To assess the effectiveness, safety and cost of Etanercept biosimilar in patients with rheumatoid arthritis (RA), spondyloarthritis (SpA) and psoriatic arthritis (PsA) compared to the standard drug in real clinical practice.
Patients and methodsRetrospective observational study. Case series of 138 patients with RA, SpA or PsA treated with at least one dose of Benepali® (n = 79) or Enbrel® (n = 59). Drug retention time was the primary efficacy endpoint compared to the biosimilar and the original. The proportion of patients achieving low disease activity or remission after 52 weeks was used as the secondary outcome. Safety was assessed by means of the adverse effects incidence rate. A cost minimization analysis was performed.
ResultsNo differences were observed regarding treatment retention time between drugs (median [95% confidence interval, 95% CI] at 12.0 months [10.2–12.0] for the biosimilar and 12.0 months [12.0–12.0] for the original). Similar improvements, in terms of inflammatory activity and physical function, were obtained after 52 weeks except for patients with SpA and PsA who, in general, experienced improvements of BASDAI and ASDAS with the original compared with the biosimilar. No significant differences were observed in the total number of adverse effects (.43 events/patient-years versus the biosimilar and .53 versus the original). Using the biosimilar in place of the original drug resulted in a net savings of 118,383.55 € (1,747.20 €/patient-years) for the hospital.
ConclusionThe biosimilar Benepali is as effective and safe as the original and much more cost-effective.
Evaluar la efectividad, la seguridad y los costes de etanercept biosimilar (BS) en pacientes con artritis reumatoide (AR), espondiloartritis (EspA) y artritis psoriásica (APs) y en comparación con su original, en condiciones de práctica clínica habitual.
Pacientes y métodosEstudio observacional retrospectivo. Se incluyeron 138 pacientes con AR, EspA o APs tratados con al menos una dosis de Benepali® (n = 79) o Enbrel® (n = 59). Como desenlace principal de efectividad del BS o de su original se usó el tiempo de retención del fármaco. Como desenlace secundario de efectividad se midió la proporción de pacientes que alcanzaban baja actividad o remisión a las 52 semanas. La seguridad fue evaluada mediante tasas de incidencia de efectos adversos. Se hizo un análisis de minimización de costes.
ResultadosNo se observaron diferencias en cuanto a retención del tratamiento (mediana [intervalo de confianza del 95%, IC 95%] de 12,0 meses [10,2–12,0] para BS y 12,0 meses [12,0–12,0] para el original). Se obtuvieron mejorías similares después de 52 semanas en actividad inflamatoria y función física, excepto en los pacientes con EspA y APs, que en general obtuvieron mejores valores de BASDAI y ASDAS con el BS. No se registraron diferencias en el número total de efectos adversos (0,43 eventos/pacientes-año con BS frente a 0,53 con original). El uso del BS, en lugar de su original, supuso un ahorro neto para el centro de 118.383,55 € (1.747,2€/pacientes-año).
ConclusionesEl uso del BS parece tan eficaz y seguro como su original y mucho más coste-efectivo.
Inflammatory joint diseases, such as rheumatoid arthritis (RA), axial spondyloarthritis (SpA) and psoriatic arthritis (PsA), are characterised by chronic inflammation, joint destruction, and functional disability.1
Conventional synthetic disease-modifying drugs (csDMARD) are the mainstay of treatment for these patients. However, approximately one third of patients do not respond to them and require targeted synthetic (tsDMARD) or biological (bDMARD) disease modifying drugs.2 These drugs have been shown to control inflammatory activity and improve physical function and quality of life in these patients over the long term. A significant proportion of patients treated with a bDMARD do not respond to initial treatment or efficacy is lost over time,3 and treatment is discontinued in more than 10% due to side effects.4 Despite their advantages, the high cost of these treatments limits their use.5 Therefore, the biosimilar (BS) agents, because of their lower cost, are making biological therapies prompter and more accessible to a greater number of patients due to their greater efficiency.
