with RA, probably because they do not include RA-specific factors that also provide prognostic information on cardiovascular risk.6 Thus, more specific tools, such as the Expanded cardiovascular Risk prediction Score for Rheumatoid Arthritis (ERS-RA), have been developed to better capture the increased risk in patients with RA.7 While ERS-RA has shown promising results in European cohorts, its utility in low-resource settings or high-altitude regions remains underexplored. Understanding the concordance between these risk scores is critical to determine whether they provide a comparable estimate of cardiovascular risk and whether they similarly stratify high-risk patients who might benefit from preventive interventions (e.g. statins).4 We aimed to evaluate the concordance between three cardiovascular risk scores (ERS-RA and two general risk scores) in an Andean population with RA. By comparing the scores, the study seeks to identify potential discrepancies in risk stratification, particularly for patients classified as high risk.
MethodsStudy design and populationWe conducted a cross-sectional study at the Hospital Nacional Adolfo Guevara Velasco, Cusco, Peru, between January and May 2024. The study included adult patients (≥18 years) with a confirmed diagnosis of RA, according to the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria.8 Patients were consecutively recruited from the rheumatology outpatient clinic. Patients with a history of cardiovascular disease were excluded.
Data collectionData were obtained through structured interviews and physical examination which were collected by the research team. Laboratory data were extracted from medical records. Demographic information, such as age, sex, education level, and civil status, was collected. Clinical variables included comorbidities (e.g., hypertension, diabetes, dyslipidemia), smoking status, alcohol consumption, body mass index (BMI), blood pressure measurements, and disease duration. RA-specific characteristics, including the RA-specific treatment, disease activity score in 28 joints (DAS-28), the modified health assessment questionnaire (mHAQ) score, and the presence of bone erosions or subcutaneous nodules, were recorded. Laboratory data, including glucose, creatinine, lipid profile, uric acid, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), rheumatoid factor, and anti-cyclic citrullinated peptide (anti-CCP) levels, were also obtained.
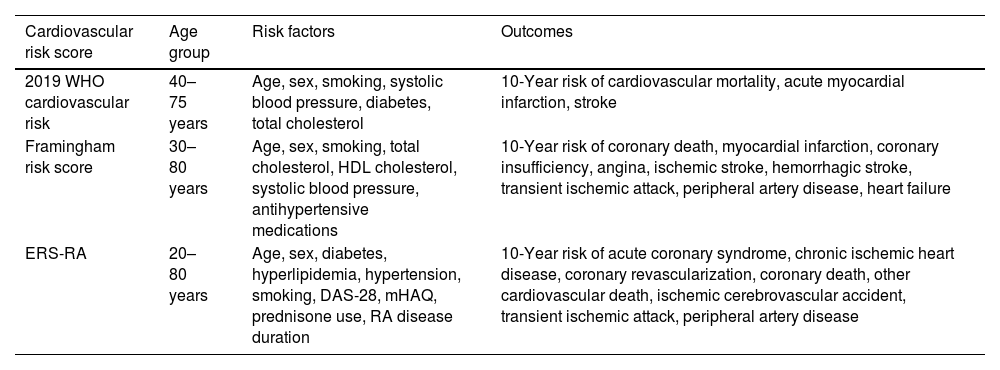
Cardiovascular risk scoresThe following three risk scores were used to estimate the 10-year risk of cardiovascular disease: the Framingham risk score (FRS),9 the 2019 World Health Organization (WHO) cardiovascular risk score,10 and the ERS-RA (Table 1).11 The FRS was calculated based on age, sex, smoking status, total cholesterol, HDL cholesterol, diabetes, hypertension treatment, and systolic blood pressure, as described by Wilson et al.9 There are two versions of the 2019 WHO cardiovascular risk score (laboratory-based and non-laboratory based), of which we use the first one that incorporates information on age, sex, smoking status, systolic blood pressure, total cholesterol, and diabetes status. The ERS-RA included RA-specific variables (such as DAS-28 score, mHAQ score, and duration of disease) in addition to traditional cardiovascular risk factors, and was calculated using the algorithm proposed by Crowson et al.11 Patients were stratified into low risk (<5%), moderate risk (5 to <10%), and high risk (≥10%) groups for each score.
