Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects multiple organs and systems. B cells have a critical role in the pathogenesis of SLE. Rituximab (RTX) is a drug composed of chimeric monoclonal antibodies against the CD20 protein, producing a depletion of B lymphocytes.
ObjectiveTo analyze the effectiveness and safety of RTX in patients with SLE in clinical practice.
MethodsCollection of retrospective variables of the medical records of 20 patients with SLE treated with RTX in 2 hospitals (Hospital de la Santa Creu i Sant Pau, and Hospital del Mar, in Barcelona, Spain). We evaluated demographic, clinical, serological and treatment variables.
ResultsThere was a statistically significant association in the following variables collected in the study before and after treatment: there was a decrease in the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (P<.001), erythrocyte sedimentation rate (P=.017), use of glucocorticoids (P=.025) and IgM values (P=.031), as well as an increase in the C4 values (P=.014) after treatment with RTX. A patient with SLE, antiphospholipid syndrome, complex comorbidity and multiorgan lupus involvement died after developing a septic process, months after receiving a single treatment cycle with RTX.
ConclusionsAlthough RTX currently has no official indication approved for SLE, our data suggest that it may be effective in reducing the activity of the disease and as a steroid-sparing agent, with an acceptable safety profile. However, larger follow-up periods with a greater number of patients are needed to solve the remaining doubts about the use of RTX in SLE.
El lupus eritematoso sistémico (LES) es una enfermedad crónica autoinmune que afecta a múltiples órganos y sistemas. Las células B tienen un papel crítico en la patogénesis del LES. El rituximab (RTX) es un fármaco compuesto por anticuerpos monoclonales quiméricos contra la proteína CD20, produciendo una depleción de linfocitos B.
ObjetivoAnalizar la efectividad y la seguridad de RTX en pacientes con LES en práctica clínica.
MétodosRecogida de variables retrospectiva de los historiales médicos de 20 pacientes con LES tratados con RTX en 2centros hospitalarios (Hospital de la Santa Creu i Sant Pau y Hospital del Mar, en Barcelona). Se evaluaron variables demográficas, clínicas, serológicas y de tratamiento.
ResultadosHubo asociación estadísticamente significativa entre las variables a estudio pre y postratamiento siguientes: descenso de SLEDAI (p<0,001), de VSG (p=0,017), en uso de glucocorticoides (p=0,025), de IgM (p=0,031) y aumento de C4 (p=0,014) tras el tratamiento con RTX. Un paciente con LES, síndrome antifosfolipídico, importante comorbilidad y afectación lúpica multiorgánica falleció tras un proceso séptico meses después de haber recibido un único ciclo de tratamiento con RTX.
ConclusionesA pesar de que actualmente RTX no tiene indicación aprobada en ficha técnica para LES, podemos indicar que es efectivo en cuanto a la reducción de la actividad de la enfermedad, ahorrador de corticoides y con un perfil de seguridad aceptable. Se necesitan mayor tiempo de seguimiento y mayor número de pacientes para resolver las dudas todavía existentes sobre el uso de RTX en LES.
Systemic lupus erythematosus (SLE) is a chronic autoimmune heterogeneous disease characterized by the possible involvement of multiple organs and systems.1 In Spain its estimated prevalence is 9 out of every 10,000 inhabitants.2 Although its prognosis has improved in recent years, the quality of life of the patient with SLE is clearly inferior to that of the general population3 and mortality is 2–3 times higher.4 In Spain few studies have been made with SLE patients that have provided consistent data, either because of their local character or because the follow-up periods have been very short.5
B cells have a critical role in SLE pathogenesis, including the production of cytokines, the presentation of auto antigens, T cell activation and the production of auto antibodies. The loss of tolerance of B cells may be an essential element in the SLE pathogenesis, with this as a strong justification for the study of treatments aimed at modifying the effects of B cells on immunity.6
Rituximab (RTX) is a drug composed of chimeric monoclonal antibodies against the CD20 protein, which is mostly found in the surface area of B lymphocytes.7 This drug produces a depletion of B lymphocytes with potential effect on immune-mediated diseases characterized by an excess of B cell colonies, hyperactive B cells or dysfunctional B cells.8 To date, only 2 clinically controlled trials have been published which have assessed the efficacy and safety of RTX in moderate-severe SLE (EXPLORER) and SLE with active lupus nephritis (LUNAR), neither of which detected any significant differences between RTX and the placebo after one year of treatment.9,10 Despite this, patient series have been published in which RTX for SLE provides benefits in cases which are refractory to standard treatment.11–13
We present a series of patients with SLE treated with RTX in 2 centres in the same Urbana area. Our aim was to analyze the clinical characteristics of the patients treated and to assess their effectiveness and safety.