According to the European Medicines Agency (EMA), a BS is a biological drug that contains a version of the active substance of an already authorised original biological product (the reference drug). It must demonstrate similarity to the reference drug in terms of quality characteristics, biological activity, safety, and efficacy, following a full comparability exercise. A BS is not like a generic drug that has a simpler structure and is identical to its reference drug.6
Enbrel® (etanercept) is an anti-TNF agent approved for use in adult patients with moderate to severe active and/or progressive RA, active and progressive, severe axial PsA, in young people with juvenile idiopathic arthritis, including polyarticular, extended oligoarticular, PsA and enthesitis-related arthritis, and severe plaque psoriasis.7 Several etanercept BS have been evaluated in clinical trials (CTs), as cheaper alternatives to the reference product. Reducing the cost of treatment is the main attraction for the BS that are entering the global market.8 Biogen's Benepali® (SB4) was the first etanercept BS approved for use in January 2016. Several studies have been published in the lead-up to its approval and marketing. A phase III parallel-group, randomised, double-blind, trial in 596 patients with moderate-severe RA previously treated with methotrexate showed equal efficacy to Enbrel, with a similar ACR20 response at week 24 in both groups (78.1% with Benepali vs. 80.3% with Enbrel) and with a comparable incidence of adverse events.9,10 In this regard, a phase I trial in a Korean population (n = 138) demonstrated an equivalent safety, pharmacokinetic and tolerability profile between Benepali and Enbrel. However, Benepali had a lower immunogenicity profile than Enbrel.11
However, other than these CTs, to date there have been no studies conducted under clinical practice conditions to confirm these results. Therefore, our aim was to evaluate the effectiveness, safety, and costs of the Benepali BS in patients with chronic inflammatory arthritis, and compare with the reference drug, under routine clinical practice conditions.
Patients and methodsStudy design and scopeA retrospective observational study based on a case series of patients with RA, axial SpA and PsA treated with Benepali or Enbrel. A cost-minimisation pharmacoeconomic analysis was also carried out. The study was conducted at IBIMA by the Rheumatology Department of the Hospital Regional Universitario de Málaga (HRUM), Spain. The protocol for the research project was approved by a provincial ethics committee of Malaga in which the work was performed, and it conforms to the provisions of the Declaration of Helsinki. The study was approved by the hospital's clinical research ethics committee (CEIC). Informed consent was obtained from all individual participants included in the study.
PatientsAll patients were included consecutively who had started treatment with the BS (from March 2017 to August 2018) or their original drug (from February 2015 to August 2018) and had received at least one dose of treatment. Selection criteria were age ≥18 years, RA according to American College of Rheumatology/European League Against Rheumatism 2010 criteria,12 or axial SpA according to ASAS/EULAR 2010 criteria,13 or PsA according to CASPAR 2006 criteria.14 Patients with any other inflammatory or rheumatic disease, except secondary Sjögren's syndrome, were excluded.
ProtocolAll patients who met the inclusion criteria and none of the exclusion criteria were recruited. All patients were prospectively followed up in a specific biological therapy unit (BTU) according to a pre-established protocol of systematic data collection. This protocol includes, among other variables, data on disease activity, physical function, and adverse effects. The BTU reviews patients treated with biological therapies every 3 months in general and specific consultations (for subcutaneous biologicals) alternately. Benepali or Enbrel were prescribed according to each physician’s judgement.
Variables and definitionEffectivenessThe primary efficacy endpoint of the BS or its original was drug retention (survival) time, measured as the time from initiation to discontinuation of treatment or loss to follow-up or study end date at 52 weeks. Secondary outcome variables of effectiveness included the effectiveness of the BS and its original measured as the proportion of patients achieving low disease activity or remission at 52 weeks, based on DAS28, BASDAI and ASDAS.