Characteristics of cardiovascular risk scores.
| Cardiovascular risk score | Age group | Risk factors | Outcomes |
|---|---|---|---|
| 2019 WHO cardiovascular risk | 40–75 years | Age, sex, smoking, systolic blood pressure, diabetes, total cholesterol | 10-Year risk of cardiovascular mortality, acute myocardial infarction, stroke |
| Framingham risk score | 30–80 years | Age, sex, smoking, total cholesterol, HDL cholesterol, systolic blood pressure, antihypertensive medications | 10-Year risk of coronary death, myocardial infarction, coronary insufficiency, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral artery disease, heart failure |
| ERS-RA | 20–80 years | Age, sex, diabetes, hyperlipidemia, hypertension, smoking, DAS-28, mHAQ, prednisone use, RA disease duration | 10-Year risk of acute coronary syndrome, chronic ischemic heart disease, coronary revascularization, coronary death, other cardiovascular death, ischemic cerebrovascular accident, transient ischemic attack, peripheral artery disease |
WHO: World Health Organization; ERS-RA: Expanded cardiovascular Risk prediction Score for Rheumatoid Arthritis.
The study was approved by the ethics committee of the Hospital Nacional Adolfo Guevara Velasco (approval date: October 02, 2023; code: CE/070-10-23). Written informed consent was obtained from all participants prior to enrollment in the study, in accordance with the principles of the Declaration of Helsinki. The confidentiality of the information was guaranteed and only the authors had access to the data.
Statistical analysisDescriptive statistics were used to summarize the demographic and clinical characteristics of the population. Continuous variables were expressed as median (percentile 25–percentile 75) and categorical variables as frequencies and percentages. Bland–Altman plots were generated to evaluate the agreement between the score. We used a nonparametric method to estimate the limits of agreement because the differences between scores did not meet the assumption of normality (Fig. S1). In addition, 95% confidence intervals (CI) for the median of differences and limits of agreement were estimated. Kappa statistics with their 95% CI were also estimated to assess the concordance between the classification of patients as high risk according to the pairwise comparison of cardiovascular risk scores. All analyses were performed using the R 4.4.1 software (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p-value <0.05 was considered statistically significant.
ResultsStudy populationA total of 145 patients were included in the study. The median age was 56 (47–65) years, and the majority were female (91.7%). Most participants had a superior level of education (62%) and were married (48.2%) (Table 2). The most prevalent comorbidities included hypertension (16.7%), dyslipidemia (4.9%), and diabetes (4.2%). Furthermore, 24.3% of patients reported a family history of autoimmune diseases. In terms of treatment, 96.5% of patients were receiving synthetic disease-modifying antirheumatic drugs (DMARDs), and 68.1% were on corticoids (Table 2). The median duration of RA was 10 (3–20) years, 56% of patients had DAS-28 score ≥3.2, and 58.3% had a mHAQ score >0.3.
Characteristics of included patients (n=145).
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| Median (p25–p75) | 56 (47–65) |
| Range | 23–82 |
| Sex | |
| Female | 132 (91.7%) |
| Male | 12 (8.3%) |
| Education level | |
| None | 2 (1.4%) |
| Primary | 14 (9.9%) |
| Secondary | 38 (26.8%) |
| Superior | 88 (62.0%) |
| Civil status | |
| Single | 32 (22.7%) |
| Cohabitant | 23 (16.3%) |
| Married | 68 (48.2%) |
| Widowed | 11 (7.8%) |
| Divorced | 7 (5.0%) |
| Comorbidities | |
| Hypertension | 24 (16.7%) |
| Diabetes | 6 (4.2%) |
| Dyslipidemia | 7 (4.9%) |
| Smoking | 9 (6.3%) |
| Alcohol consumption | 17 (11.8%) |
| Asthma | 6 (4.2%) |
| COPD | 4 (2.9%) |
| Cancer | 5 (3.5%) |
| Hypothyroidism | 15 (10.4%) |
| Atrial fibrillation | 3 (2.1%) |
| Family history of CAD | 5 (3.5%) |
| Previous fracture | 20 (14.0%) |
| Family history of autoimmune disease | 35 (24.3%) |
| Medications | |
| NSAIDs | 88 (61.1%) |
| Corticoids | 98 (68.1%) |
| Synthetic DMARDs | 139 (96.5%) |
