Different prediction rules have been applied to patients with undifferentiated arthritis (UA) to identify those that progress to rheumatoid arthritis (RA). The Leiden Prediction Rule (LPR) has proven useful in different UA cohorts.
ObjectiveTo apply the LPR to a cohort of patients with UA of northeastern Mexico.
MethodsWe included 47 patients with UA, LPR was applied at baseline. They were evaluated and then classified after 1 year of follow-up into 2 groups: those who progressed to RA (according to ACR 1987) and those who did not.
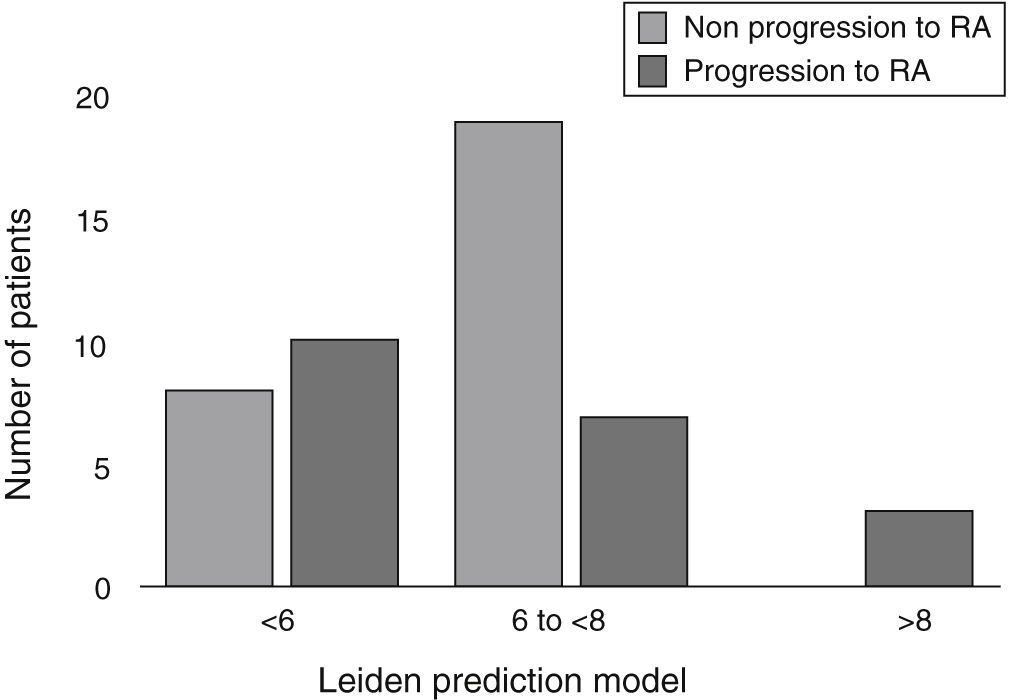
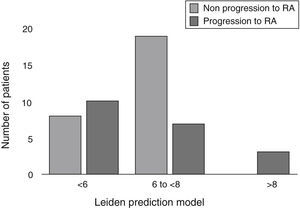
Results43% of the AI patients developed RA. In the RA group, 56% of patients obtained a score ≤6 and only 15% ≥8. 70% who did not progress to RA had a score between 6 and ≤8. There was no difference in median score of LPR between groups, P=.940.
ConclusionMost patients who progressed to RA scored less than 6 points in the LPR. Unlike what was observed in other cohorts, the model in our population did not allow us to predict the progression of the disease.
Distintos modelos de predicción han sido aplicados en pacientes con artritis indiferenciada (AI) con el objetivo de identificar a aquellos que progresarán a artritis reumatoide (AR). El modelo de predicción de Leiden (MPL) ha demostrado su utilidad en distintas cohortes de AI.
ObjetivoAplicar el MPL a una cohorte de pacientes con AI del noreste de México.
MétodosSe incluyó a 47 pacientes con AI; al ingreso se aplicó el MPL, después de un año de seguimiento se clasificaron en 2 grupos: los que progresaron a AR (de acuerdo con los criterios ACR 1987) y los que no progresaron.
ResultadosEl 43% de los pacientes con AI progresó a AR. De los pacientes que progresaron a AR, el 56% obtuvo una puntuación ≤ 6 y solo el 15% ≥ 8 puntos. El 70% de los que no progresaron alcanzaron una puntuación entre 6 y ≤ 8. No existió diferencia en la mediana de la puntuación del MPL entre los grupos, p=0,940.