SafetySafety was assessed by calculating the incidence rates of adverse effects between 2015 and 2018. This was done by dividing the total number of adverse effects by the total follow-up time of all patients in years (number of events/patient-year). Adverse events were classified as mild-moderate, severe, and serious, with mild-moderate being understood as signs or symptoms that are easily tolerated, could interfere with usual activities, and require medical intervention or treatment; severe as disabling and incapacitating for usual activities and requiring medical intervention or therapy; and serious as life-threatening, requiring, or prolonging hospitalisation, causing congenital anomaly or causing significant persistent disability.15
Economic studyThe costs of each treatment were calculated by patient-week cost, considering the procurement price of the drug, corresponding to the latest tender of the provincial purchasing platform. The costs of administering the drug in the hospital were not considered. This resulted in a price of €5,540 per year of treatment with the BS and €7,306 per year of treatment with the original drug. To analyse the costs overall, the total cost of treatment was calculated by the sum of the partial costs for each patient throughout the study. For the BS patients, in addition, a calculation was made of the cost that would have been incurred by each patient if they had been treated with the original drug. The official price of each drug was calculated based on the laboratory's sale price (LSP): €106.55 per 50 mg injection of the BS, and €140.5 of Enbrel, which corresponded to the weekly cost of treatment per patient. Total cost: the result of multiplying the weekly cost by the patient-week total. Actual total cost: final cost in Euros of the treatment administered to the patients included in the study.
Other variablesAge (years), sex, duration of symptoms, date of starting the BS/original and date of event and date of discontinuation of treatment were included for all patients. Disease Activity Score 28 joints (DAS28) with erythrocyte sedimentation rate (DAS28-ESR) (continuous, range 0–9) (<2.6 points: remission, ≤3.2: low disease activity), Clinical Disease Activity Index (CDAI; continuous, range 0–76) and Simplified Disease Activity Index (SDAI; continuous, range 0–86),16 Ankylosing Spondylitis Disease Activity Score (ASDAS; continuous; <1.3 low disease activity, 1.3–2.1 moderate activity, 2.1–3.5 high activity, >3.5 very high activity), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI; continuous, range 0–10; <4 low activity or remission)17 as appropriate, collected at baseline visit and 1-year visit. Physical function: Health Assessment Questionnaire (HAQ) Spanish version (continuous, range 0–3)18 and Bath Ankylosing Spondylitis Functional Index (BASFI; continuous, range 0–10).19 The presence of radiological erosions (categorical, yes/no) and laboratory variables were also collected: rheumatoid factor, measured in U/mL, was considered elevated if >20 U/mL; presence of ACPA, measured in U/mL, was considered positive if >10 U/mL; and HLA-B27 positive or not.
Statistical analysisWe performed a descriptive analysis. Qualitative variables were expressed as absolute number and percentage and quantitative variables as mean and standard deviation (SD) or median and interquartile range (IQR), according to their distribution. Normality adjustment of continuous variables was confirmed with the Kolgomorov–Smirnov test. The Student’s t-test for independent samples or Mann-Whitney test was used in cases of non-normality between mean DAS28-ESR, HAQ, CRP, ESR, BASDAI and BASFI between baseline and 52 weeks. The survival time of Benepali and Enbrel was analysed using Kaplan Meier curves. Survival likelihoods and their 95% confidence interval were calculated. For all analyses, a value of P < .05 was considered significant. All data were analysed using R2.4.0 statistical software.
ResultsBetween January 2015 and August 2018 (inclusive) 138 patients with different rheumatic diseases started treatment with etanercept: 85 (61.5%) patients with RA, 29 (21.0%) with axial SpA and 24 (17.3%) with PsA. Of these, 79 (57.2%) received the BS (43 [54.4%] RA, 20 [25.3%] AS and 16 [20.2%] PsA) and 59 (42.7%) the original drug (42 [71.2%] RA, 9 [15.2%] AS and 8 [13.5%] PsA).