MethodsPatientsA retrospective observational study in the form of a case series. The cases were obtained from patients with SLE who regularly went to the rheumatology outpatients department, the monographic Lupus consultation and rheumatology day Hospital of 2 centres in Catalonia (Spain) between March 2007 and September 2015 in the Hospital de la Santa Creu y Sant Pau and the Hospital del Mar/Parc de Salut Mar-IMAS of Barcelona. The patients had to comply with at least 4 of the 11 classification criteria for SLE of the American College of Rheumatology de 1997.14 Data collection of the patients was undertaken in accordance with that stipulated in the Declaration of Helsinki.
VariablesSociodemographic variables were: age, sex, duration of the disease. Clinical data were: activity (SLEDAI15) before and after treatment with RTX (1g/iv on days 1 and 15, administered every 6 months by protocol). Serological data were: anti-ADNdc (0–300UI/ml Hospital del Mar; 1/10UI Hospital de la Santa Creu i Sant Pau) antibodies, erythrocyte sedimentation rate (ESR) (0–37mm/h both centres), reactive C protein (0–0.8mg/dl both centres), C3 complement (0–90mg/dl) (85–193mg/dl) and C4 (0–10mg/dl) (12–36mg/dl), immunoglobulin levels IgA, IgG, IgM (IgA 70–400mg/dl, IgG 700–1.600mg/dl, IgM 40–230mg/dl) (IgA 69–382mg/dl, IgG 723–1685mg/dl, IgM 40–230mg/dl), blood differential (leukocytes 4.49–12.68×10E9/l, neutrophils 2.10–8.89×10E9/l, lymphocytes 1.26–3.35×10E9/l) (leukocytes 3.80–11.00×10E9/l, neutrophils 1.80–7.00×10E9/l, lymphocytes 1.00–4.00×10E9/l), total depletion of lymphocytes B (CD 19<1%), prednisone dose (mg/day). Treatment data were: number of RTX cycles received by each patient during follow-up. Data were collected on adverse events, serious infections (defined as those which required hospital admission or intravenous medication) and deaths.
Statistical analysisPre (immediately prior to the administration of a new cycle of RTX) and post-treatment data (33 months after administration of treatment) were analyzed, with categorical variables being described with frequencies and percentages and continuous variables with median and percentiles of 25 and 75. The association between clinical variables before and after treatment with RTX was assessed using the Pearson or Mcnemar Chi-square test, as applicable for the categorical variables and the Mann–Whitney U test for the continuous variables. All analyses were 2-tailed and P values were considered significant if P<.05. Due to the different units and normality ranges for the variable data: anti-ADNdc, C3, C4, immunoglobulin's IgA, IgG, IgM and blood differential of the patients of both centres, only the upper, lower or those within the normal distribution range were considered, without including the exact numerical value of each variable in the analysis. As a result, we were obliged to treat these continuous variables as categorical to be able to analyze the results of both centres jointly. Statistical analysis was performed with the SPSS 18.0 (IBM Inc., SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.) software.
ResultsA total of 20 patients were included in the study, 16 women and 4 men, with a mean age of 43.9±15.8 years, with a follow-up time from diagnosis of SLE up to the beginning of treatment with RTX of 10.9±8.8 years, and they received a mean of 3.5±1.7 cycles of RTX. In the 20 patients, the manifestations resistant to standard immunosuppressant treatment and for whom treatment with RTX was indicated were: arthritis (n=10), nephropathy (n=8), haematological involvement (n=6), cutaneous (n=5), pericarditis (n=3), shrink lung (n=1) and central nervous system involvement (n=1). The immunosuppressant drugs which they had previously received and those which had not presented with a response were: mycophenolate mofetil (MMF) (n=8), chloroquine hydroxichloroquine (n=8) and methotrexate (n=3). Of the 20 patients, depletion of B lymphocytes was monitored in 15 patients (% CD 19 3 months after the perfusion). Of these 15 patients, 8 presented with a total depletion of B lymphocytes (CD 19<1%).