ConclusiónLa mayoría de los pacientes que progresó a AR obtuvieron menos de 6 puntos en el MPL. A diferencia de lo observado en otras cohortes, en nuestra población el modelo no permitió predecir la progresión de la enfermedad.
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by progressive joint deterioration that can generate permanent disability, which affects the patient's quality of life and economy due to absenteeism and the high cost of treatment.1 Diagnosis and treatment in its early stages prevent disease progression.2 Identification of RA patients at increased risk of progression is critical to the rapid onset of treatment and is one of the objectives of the Early Arthritis Clinic (EAC), which usually include patients with undifferentiated arthritis (UA), a type of arthritis defined as not meeting criteria for classifying as a specific inflammatory disease.3 Patients with UA can progress into remission, remain as UA, or progress to a definite inflammatory disease such as RA.4 Different prediction models have been developed to evaluate patients with UA who are at risk of developing RA5,6; Leiden's University developed a prediction model (LPM) to identify these patients with a previously described methodology.7 The LPM includes the following clinical variables: age, gender, distribution of affected joints, severity of morning stiffness, painful and swollen joints, measurement of C-reactive protein (CRP), the presence of rheumatoid factor (RF), and anti-cyclic citrullinated peptide antibodies (anti-CCP). This model establishes an 84% probability of progression to RA in the first year in patients presenting a baseline assessment equal to or greater than 8. The LPM has been applied and validated in several cohorts in the world, with different inclusion criteria.8–10 In Mexico, there are no reports of the application of this model, so the objective of this study was to determine if the LPM is useful in our population to predict progression to UA and RA.
Materials and MethodsPatients belonging to a EAC established in a university tertiary hospital, serving patients in northeastern Mexico were selected. The EAC includes individuals over 18 years, from an epidemiological study of the state of Nuevo Leon, performed using the Community Oriented Program for the Control of the Rheumatic Diseases, COPCORD) methodology, which has been previously described,11 and by patients who are referred from other hospitals. Patients being admitted to the EAC, are classified into one of the following 3 groups: those with RA of less than 1 year since onset of symptoms, those with UA defined by the presence of 1 or more swollen joints for more than a week and less than 1 year of evolution, and another group of patients at high risk of arthritis (joint pain patients without the presence of arthritis). Patients who meet well-defined criteria for classification of other inflammatory diseases at admission are excluded. Two certified rheumatologists assess patients in the EAC at each visit, in which physical examination is performed with evaluation of swollen and tender joints.
In this study, between 2008 and 2011, we included only patients with UA who were administered the LPM upon admission to the clinic. All patients underwent, on the baseline visit, RF determined by nephelometry (Behring Nephelometer laser no. 441197/71160) and anti-CCP antibodies by first generation ELISA (EUROINMUN®), and at each assessment we determined the erythrocyte sedimentation rate and CRP.
Later, at 1 year, patients were reclassified on the basis of progression or not to RA according to 1987 classification criteria of the American College of Rheumatology.12
Statistical analysis: numeric variables were expressed as mean±standard deviation or median and interquartile range (IQR), according to their distribution. Categorical variables were expressed as percentages. A bivariate analysis of the numerical variables comparing them with the Student's t test or the Mann Whitney as a function of its distribution, was performed. For categorical variables, the chi-square test was used. The LPM was analyzed by comparing the medians of the scores obtained with the test of Mann–Whitney U; subsequently, patients were subdivided according to the result (more or less than 8 points) and evaluated by the Kaplan–Meier survival curve after a year to progress to RA. Finally, a plot of distribution was performed in accordance with the LPM score obtained and whether or not the patient progressed to RA.
ResultsThe study included 47 patients with UA, with a mean age of 51.6±9.5 years, 46 patients (98%) were women; 42 (89%) patients came from the COPCORD study. At follow-up, 20 patients (43%) had developed RA (RA group) and 27 (57%) did not progress to RA (non-RA group). In the non-RA group, 6 patients (13%) had resolution of arthritis, 12 patients (25%) persisted with UA, 9 patients (19%) met criteria for classification of other rheumatic diseases (6 with osteoarthritis, 1 with fibromyalgia, 1 with Sjögren's syndrome and 1 enteropathic arthritis).