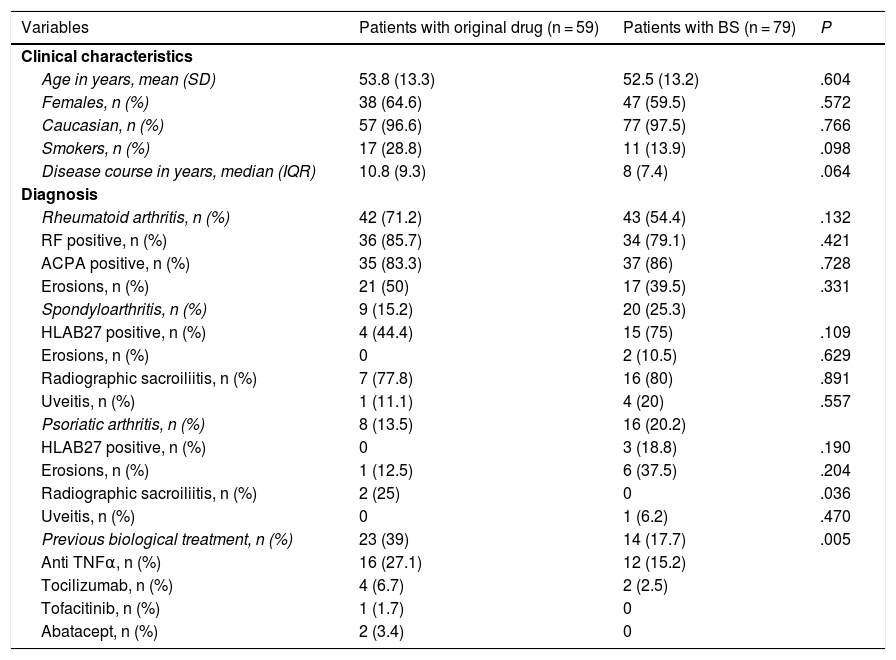
Patient characteristicsThe main characteristics of the patients in both treatment groups are described in Table 1. The majority were women around 52 years of age with long-standing disease. Patients on the original drug tended to have a longer disease duration and more were smokers than those on the BS.
Baseline characteristics of the patients.
| Variables | Patients with original drug (n = 59) | Patients with BS (n = 79) | P |
|---|---|---|---|
| Clinical characteristics | |||
| Age in years, mean (SD) | 53.8 (13.3) | 52.5 (13.2) | .604 |
| Females, n (%) | 38 (64.6) | 47 (59.5) | .572 |
| Caucasian, n (%) | 57 (96.6) | 77 (97.5) | .766 |
| Smokers, n (%) | 17 (28.8) | 11 (13.9) | .098 |
| Disease course in years, median (IQR) | 10.8 (9.3) | 8 (7.4) | .064 |
| Diagnosis | |||
| Rheumatoid arthritis, n (%) | 42 (71.2) | 43 (54.4) | .132 |
| RF positive, n (%) | 36 (85.7) | 34 (79.1) | .421 |
| ACPA positive, n (%) | 35 (83.3) | 37 (86) | .728 |
| Erosions, n (%) | 21 (50) | 17 (39.5) | .331 |
| Spondyloarthritis, n (%) | 9 (15.2) | 20 (25.3) | |
| HLAB27 positive, n (%) | 4 (44.4) | 15 (75) | .109 |
| Erosions, n (%) | 0 | 2 (10.5) | .629 |
| Radiographic sacroiliitis, n (%) | 7 (77.8) | 16 (80) | .891 |
| Uveitis, n (%) | 1 (11.1) | 4 (20) | .557 |
| Psoriatic arthritis, n (%) | 8 (13.5) | 16 (20.2) | |
| HLAB27 positive, n (%) | 0 | 3 (18.8) | .190 |
| Erosions, n (%) | 1 (12.5) | 6 (37.5) | .204 |
| Radiographic sacroiliitis, n (%) | 2 (25) | 0 | .036 |
| Uveitis, n (%) | 0 | 1 (6.2) | .470 |
| Previous biological treatment, n (%) | 23 (39) | 14 (17.7) | .005 |
| Anti TNFα, n (%) | 16 (27.1) | 12 (15.2) | |
| Tocilizumab, n (%) | 4 (6.7) | 2 (2.5) | |
| Tofacitinib, n (%) | 1 (1.7) | 0 | |
| Abatacept, n (%) | 2 (3.4) | 0 | |
ACPA: Anti-citrullinated Protein Antibodies; IQR: Interquartile Range; RF: Rheumatoid Factor; SD: Standard Deviation; TNF: Tumour Necrosis Factor.