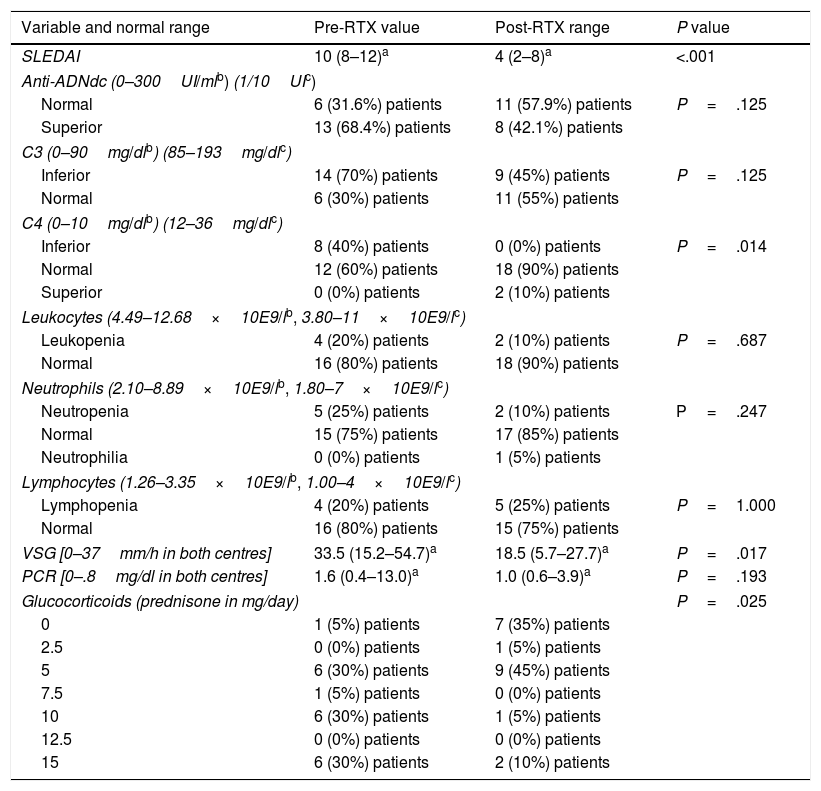
There was a statistically significant association in the following variables collected before and after treatment: a mean drop in SLEDAI from 10 (8–12) (p25–p75) to 4 (2–8) points (P<.001), a drop in ESR from a mean of 33.5 (15.2–54.7) to 18.5 (5.7–27.7) (P=.017), a drop in the use of glucocorticoids where before treatment 65% of patients took doses over 5mg/day and after treatment 85% of patients took doses under or equal to 5mg/day (P=.025), a drop in IgM from 1 to 7 patients with values under the normal range (P=.031) and an increase in C4 values with 100% of patients with normal or high C4 values after treatment with RTX (P=.014) (Table 1). There was no significant reduction in plasmatic levels of anti-DNAdc antibodies but there was a trend towards a reduction (this fell in 8 out of the 13 patients for whom it was high prior to treatment with RTX) (P=.125). For the results of the categorical variables in Table 1 which indicate “low”, “high” or “normal” reference is made to how many patients in percentage are found with values below, above or within the normal range of each variable, respectively.
Associations between treatment with RTX and the different variables before and after treatment of the patients with SLE in both centres.
| Variable and normal range | Pre-RTX value | Post-RTX range | P value |
|---|---|---|---|
| SLEDAI | 10 (8–12)a | 4 (2–8)a | <.001 |
| Anti-ADNdc (0–300UI/mlb) (1/10UIc) | |||
| Normal | 6 (31.6%) patients | 11 (57.9%) patients | P=.125 |
| Superior | 13 (68.4%) patients | 8 (42.1%) patients | |
| C3 (0–90mg/dlb) (85–193mg/dlc) | |||
| Inferior | 14 (70%) patients | 9 (45%) patients | P=.125 |
| Normal | 6 (30%) patients | 11 (55%) patients | |
| C4 (0–10mg/dlb) (12–36mg/dlc) | |||
| Inferior | 8 (40%) patients | 0 (0%) patients | P=.014 |
| Normal | 12 (60%) patients | 18 (90%) patients | |
| Superior | 0 (0%) patients | 2 (10%) patients | |
| Leukocytes (4.49–12.68×10E9/lb, 3.80–11×10E9/lc) | |||
| Leukopenia | 4 (20%) patients | 2 (10%) patients | P=.687 |
| Normal | 16 (80%) patients | 18 (90%) patients | |
| Neutrophils (2.10–8.89×10E9/lb, 1.80–7×10E9/lc) | |||
| Neutropenia | 5 (25%) patients | 2 (10%) patients | P=.247 |
| Normal | 15 (75%) patients | 17 (85%) patients | |
| Neutrophilia | 0 (0%) patients | 1 (5%) patients | |
| Lymphocytes (1.26–3.35×10E9/lb, 1.00–4×10E9/lc) | |||
| Lymphopenia | 4 (20%) patients | 5 (25%) patients | P=1.000 |
| Normal | 16 (80%) patients | 15 (75%) patients | |
| VSG [0–37mm/h in both centres] | 33.5 (15.2–54.7)a | 18.5 (5.7–27.7)a | P=.017 |
| PCR [0–.8mg/dl in both centres] | 1.6 (0.4–13.0)a | 1.0 (0.6–3.9)a | P=.193 |
| Glucocorticoids (prednisone in mg/day) | P=.025 | ||
| 0 | 1 (5%) patients | 7 (35%) patients | |
| 2.5 | 0 (0%) patients | 1 (5%) patients | |
| 5 | 6 (30%) patients | 9 (45%) patients | |
| 7.5 | 1 (5%) patients | 0 (0%) patients | |
| 10 | 6 (30%) patients | 1 (5%) patients | |
| 12.5 | 0 (0%) patients | 0 (0%) patients | |
| 15 | 6 (30%) patients | 2 (10%) patients | |
| IgA (70–400mg/dlb, 69–382mg/dlc) | |||
| Inferior | 3 (15.8%) patients | 2 (10.5%) patients | P=.368 |
