The aim is to describe clinical and serological features, previous and current therapies, and adverse events in four systemic sclerosis patients (SSc) who undergo autologous hematopoietic stem cells transplantation (AHSCT).
MethodsDescriptive, cross-sectional study including SSc patients according ACR/EULAR 2013 criteria. Clinical and serological data, data related to current and previous therapy and adverse events were collected from 2014 to 2023.
ResultsFour female patients were included, with a mean age of 49 (6.78) years old and a mean of 32.5 (7) months since diagnosis to AHSCT. Mean mRodnan score (mRSS) was 33.75. All cases underwent a skin improvement measured by mRSS (mean difference of 14.75) and lung function (DLCO) remained stable. Only one patient presented a pneumonia post-AHSCT, that required admission to intensive care unit, with improvement and complete resolution of infection a few days after admission.
ConclusionsAHSCT is an appropriate therapeutic option in rapidly progressive diffuse SSc patients, with a good safety profile in our cohort.
El objetivo es describir las características clínicas, serológicas, los tratamientos previos/actuales y los eventos adversos de 4 pacientes con esclerosis sistémica (ES) que se han sometido a un auto-TPH.
Material y métodosEstudio descriptivo, transversal de los pacientes con ES (criterios ACR/EULAR 2013). Se recogieron los tratamientos previos y actuales, eventos adversos y datos clínicos/serológicos (2014-2023).
ResultadosSe incluyeron 4 mujeres (edad media de 49 [6,78 años) y mediana de 32,5 [7 meses] desde el diagnóstico hasta el auto-TPH. El m-Rodnan score (mRSS) medio fue de 33,75. En todos los casos se produjo una mejoría (diferencia media de 14,75) y la función pulmonar (DLCO) se mantuvo estable. Solo una paciente presentó una neumonía tras el trasplante, requiriendo ingreso en la unidad de cuidados intensivos, con mejoría y resolución completa del cuadro infeccioso.
ConclusionesEl auto-TPH es una estrategia terapéutica adecuada en los pacientes con diagnóstico de ES difusa rápidamente progresiva, con un buen perfil de seguridad en nuestra cohorte.
Systemic sclerosis (SSc) is an autoimmune, multisystem disease the pathophysiology of which is based on three pillars: 1) structural and functional vasculopathy of the microcirculation; 2) dysregulation of the immune system and production of autoantibodies; and 3) vascular and interstitial fibrosis of the skin and internal organs.1 The clinical manifestations are heterogeneous, although most patients present cutaneous hardening, the extent of which determines the prognosis.1,2
In the absence of disease-modifying drugs, autologous hematopoietic stem cell transplantation (AHSCT) has gained more relevance in recent years, and has proven to be an effective and generally safe procedure.1,2
The objective was to describe the clinical and serological characteristics, current and previous treatment, adverse events and evolution of 4 patients with SSc after AHSCT.
Clinical observationDescriptive, cross-sectional study, which included four patients diagnosed with SSc according to ACR/EULAR 2013 criteria. Data regarding previous and current treatments, the clinical course of the disease post-HSCT, adverse events and clinical and serological data were collected from 2014 to 2023.
Patient 1Fifty-two-year-old woman diagnosed with diffuse systemic sclerosis since 2011, mainly cutaneous symptoms. In 2013, rituximab and methotrexate were prescribed, but she only received a couple of cycles of rituximab, which were discontinued at the patient’s request. Finally, methotrexate was also discontinued due to intolerance.
An autologous hematopoietic stem cell transplant was performed in November 2014 due to progressive skin deterioration with severe limitation in the hands. After the procedure and thanks to the clinical improvement obtained, immunosuppressive treatment was withdrawn, and she remains without treatment at present.
Patient 2A 52-year-old woman diagnosed with diffuse systemic sclerosis and interstitial lung disease (ILD) in 2013.
Clinically, she presented with Raynaud’s phenomenon in 2009 and progressive skin hardening. In 2012, she began to have dyspnoea with minimal effort, being diagnosed with ILD and then recording a DLCO of 42% (11.22).
In April 2014, cyclophosphamide was prescribed and she completed 6 doses. Due to the limitation caused by the skin involvement, the first cycle of rituximab was administered in August 2014. From then on, the patient improved at skin level but at respiratory level, the worsening continued, so she was finally proposed as a candidate for AHSCT, which was performed in September 2015. Currently, the patient is without treatment.
Patient 3A 55-year-old woman diagnosed with diffuse systemic sclerosis in February 2021, which presented with predominantly cutaneous symptoms. In December 2021, she completed 5 cycles of cyclophosphamide. Subcutaneous tocilizumab was subsequently prescribed, which was withdrawn due to lack of response and poor tolerance (digestive discomfort).