Twenty-three patients (39%) with the original drug had previously had another biological therapy, compared to 14 patients (17.7%) of those treated with the BS (P = .005), which was mostly another anti-TNF, in 16 patients (27.1%) in the original group versus 12 (15.2%) patients in the BS group. Most patients were concomitantly taking a DMARD, mainly methotrexate: 32 (54.1%) with the original drug and 38 (48.8%) with the BS; P = .521. Only 21% of patients in both groups started treatment in monotherapy (P = .680). Twenty-two patients (37.5%) on the original drug and 28 (35.4%) on the BS (P = .823) were taking low-dose corticosteroids at baseline (mean [SD] prednisone treated with the original of 3.7 [5.9] vs. 4.4 [7.5] with the BS; P = .579).
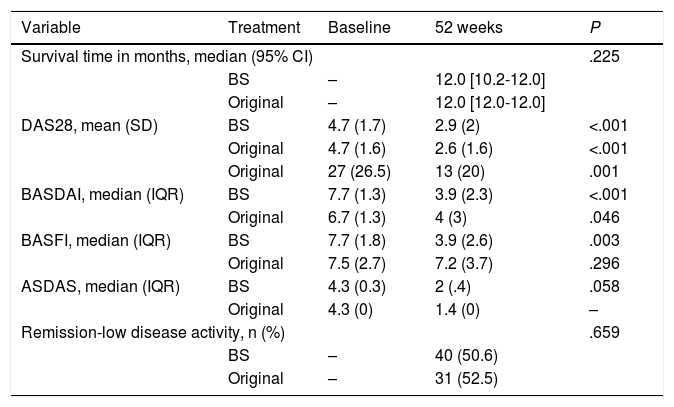
Effectiveness of the biosimilar vs. the original drugNo differences were observed in treatment retention (median [95% confidence interval, 95% CI] 12.0 months [10.2–12.0] for the BS and 12.0 months [12.0-12.0] for the original drug; logrank test P = .225). Neither were there differences between diagnoses or in corticosteroid consumption. After the 52 weeks, 9 patients (13.2%) with the BS and 7 (13.5%) with the original drug (P = .971) continued to take corticosteroids at low doses (mean [SD] prednisone equivalent dose with the BS 11.8 mg/day8 vs. 10 mg/day5,7 with the original; P = .341).
Fifty-five patients (70%) in the BS group and 48 patients (81.4%) treated with the original drug (P = .117) continued treatment at 52 weeks. The reason for discontinuation was mostly primary failure in 15/24 (62.5%) with the BS and 5/11 (45.5%) with the original (P = .039). Eight (33.3%) patients discontinued treatment due to adverse effects in the BS group versus 5 (45.5%) in the original group. One patient (4.2%) with a desire for pregnancy and good disease control discontinued treatment in the BS group. One patient in each group discontinued treatment due to loss of efficacy.
As shown in Table 2, both treatment groups obtained similar improvements after 52 weeks of treatment in terms of inflammatory activity, physical function, and acute phase reactants (CRP and ESR), except the patients with SpA and PsA, who, in general, obtained better results with the BS, as can be seen from the BASDAI and ASDAS scores. There was significant improvement and no differences in either group in SDAI, CDAI, NAJ, NIJ, VAS, physician and patient VAS and HAQ. The proportion of patients achieving remission or low disease activity at week 52 was 40 patients (50.6%) with the BS and 31 patients (52.5%) with the original (P = .659). After one year of treatment, the drug dose was optimised in 8 patients (10.4%) treated with the BS and in 2 patients (3.4%) treated with the original (P = .121).
Effectiveness data of both drugs.
| Variable | Treatment | Baseline | 52 weeks | P |
|---|---|---|---|---|
| Survival time in months, median (95% CI) | .225 | |||
| BS | – | 12.0 [10.2-12.0] | ||
| Original | – | 12.0 [12.0-12.0] | ||
| DAS28, mean (SD) | BS | 4.7 (1.7) | 2.9 (2) | <.001 |
| Original | 4.7 (1.6) | 2.6 (1.6) | <.001 | |
| Original | 27 (26.5) | 13 (20) | .001 | |
| BASDAI, median (IQR) | BS | 7.7 (1.3) | 3.9 (2.3) | <.001 |
| Original | 6.7 (1.3) | 4 (3) | .046 | |
| BASFI, median (IQR) | BS | 7.7 (1.8) | 3.9 (2.6) | .003 |
| Original | 7.5 (2.7) | 7.2 (3.7) | .296 | |
| ASDAS, median (IQR) | BS | 4.3 (0.3) | 2 (.4) | .058 |
| Original | 4.3 (0) | 1.4 (0) | – | |
| Remission-low disease activity, n (%) | .659 | |||
| BS | – | 40 (50.6) | ||
| Original | – | 31 (52.5) | ||
BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; DAS28: Disease Activity Score 28 joints.