| Normal | 13 (68.4%) patients | 15 (78.9%) patients | |
| Superior | 3 (15.8) patients | 2 (10.5%) patients | |
| IgM (40–230mg/dlb, 0–230mg/dlc) | |||
| Inferior | 1 (5.3%) patients | 7 (36.8%) patients | P=.031 |
| Normal | 18 (94.7%) patients | 12 (63.2%) patients | |
| IgG (700–1.600mg/dlb, 723–1.685mg/dlc) | |||
| Inferior | 0 (0%) patients | 1 (5.3%) patients | P=.135 |
| Normal | 10 (52.6%) patients | 12 (63.1%) patients | |
| Superior | 9 (47.4%) patients | 6 (31.5%) patients | |
One patient with SLE, antiphosphalipid syndrome, serious comorbidities (hypertension, diabetes, a history of ischaemic heart disease, polymedicated) and multiple organ lupus involvement died after a septic process month, having received a single cycle of treatment with RTX. The other patients suffered from no major events of serious infections or mortality during follow-up.
DiscussionThe experience of the 2 centres led to significant results in favour of the use of RTX in SLE patients refractory to standard treatment with regards to the effectiveness expressed as a reduction in the SLEDAI data after treatment. This was in keeping with some of the previously published experiences.13,16,17
To date, there have only been 2 clinically controlled trials which have assessed the efficacy and safety in the use of RTX in SLE, the LUNAR9 and EXPLORER10 studies. In total, 401 patients were included, without being able to demonstrate the efficacy of RTX on comparing them with the control group. It is of note that the selected patients in the 2 trials maintained the previously prescribed immunosuppressant treatment regime. In the LUNAR study response was compared between patients with lupus nephritis treated with glucocorticoids and MMF randomized to placebo or RTX, with detection of a partial, but not complete renal response.9 In the EXPLORER study, the RTX group did not present a significant British Isles Lupus Assessment Group (BILAG)18 response compared with the placebo group treated with AZA, MMF and glucocorticoids.10 In both studies RTX was compared with placebo in patients in treatment with immunosuppressant's and glucocorticoids, which could have minimized the differences regarding efficacy results.12 Furthermore, activity of the SLE was measured with simpler indexes than those in other randomized clinical trials, such as for example in the efficacy study of belimumab, the BLISS study, in which a composite activity index was used called the SLE Responder Index (SRI).19 Therefore, despite not having demonstrated a clearly positive effect when added to treatment with MMF in clinical trials, RTX may be an effective drug in the control of patients refractory to standard immunosuppressant treatment, and particularly cyclophosphamide and MMF.
It is increasingly evident that RTX is effective in the treatment of as SLE for both extra renal manifestations and for renal ones. Numerous series and registers have proven its efficacy in the treatment of SLE, including the UK-Biogeas11 register, the BILAG-BR20 register, the SLEIMAB12 study and the French AIR13 register, where the results for efficacy vary from 49% to 71% of complete response at 6 months in overall disease manifestations.