Due to the progression of the disease and the extent of skin involvement, AHSCT was performed in November 2022. Given the slow improvement of the skin symptoms after the procedure, it was agreed with the patient to restart tocilizumab 6mg/kg intravenously in June 2023, which is currently being maintained.
Patient 4A 38-year-old woman diagnosed with diffuse systemic sclerosis in January 2020. She debuted in March 2019 with Raynaud’s phenomenon and oedema in the upper and lower limbs. The disease progressed rapidly, associated with erythema nodosum, interstitial lung disease and inflammatory arthralgia. She initially received treatment with rituximab (October 2022) and subsequently six cycles of cyclophosphamide 750mg/m2 were prescribed in November 2021, despite which the progression of the disease could not be stopped, so she was referred for AHSCT, which was performed in May 2023.
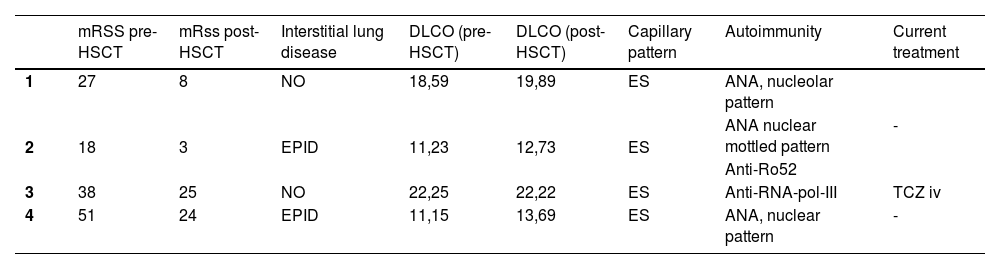
The table shows clinical-analytical data for each of the patients described (Table 1).
Clinical-analytical patient data.
| mRSS pre-HSCT | mRss post-HSCT | Interstitial lung disease | DLCO (pre-HSCT) | DLCO (post-HSCT) | Capillary pattern | Autoimmunity | Current treatment | |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 8 | NO | 18,59 | 19,89 | ES | ANA, nucleolar pattern | - |
| 2 | 18 | 3 | EPID | 11,23 | 12,73 | ES | ANA nuclear mottled pattern | |
| Anti-Ro52 | ||||||||
| 3 | 38 | 25 | NO | 22,25 | 22,22 | ES | Anti-RNA-pol-III | TCZ iv |
| 4 | 51 | 24 | EPID | 11,15 | 13,69 | ES | ANA, nuclear pattern | - |
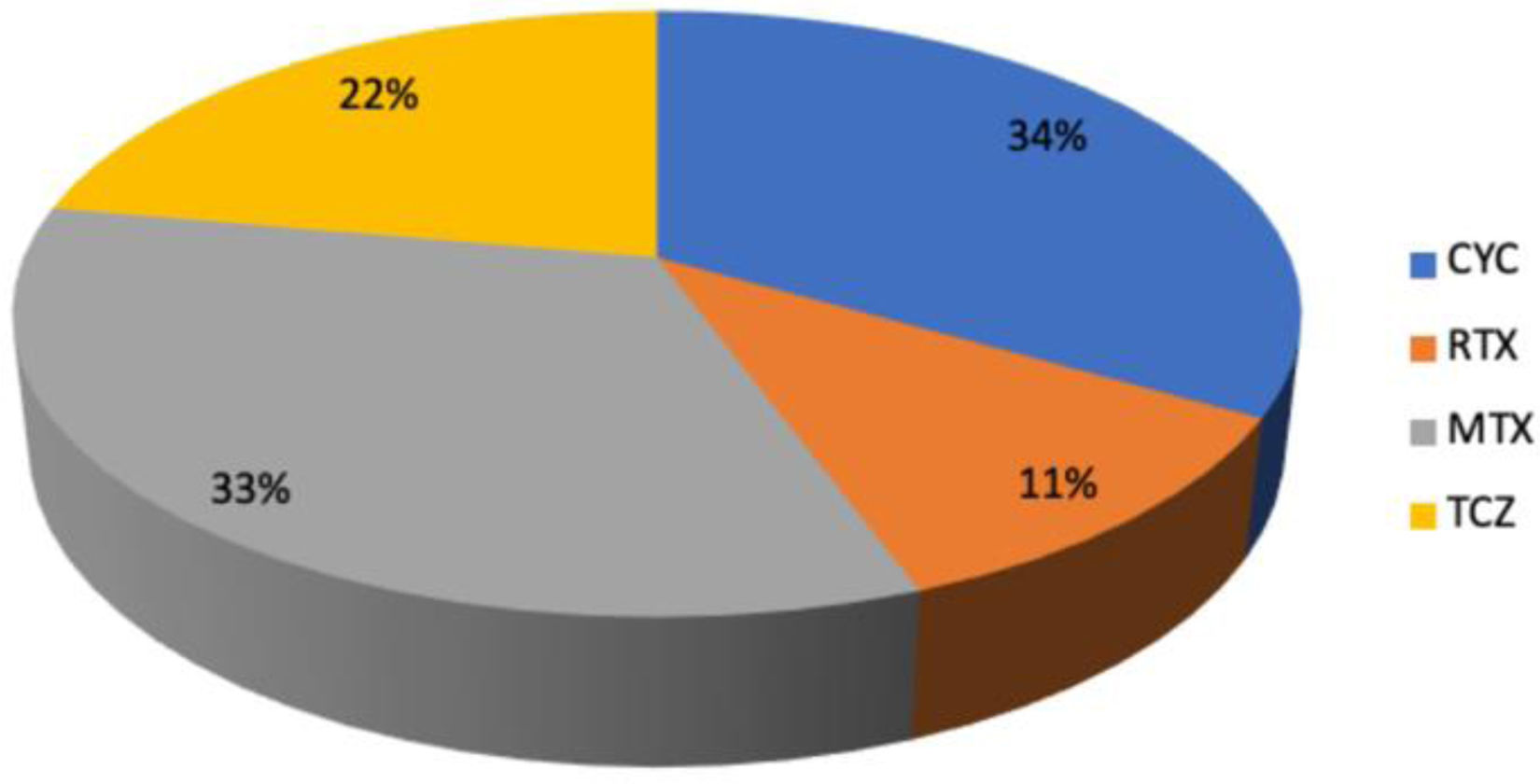
The previous treatment received is shown in the graph (Fig. 1).
Finally, with regard to treatment-related adverse events (AEs), only patient 4 presented a serious AE consisting of bibasilar pneumonia two weeks after AHSCT, which required admission to the intensive care unit, with improvement and complete resolution of the clinical picture two days after admission.
DiscussionSince 2017, the EULAR recommendations for the treatment of SSc indicate that AHSCT should be considered in patients with rapidly progressive skin/lung involvement who have poor prognostic factors and are at risk of organ failure.3 In turn, the American Society for Blood and Marrow Transplantation also recommended auto-HSCT in patients with severe SSc.4
The guidelines of the European Group for Bone Marrow Transplantation in Autoimmune Diseases (2012) specify the characteristics that should prompt the performance of AHSCT: diffuse skin involvement with <5 years of evolution since the first non-Raynaud symptom, mRSS score >15 and with major organ involvement, defined as respiratory involvement in functional tests (PFR) or imaging; heart disease with conduction abnormalities/pericarditis or renal involvement with proteinuria attributable to SSc.5