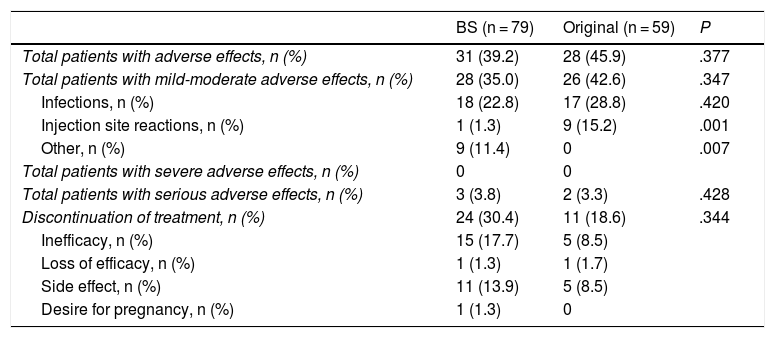
As shown in Table 3, there was no difference between the two treatments in the total number of adverse effects (.43 events/patient-year [95% CI: .27–.58] with the BS versus .53 [95% CI: .33–.73] with the original). The most frequent adverse effects were mild-moderate (rate .41 [95% CI: .26–.57] BS vs. .49 [95% CI: .3–.68] original); most were infections (bronchitis, colds, urine infections, herpes simplex, shingles). One patient (1.3%) with the BS developed paradoxical psoriasis. Injection site skin reactions were more frequent with the original (1 [1.3%] with the BS vs. 9 [15.3%] with the original; P = .001), while headaches were more frequent with the BS (9 [11.4%] vs. 0 with the original; P = .007).
Comparison of adverse effects of the biosimilar (BS) and the original drug.
| BS (n = 79) | Original (n = 59) | P | |
|---|---|---|---|
| Total patients with adverse effects, n (%) | 31 (39.2) | 28 (45.9) | .377 |
| Total patients with mild-moderate adverse effects, n (%) | 28 (35.0) | 26 (42.6) | .347 |
| Infections, n (%) | 18 (22.8) | 17 (28.8) | .420 |
| Injection site reactions, n (%) | 1 (1.3) | 9 (15.2) | .001 |
| Other, n (%) | 9 (11.4) | 0 | .007 |
| Total patients with severe adverse effects, n (%) | 0 | 0 | |
| Total patients with serious adverse effects, n (%) | 3 (3.8) | 2 (3.3) | .428 |
| Discontinuation of treatment, n (%) | 24 (30.4) | 11 (18.6) | .344 |
| Inefficacy, n (%) | 15 (17.7) | 5 (8.5) | |
| Loss of efficacy, n (%) | 1 (1.3) | 1 (1.7) | |
| Side effect, n (%) | 11 (13.9) | 5 (8.5) | |
| Desire for pregnancy, n (%) | 1 (1.3) | 0 |
Eight patients (10.1%) discontinued the BS due to non-serious adverse effects (2 disabling headaches, 1 increased baseline dyspnoea, 1 gastritis, 3 patients with recurrent infections and 1 neutropenia). Five patients (8.5%) with the original discontinued the drug also due to adverse effects (1 pneumonia, 3 patients with skin reactions and 1 adenocarcinoma of the ovary). Three patients discontinued the drug due to a serious adverse effect (rate .04 [95% CI: .0–.09] BS vs. .04 [95% CI: .01–.09] original): one patient treated with the BS suffered severe neutropenia, another patient due to pneumonia and the third due to undifferentiated gynaecological adenocarcinoma, both treated with the original.