| Biologic DMARDs | 9 (6.3%) |
| Antiplatelets | 7 (4.9%) |
| Statins | 6 (4.2%) |
| Body mass index (kg/m2) | |
| Median (p25–p75) | 25.1 (22.7–27.6) |
| Range | 15.2–37.2 |
| Systolic blood pressure (mmHg) | |
| Median (p25–p75) | 117 (109–128) |
| Range | 87–177 |
| Diastolic blood pressure (mmHg) | |
| Median (p25–p75) | 73 (68–80) |
| Range | 39–115 |
| Duration of disease (years) | |
| Median (p25–p75) | 10 (3–20) |
| Range | 0–50 |
| Bone erosion | 22 (15.6%) |
| Subcutaneous nodules | 6 (4.2%) |
| DAS-28 score | |
| Median (p25–p75) | 3.34 (2.69–4.11) |
| Range | 1.55–5.71 |
| DAS-28 ≥3.2 | 75 (56.0%) |
| mHAQ score | |
| Median (p25–p75) | 0.38 (0.13–0.75) |
| Range | 0.00–2.75 |
| mHAQ >0.3 | 84 (58.3%) |
| Laboratory | |
| Glucose (mg/dL) | |
| Median (p25–p75) | 93 (86–99) |
| Range | 68–147 |
| Creatinine (mg/dL) | |
| Median (p25–p75) | 0.67 (0.58–0.77) |
| Range | 0.35–1.27 |
| Total cholesterol (mg/dL) | |
| Median (p25–p75) | 177 (153–207) |
| Range | 87–399 |
| LDL cholesterol (mg/dL) | |
| Median (p25–p75) | 111 (91–137) |
| Range | 42–248 |
| HDL cholesterol (mg/dL) | |
| Median (p25–p75) | 47 (41–57) |
| Range | 31–170 |
| Triglycerides (mg/dL) | |
| Median (p25–p75) | 118 (92–168) |
| Range | 54–361 |
| Uric acid (mg/dL) | |
| Median (p25–p75) | 3.73 (2.99–4.50) |
| Range | 1.97–7.27 |
| C-reactive protein (mg/dL) | |
| Median (p25–p75) | 0.40 (0.20–1.06) |
| Range | 0.04–15.24 |
| Erythrocyte sedimentation rate (mm/h) | |
| Median (p25–p75) | 23 (17–32) |
| Range | 2–56 |
| Rheumatoid factor (UI/mL) | |
| Median (p25–p75) | 151 (76–395) |
| Range | 2–886 |
| Anti-cyclic citrullinated peptide (UI/mL) | |
| Median (p25–p75) | 780 (251–1000) |
| Range | 0–1000 |
| Cardiovascular risk scores | |
| 2019 WHO cardiovascular risk | |
| Median (p25–p75) | 3 (2–5) |
| Range | 1–15 |
| Framingham risk score | |
| Median (p25–p75) | 5.4 (2.8–7.9) |
| Range | 0.4–41.2 |
| ERS-RA | |
| Median (p25–p75) | 5.0 (2.3–9.4) |
| Range | 0.6–55.6 |
COPD: chronic obstructive pulmonary disease; CAD: coronary artery disease; NSAIDs: non-steroidal anti-inflammatory drugs; DMARDs: disease-modifying antirheumatic drugs; DAS-28: disease activity score in 28 joints; mHAQ: modified health assessment questionnaire; WHO: World Health Organization; ERS-RA: Expanded cardiovascular Risk prediction Score for Rheumatoid Arthritis.
The median 10-year cardiovascular risk scores were 3% (2–5) using the 2019 WHO cardiovascular risk score, 5.4% (2.8–7.9) using the FRS, and 5% (2.3–9.4) using the ERS-RA (Table 1). The distribution of risk categories varied across the different scores (Fig. 1). The ERS-RA identified the highest proportion of high-risk patients (23.2%), compared to 16.7% with the FRS and 7.6% with the 2019 WHO cardiovascular risk score (Fig. 1).
Agreement between cardiovascular risk scoresBland–Altman plots showed disagreement between cardiovascular risk scores, particularly in the identification of high-risk patients (Fig. 2). The median of the difference between the 2019 WHO cardiovascular risk score and the ERS-RA was −1.9% (95% CI −2.7 to −1.2), between the 2019 WHO cardiovascular risk score and the FRS was −1.9% (95% CI −2.4 to −1.6), and between the FRS and the ERS-RA was −0.1% (95% CI −0.9 to 0.3) (Fig. 2). The limits of agreement were wide between risk scores: −16.8% to 1.4% for 2019-WHO-CRS vs. ERS-RA, −12.8% to 2.3% for 2019-WHO-CRS vs. FRS, and −11.8% to 7.7% for FRS vs. ERS-RA (Fig. 2). The highest agreement in predicting patients at high risk was between the 2019 WHO cardiovascular risk vs. FRS (Kappa statistic: 0.56, 95% CI 0.35–0.76) and between FRS vs. ERS-RA (Kappa statistic: 0.54, 95% CI 0.36–0.71) (Fig. 3).
This study assessed the agreement between three cardiovascular risk scores in a cohort of patients with RA from a high-altitude Andean population. In terms of clinical significance, our findings revealed disagreement between the assessed risk scores, particularly in identifying patients at high cardiovascular risk, with the ERS-RA consistently predicting higher risk estimates. Overall, the agreement in predicting high risk was moderate, with maximum agreement between the 2019 WHO cardiovascular risk and FRS scores.