If we assess the results of the different studies regarding how organs and systems are involved, we may detail the benefits of RTX in different conditions of SLE. In the SLEIMAB cohort 93% of patients who presented with active arthritis on treatment initiation responded to RTX12; a review of 100 patients treated with RTX also observed this efficacy of RTX at joint level,21 and we also found favourable data in the treatment of arthritis in the AIR register.13 Regarding its effect on the central nervous system, the experience with RTX is low and limited to various series of published cases.22 Results on haematological treatment have also been positive, especially in thrombocytopenias.17,21 Regarding nephropathy, there is broad evidence of its effectiveness,23,24 and even the new trends for researching the termination in the use of glucocorticoids in maintenance treatment of this condition. The treatment regime descried is “rituxilup”, in which it is proven in 50 patients that the use of the consistent treatment regime of 2 doses of RTX (1g/iv) combined with methylprednisolone (500mg/iv) on days 1 and 15, and maintenance treatment with oral MMF in patients with nephropathy, may avoid the need for glucocorticoids in maintenance treatment.25
For all of the above, RTX should be reserved for patients with moderate or severe activity and insufficient response, or patients who are resistant to standard treatment with glucocorticoids and immunosuppressants.
The need for high doses of glucocorticoids for disease maintenance may also constitute another possible indication.26 in our study we obtained significant results regarding a reduction in the mean dose of pre and post treatment coracoids, as has been observed in other series.13,17,20,27,28 This is important because it would avoid the side effects of the high doses of glucocorticoids over long periods of time.
Other data which support RTX reducing the inflammatory activity in SLE is our study obtaining significant results in the reduction of pre and post treatment ESR and the increase of C4 values. Regarding anti-DNAdc values, there was no significant reduction in plasmatic levels pre and post treatment, but there was a tendency towards reduction (it fell in 8 of the 13 patients where it was high prior to treatment with RTX). This was previously seen in other published series.9,11
With regards to safety, no large series on pharmaco-vigilance and risk management in SLE exist but it has been seen that RTX use is safe for at least 6–12 months.29 It has been broadly documented that repetitive treatment with RTX induces hypogammaglobuliaemia.30 The consequences of sustained hypogammaglobuliaemia with repeated use of RTX is as yet unknown. In our cohort of patients there was a significant relationship for the drop in pre and post treatment IgM, without this being related to any type of infectious process. Moreover, we found no serious adverse effects, and no cases of hepatitis B in our series. Regarding deaths, one patient with SLE and antiphospholipidic syndrome with multiple comorbidities and multi-organ involvement died from a septic process months after treatment with RTX.
The treatment response marker with RTX in our study was to monitor B cell levels, which has traditionally been performed in patients with rheumatoid arthritis treated with RTX.8 Of the 20 patients; the depletion of B lymphocytes was monitored (percentage of CD 19 after 3 months of perfusion) in 15. Of these 15 patients, 8 presented with a total depletion of B lymphocytes (CD 19<1%).
Several questions remain unresolved. For example, whether previous immunosuppressant therapy should be maintained or how many RTX cycles should be administered after treatment initiation. In general, it is recommended that previous immunosuppressant therapy be maintained n those patients where response has been insufficient, but particular attention should be paid to the risk-benefit of combined use with certain immunosuppressant's, since there is a high risk of toxicity, with the RTX-CFM combination, for example. In absence of lupus activity, the systematic administration of new cycles of RTX is not recommended, except in patients with particularly severe outbreaks of the disease.26
The main study limitations were the small sample size, the difference between the centres’ data collection, the different normalcy ranges, and the units of each variable depending on the centre from which they came. All of this led to a consequent loss of internal and external validity.
To conclude, and despite the fact RTX currently has no approved indication in the summary of product characteristics for SLE, after the results of our study we report that it is effective with regards to the reduction of several measurements of activity and as a glucocorticoids-sparing agent. Regarding safety, in our series one patient died but this was a complex case with major SLE activity and secondary antiphospholipid syndrome, but the use of RTX presented an acceptable safety profile in the other patients. A longer follow-up period and a greater number of patients are needed to solve the remaining doubts about the use of RTX in SLE.
Conflict of interestsThe authors have no conflict of interests to declare for this study.
Please cite this article as: Gómez VJ, Carrión-Barberá I, Salman Monte TC, Acosta A, Torrente-Segarra V, Monfort J. Efectividad y seguridad de rituximab en el lupus eritematoso sistémico. Serie de casos. Experiencia de 2 centros. Reumatol Clin. 2020;16:391–395.