Costs of the biosimilarThe cost of the BS during the follow-up period was €5,540 patient-year, compared to €7,306 patient-year for the original. The real total cost of treatment for all BS patients followed for 3,523.32 weeks was €375,409.75, while the total cost using the original drug would have been €493,793.3. This resulted in a net saving for the centre during follow-up of €118,383.55 (€1,747.2/patient-year).
DiscussionThe economic impact of the different rheumatic diseases is largely due to the costs of treatment. Therefore, the choice of each drug should not only depend on its efficacy, but also its efficiency and safety. We conducted a study to evaluate the efficacy, safety, and cost of the Benepali BS in a group of patients with different rheumatic diseases, based on our clinical practice. To date, no study had been conducted in clinical practice to compare efficacy between the BS and reference drugs. Nor has the survival of the BS in inflammatory rheumatic diseases been analysed. In our study, all patients showed significant improvement in disease activity and physical function parameters assessed at 52 weeks of treatment with Benepali or Enbrel. Furthermore, in both groups, half of the patients achieved a state of remission-low disease activity by DAS28 and BASDAI. In addition, we found no significant differences between the two treatment groups in the improvement observed in patients after 52 weeks. At 12 months we only observed differences between both groups in higher median CRP in patients treated with Benepali, but despite significant differences, the CRP value was lower than 5 mg/l in both groups. Neither could these results be explained by higher corticosteroid use in the Enbrel patients, as the mean dose used in both groups was similar. We observed a higher BASFI score in patients treated with Enbrel, which may be explained by the longer disease course of these patients.
The comparability in efficacy that we observed is similar to that described in other CTs. The study by Chadwick et al.20 shows the clinical efficacy of etanercept for RA evaluated in several randomised controlled trials (RCTs) and extension studies.20,21 It also evaluates the efficacy of different BS such as Benepali or Erelzi. In a phase III RCT conducted in 596 patients randomised to Benepali or Enbrel,22 a significant improvement in DAS28 at week 24 was observed in both groups of patients. About 50% of patients in this study achieved low disease activity or remission status at week 24 according to DAS28. Furthermore, no significant differences were observed between the two treatments in the improvement measured by DAS28 or in other activity variables such as ACR20, ACR50, or physical function by HAQ.22 Similarly, another phase III RCT23 compared the efficacy and safety of Benepali and Enbrel at week 52 in patients with RA. In this study they observed that 41% of patients with Enbrel achieved low disease activity-remission at 52 weeks versus 35% with Benepali, showing no significant differences either group. Furthermore, at 52 weeks, a significant improvement was also observed in all activity and physical function parameters measured in both groups of patients. However, we were not able to compare these data with other studies in routine clinical practice because so far, we only have the results from CTs on the effectiveness of these drugs and the different reference biologicals. Therefore, more data are needed in real clinical practice to corroborate this benefit.
There are many factors that influence a drug’s retention time, including the availability of treatment alternatives, effectiveness, drug toxicity, disease severity and adherence to treatment.24 Longer treatment retention time usually translates into greater effectiveness and safety of treatment. There are currently no survival studies in rheumatology with BS, but there are studies with its reference drug. Zink et al.25 conducted a survival study of different anti-TNFs in RA, in which they observed that after one year of treatment 70% of patients continued with Enbrel. In other studies, such as that by Khraishi et al.,26 this figure was 66%. In our study, these data are similar to the above, and after one year of follow-up 81% of patients were still on Enbrel and 72% were still on Benepali. In these data we can see that both treatment groups are similar in survival, with a slightly higher percentage with Enbrel.
As mentioned above, drug safety is a fundamental factor in influencing this survival. The use of BS has been the subject of debate in recent years and they have been used in clinical practice with caution, mainly due to a lack of knowledge of their possible adverse effects.27 In this regard, adverse effects were somewhat more frequent in the Enbrel group than in the Benepali group (46% vs. 36%), than mild-moderate adverse effects. However, drug discontinuation was higher with Benepali than with Enbrel (30.4% vs. 18.6%) due to inefficacy rather than adverse effects. This higher drug discontinuation rate may be the reason why the survival we observed with Benepali was somewhat lower than with Enbrel. In this regard, although both drugs showed efficacy, it is known that a significant proportion do not respond to initial treatment or efficacy is lost over time.3 In the phase III RCT by Emery et al.23 the percentage of adverse events in the Benepali group was 58% compared to 60% with Enbrel during the study up to week 52. Also, in the phase III CT28 the incidence of treatment emergent adverse events was comparable between the two groups (55.2% vs. 58.2%). In our data, the safety profile of Benepali was comparable to that of Enbrel and similar to those observed in these and other etanercept studies.22,29 They are also consistent in that most adverse effects are mild-moderate. The most frequently described adverse effects are always respiratory infections, liver profile disturbances and injection site reactions.30–32
In our study we show that, considering the cost-effectiveness of Benepali, the use of this BS is as effective as the reference drug, but more cost-effective, representing a great saving for the hospital.