Our findings highlight the challenges in cardiovascular risk stratification in patients with RA. Traditional risk models, such as the FRS, tend to underestimate cardiovascular risk in this population due to the exclusion of RA-related characteristics, such as disease activity and the use of corticosteroids, which have been shown to contribute to increased cardiovascular risk in patients with RA.12 The ERS-RA, which includes RA-specific variables,11 has demonstrated a higher sensitivity in detecting high-risk individuals. Similarly, in a Nigerian RA cohort (n=85),13 the ERS-RA score was found to significantly identify a higher proportion of high-risk patients (42%) compared with FRS (18%) and QRISK3 (15%), showing fair to moderate agreement with both scores. In contrast, in another large cohort of RA patients from seven different countries (n=1796),7 it was found that despite the inclusion of RA-specific factors, most patients had lower cardiovascular risk estimates by ERS-RA (mean 8.8%) compared to FRS (9.1%) and QRISK2 (15.5%). The discrepancy between the two studies is probably due to differences in population characteristics and health infrastructure.13 In contrast, the multinational cohort may include populations with better controlled RA and lower disease activity, as well as better control of traditional cardiovascular risk factors, resulting in lower cardiovascular risk estimates by ERS-RA.7
Overall, a tailored approach to cardiovascular risk stratification in RA patients is warranted.14,15 Underestimating cardiovascular risk in patients with RA using traditional models like the FRS could result in missed opportunities for preventive interventions, such as statin therapy.14 Conversely, overestimation by ERS-RA might lead to unnecessary treatment in patients who are not truly at high risk. In addition, the influence of chronic hypoxia at high altitudes may exacerbate the cardiovascular risk in RA, potentially amplifying the inflammatory effects already present.16 Some reports have shown that patients living at high altitudes are at increased risk of developing RA or having exacerbations due to environmental factors and increased expression of hypoxia-inducible factor.16,17 This may also contribute to the higher cardiovascular risk estimate found using the ERS-RA score. However, it is important to note that some studies, such as the work by Mallet et al.,18 have reported potential protective effects of high-altitude residence on cardiovascular health. These differences may arise from varying population characteristics, the duration of altitude exposure, or specific comorbid conditions.
Our study highlights the need for prospective studies to evaluate the actual prognostic performance of cardiovascular risk scores in patients with RA. Given the significant variability in risk classification between the scores, future research should focus on refining existing RA-specific models to improve accuracy while avoiding overestimation.19–21 Additionally, further exploration of the impact of environmental factors, such as altitude, is also needed. Incorporating such factors may enhance the precision of risk assessment tools for these populations.
A major strength of our study is the inclusion of a well-characterized cohort of RA patients from a unique geographic region and the evaluation of general and specific risk scores for RA. In addition, the variables included in the risk models were measured as they would be assessed in a real-world setting. However, some limitations should be noted. The relatively small sample size of our study, while adequate for preliminary analyses, may limit the statistical power and precision of our findings, particularly in detecting subtle differences in agreement between cardiovascular risk scores. The cross-sectional design of our study limits the ability to assess the predictive value of these risk scores for long-term cardiovascular events. Furthermore, the study was conducted at a single center, which may limit the generalizability to broader populations or different settings, particularly to non-Andean populations. Another limitation of our study is the absence of a gold standard measure, such as carotid intima-media thickness (CIMT), for cardiovascular risk assessment. Without such a reference, it is not possible to determine which of the evaluated cardiovascular risk scores provides the most accurate estimation of risk. Future studies incorporating direct measures of subclinical atherosclerosis, like CIMT or coronary artery calcium, would be valuable to validate and compare the performance of these risk scores in patients with RA. Finally, the lack of longitudinal data restricts our understanding of how cardiovascular risk may evolve in patients with RA, particularly in relation to disease progression or adjustments in treatment.
ConclusionsIn an Andean high-altitude population with RA, our results suggest that the 2019 WHO cardiovascular risk score, ERS-RA, and FRS showed clinically significant disagreement in the estimation of absolute cardiovascular risk at 10 years, especially when the estimates were high. The identification of high-risk patients varied between risk scores, with the ERS-RA score resulting in the highest risk estimates.
Ethical responsibilitiesApproved by the committee of the Hospital Nacional Adolfo Guevara Velasco (approval date: October 02, 2023; code: CE/070-10-23).
FundingThis study was funded by the research grant awarded by the Red Asistencial EsSalud Cusco in the “IV Concurso de Fomento del Desarrollo de Investigación en Salud”.
Conflict of interestThe authors declare that they have no competing interests.