With regard to other cases in the literature, the case of a 49-year-old woman with diffuse SSc of 17 months of evolution with an mRSS of 3, pulmonary involvement consisting of dyspnoea (functional class III/IV) with a restrictive pattern in PFR and interstitial disease in HRCT, together with pulmonary hypertension confirmed by right catheterisation was published. Following failure of methotrexate and cyclophosphamide and given the progression of the cutaneous and pulmonary disease, AHSCT was performed, with improvement at 6 months of the mRSS score greater than 50%, resolution of the restrictive pattern by PFR and ILD, as well as PHT data by echocardiography.6
In September 2021, a series of 3 cases of rapidly progressive diffuse SSc with a mean age of 35 years (lower than that recorded in our study), undergoing AHSCT (2015-2020), was published. The sex of the patients was not reported. No severe infectious complications were observed, but a relapse occurred in all 3 patients 9.6 months post-HSCT. Relapse was defined as the development of new dermal and Raynaud’s lesions, as well as an increase in the mRSS score. Given the poor response to mycophenolate mofetil and prior treatment with cyclophosphamide, rituximab 1g/2 weeks in two doses was indicated. Currently, the patients’ disease is under control and they are receiving mycophenolate as maintenance.7
Regarding clinical trials (CT), there are three published trials: the ASSIST, ASTIS and SCOT studies. In all three trials, the HSCT vs. cyclophosphamide arm was evaluated, showing an improvement in lung function, skin elasticity and a good safety profile, with a lower rate of AEs compared to the CYC group, except in the first year, when it was higher in the HSCT vs. CYC group.8–10
In our series, all patients experienced significant improvement in skin condition and stable lung function after HSCT, with no serious AEs, except in one patient, probably because she had a BMI of 11.52 and severe sarcopenia at the time of the procedure. Nutritional support was essential to enable post-HSCT hospital discharge.
ConclusionsAHSCT is an appropriate therapeutic option in patients with recently diagnosed, rapidly progressive diffuse systemic sclerosis associated with interstitial lung disease, with a good safety profile in our cohort.
Conflict of interestsThe authors declare that they have no conflict of interests.