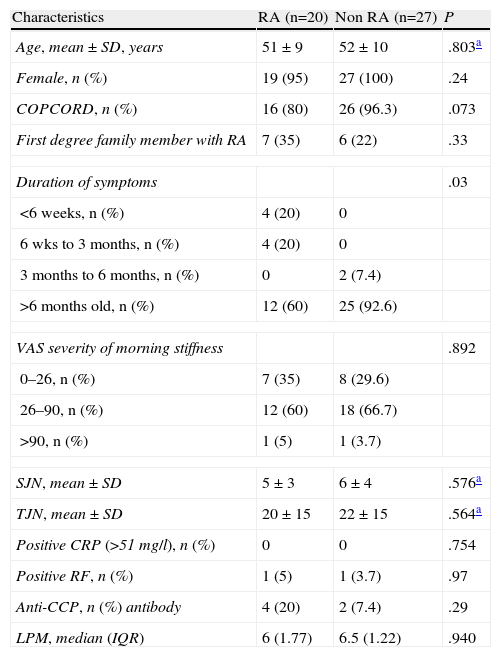
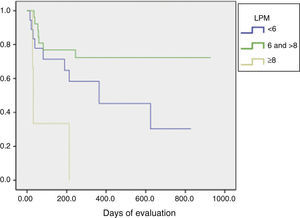
Baseline characteristics of patients and the bivariate analysis are shown in Table 1. When evaluating the score for LPM, in the RA group we found: 10 patients (50%) with a score <6, 7 patients (35%) with a score between 6 and <8, and finally 3 patients (15%) with ≥8 points. In the non-RA group we found: 8 patients (30%) with <6 points, 19 patients (70%) with a score between 6 and <8, and none with 8 or more points (Fig. 1). The RA group had a mean LPM score of 6 (IQR 1.77), whereas in the non-RA group the score was 6.5 (RIQ 1.22) (P=.940).
Baseline Characteristics and Bivariate Analysis.
| Characteristics | RA (n=20) | Non RA (n=27) | P |
| Age, mean±SD, years | 51±9 | 52±10 | .803a |
| Female, n (%) | 19 (95) | 27 (100) | .24 |
| COPCORD, n (%) | 16 (80) | 26 (96.3) | .073 |
| First degree family member with RA | 7 (35) | 6 (22) | .33 |
| Duration of symptoms | .03 | ||
| <6 weeks, n (%) | 4 (20) | 0 | |
| 6 wks to 3 months, n (%) | 4 (20) | 0 | |
| 3 months to 6 months, n (%) | 0 | 2 (7.4) | |
| >6 months old, n (%) | 12 (60) | 25 (92.6) | |
| VAS severity of morning stiffness | .892 | ||
| 0–26, n (%) | 7 (35) | 8 (29.6) | |
| 26–90, n (%) | 12 (60) | 18 (66.7) | |
| >90, n (%) | 1 (5) | 1 (3.7) | |
| SJN, mean±SD | 5±3 | 6±4 | .576a |
| TJN, mean±SD | 20±15 | 22±15 | .564a |
| Positive CRP (>51mg/l), n (%) | 0 | 0 | .754 |
| Positive RF, n (%) | 1 (5) | 1 (3.7) | .97 |
| Anti-CCP, n (%) antibody | 4 (20) | 2 (7.4) | .29 |
| LPM, median (IQR) | 6 (1.77) | 6.5 (1.22) | .940 |
Statistical analysis performed using chi squared test.
Anti-CCP: anti-cyclic citrullinated peptide; RA: rheumatoid arthritis; SD: standard deviation; VAS: visual analogue scale; RF: rheumatoid factor; IQR, interquartile range; SJN: swollen joint number; PJN: painful joint number; CRP: C-reactive protein.