One of the most important aspects of this study is the progress that is being made with the new incorporation of these treatments, with similar efficacy to their reference drugs, resulting in a reduction in hospital costs. According to our data, the savings from treatment in the Benepali group compared to Enbrel is €1,747.2/patient-year. A study by Kowalik et al.33 describes the introduction of BS as a measure to reduce direct and indirect costs in RA. Moreover, not only do they show savings in the prescription of the BS drug but also, after marketing, a reduction in the price of the original drug. In general, biological drugs are associated with high cost, which restricts their use and leads to inequality in different countries.34 Since BS are a lower-cost alternative to reference drugs, the current inequality in healthcare provision could be gradually diminishing.35 In fact, budget impact analyses following the introduction of BS, such as infliximab, in different European countries have shown that switching to BS therapy results in significant cost savings and increased access to these therapies.36
Our study has some limitations, primarily its retrospective design. Although the design is technically retrospective, it is in fact mostly prospective, as all variables analysed were collected prospectively and systematically, based on a previously designed protocol. This explains the absence of data loss and the very consistent results. A second limitation would be the small number of patients, which would influence the statistical power. However, all the patients are from routine clinical practice, and despite being a heterogeneous sample because we include different diseases in the study (unlike the RCTs mentioned, which only include RA), we found differences in the main variables in the use of the BS. Finally, there are limitations due to different follow-up times for each patient and disease course. The choice of treatment with Enbrel or Benepali, fell to each physician according to the characteristics of the patient and, obviously, taking cost into consideration. Therefore, since Benepali’s marketing, there has been increased prescription of Benepali compared to Enbrel, therefore more patients can be treated at the same price. The recent marketing of Benepali and increase in its prescription explain why, in our study, the percentage of patients who had previously received other biological drugs was higher with Enbrel than with Benepali (39% vs. 17%, P = .005), as well as the longer disease course in the patients with Enbrel.
In conclusion, these results demonstrate the effectiveness of Benepali in safely controlling the signs and symptoms of patients with inflammatory joint diseases, comparable to Enbrel in clinical practice. These results are comparable to those of the CTs. In addition, this disease control is maintained at a lower cost. Benepali represents a reduction in hospital costs that should be considered. These benefits are homogeneous, according to our study, in the different rheumatic diseases that we have included here; however, further studies with a larger number of patients and the inclusion in clinical practice of other already marketed BS are necessary to consolidate these results.
FundingResearch grants from the Spanish Rheumatology Foundation (FER). We received no other financial support or other benefits from commercial sources.
Conflict of interestsMarta Rojas-Giménez: talks for MSD.
Natalia Mena-Vázquez: talks for MSD, UCB and Roche.
Carmen María Romero-Barco: talks/presentations for AbbVie.
Sara Manrique-Arija: talks/presentations for AbbVie, Pfizer, and MSD.
Inmaculada Ureña-Garnica has no conflict of interest to declare.
Gisela Diaz-Cordovés: talks/presentations for AbbVie, Pfizer, and MSD.
Francisco Gabriel Jiménez-Núñez: talks/presentations for AbbVie, Pfizer, and MSD.
We would like to thank the Sociedad Española de Reumatología for the translation.
Please cite this article as: Rojas-Giménez M, Mena-Vázquez N, Romero-Barco CM, Manrique-Arija S, Ureña-Garnica I, Diaz-Cordovés G, et al. Efectividad, seguridad y análisis económico de Benepali en práctica clínica. Reumatol Clin. 2021;17:588–594.