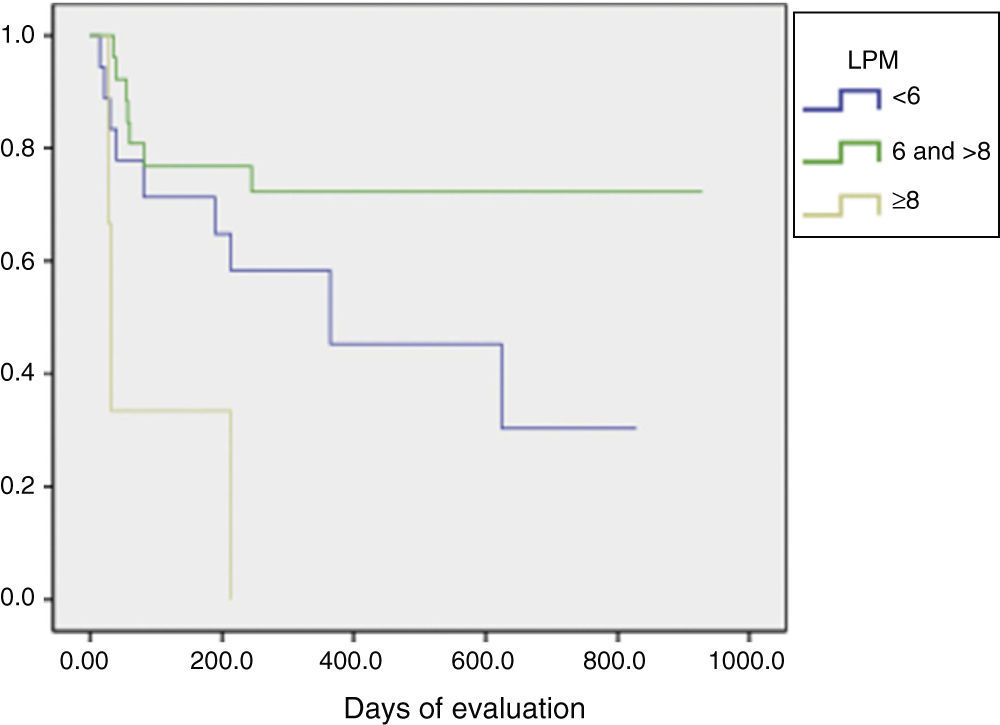
The LPM score was assessed using the Kaplan–Meier test and Log rank, and found that patients with a score ≥8 showed a statistically significant difference in their progression to RA (P=.01) (Fig. 2).
DiscussionEarly detection of UA in patients with high risk of progression to RA has become the current challenge in order to initiate treatment in a timely manner and, therefore, alter the course of the disease and prevent joint destruction, deformity and disability in RA patients.13
In the baseline characteristics of our population we found significant results in disease duration for progression to RA, noting that most of the patients who progressed to RA had a longer than 6-month evolution, but was also significant in the non RA group; likewise a low prevalence of RF and anti-CCP antibodies in the 2 groups was evaluated, finding differences between groups and progression to RA (P=.97 and P=.29, respectively).
The incidence of UA progression to RA in our clinic was similar to that reported by other studies. Unlike other cohorts in which LPM has been previously validated, where each used different inclusion criteria, patients who progressed to RA in this study obtained a lower score.8–10 In our cohort, all patients who achieved a score of 8 or more progressed to RA, although most patients in this group had a lower than 6 score; therefore, this result did not allow us to identify patients who will progress to RA using the LPM (Fig. 1). Similar to our study data is reported in a study recently published by Krabben et al., that evaluated 1219 patients with UA and risk of progression to RA in 3 different cohorts (Leiden, Amsterdam and Birmingham) using the LPM and the presence of other variables, such as autoantibodies. At the end of the evaluation, it was observed that only 0%–6% of the patients had anti-CCP antibodies and 0%–1% had high LPM scores, with these 2 variables for prediction of progression to RA resulting insufficient.14
Predictive models of UA to RA have been applied to reference clinics, but its use at the community level has not been reported. The COPCORD methodology allows the identification of patients with painful musculoskeletal conditions in the general population, even before reference to a physician.15 Most of our patients came from COPCORD and 40% of patients who progressed to RA were enrolled in the study with less than 3 months duration, which could somehow increase the likelihood of negative outcomes in the RF and anti-PCC and, therefore, on the score of LPM.
There are limitations to the study that should be considered when interpreting the results; the first is that the sample size was small, although all patients with UA in the EAC were included; this however, allowed us to determine the poor performance of LPM in our population; the second limitation is that the determination of anti-CCP antibodies was performed using first generation ELISA, with a sensitivity that is lower than the second generation,16 which could influence the low score of the LPM. The difference between tender and swollen joints in the RA group can be explained by the origin of the patients and also due to the presence of other concomitant diseases such as fibromyalgia, which can increase the perception of pain when not adjusted.
In conclusion, 43% of patients with UA progressed to RA after 1 year of follow up. 56% of patients who progressed to RA had a score that was ≤6 in the LPM, in contrast to what has been reported in the cohort of Leiden, where it was less than 10%; these results did not allow us to establish a prediction of progression to RA using the LPM.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that this study did not perform experiments on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients, and all patients included in the study have received sufficient information and gave written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained informed consent from patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Conflict of InterestThe authors declare no conflicts of interest.
Please cite this article as: Arana-Guajardo A, Pérez-Barbosa L, Vega-Morales D, Riega-Torres J, Esquivel-Valerio J, Garza-Elizondo M. Aplicación de un modelo de predicción de progresión de artritis reumatoide en pacientes con artritis indiferenciada. Reumatol Clin. 2014;10:360–363.