Analyze adverse events (AE) and survival associated with biologic therapies (BT) in the BIOBADAGUAY, the Paraguayan Uruguayan registry of adverse events.
MethodsProspective, observational study of undetermined duration. Patients on biologic therapy at initiation and controls were included. Clinical, biological and treatment variables were registered.
Results826 registers were entered (650 BT and 176 controls). 70.9% were women and rheumatoid arthritis (RA) was the most frequent diagnosis (63.2%). The BT most often used was adalimumab and the main cause of discontinuation was loss of efficacy (42.1%). The incidence of AE of patients on BT was 143.9 (128.8–160.8) per 1000 patients/year. In the comparative study of AE related to diagnosis, juvenile idiopathic arthrosis (JIA) was associated with a higher overall number of AE (RTI = 2.3, 95% confidence interval [CI] 1.6–3.4; P = 4.27 e-06), whereas RA was associated with a higher number of serious AE (RTI = 2.2, 95% CI 1.2–4.1; P = 1.17 e-0.2). On the other hand, treatment with tocilizumab was associated with a higher rate of AE (RTI = 2.69, 95% CI 1.9–3.82; P = 3.13 e-08). In JIA, treatment with corticosteroids and number of previous BT was associated with a decrease in BT survival.
ConclusionIn this first report of the BIOBADAGUAY registry, the main cause of BT discontinuation was loss of efficacy. In terms of the diagnosis involved, RA and JIA were associated with a higher risk of AE. In this registry, variables related to a shorter survival of BT were identified.
Analizar los acontecimientos adversos (AA) y la supervivencia de las terapias biológicas (TB) en el registro paraguayo-uruguayo de AA, Biobadaguay.
MétodoEstudio observacional, prospectivo de duración indeterminada. Se han incluido pacientes al inicio de la TB y controles. Se han registrado variables clínicas, biológicas y relacionadas con el tratamiento.
ResultadosSe realizaron 826 registros (650 TB y 176 controles). El 70,9% fueron mujeres y el diagnóstico más frecuente fue la artritis reumatoide (AR) (63,2%). La TB más utilizada fue el adalimumab (56,6%) y la causa más frecuente de discontinuación la ineficacia (42,1%). La incidencia de AA en pacientes con TB fue 143,9 (128,8–160,8) por 1000 pacientes/año. En el estudio comparativo de AA en función al diagnóstico se observó que la Artritis Idiopática Juvenil (AIJ) se asoció a más AA globales (RTI = 2,3, IC95%: 1,6−3,4; p = 4,27e-06) mientras que la AR se asoció a un mayor número de AA graves (RTI = 220 IC95%: 1,2–4,1 P = 1,17e-02). Por otro lado, el tratamiento con tocilizumab se asoció a una mayor tasa de AA (RTI = 2,69, IC95%1,90−3,82; p = 3,13e-08). El diagnóstico de AIJ, el tratamiento con corticoides y el número de TB previas se asociaron a la disminución de la supervivencia de las TB.
ConclusiónEn este primer informe del registro BIOBADAGUAY, la principal causa de discontinuación de la TB fue la ineficacia. En relación al diagnóstico, la AR y la AIJ se asociaron a un mayor riesgo de AA. En este registro, se identificaron variables relacionadas a una menor supervivencia de las TB.
The use of biological therapies (BT) has revolutionised the treatment of chronic rheumatic diseases. By regulating the cellular and molecular imbalance characteristic of targeted inflammation in these diseases, they have enabled a greater number of patients to achieve remission or low disease activity.1
Because the action of these agents is not limited to the joint, their use can be associated with unexpected side events. Several biological agents have currently been approved for use in rheumatology, and each has a different molecular structure and mechanism of action. This makes it possible that each agent can generate specific secondary events.2
A large number of clinical trials and observational studies have demonstrated the efficacy and safety of BT, however, long and medium-term follow-up has been limited.3 Therefore it was considered necessary to follow up these agents in real-world scenarios and in unselected patients. Thus, BT registries have emerged in different countries in recent years, most of them in developed countries.4,5 Data on BT in emerging countries are currently scarce.6–8 The socioeconomic, epidemiological and demographic situation of these countries is different to that of developed countries, which could influence the therapeutic response and presentation of certain adverse events (AE).
In view of this, in 2008, within the framework of BiobadAmérica, the Uruguayan registry of AE with BiobadaUruguay biological therapies was started as an initiative of the Uruguayan Society of Rheumatology. Later, in 2012 the Paraguayan Society of Rheumatology started the registry of AE with BiobadaParaguay biological therapies. In order to adapt to the arrival of biosimilar agents, in addition to establishing a stricter follow-up of patients, BiobadAmérica entered a new registration phase. In this context, Uruguayan and Paraguayan society joined this new phase in a joint platform from which Biobadaguay (the Paraguayan-Uruguayan registry of AE with BT) emerged.
The objective of this study is to analyse the safety and survival of BT in the joint Biobadaguay registry.
Material and methodsBiobadaguay is a prospective observational study of the safety of BT in patients with rheumatological diseases. The registry includes patients from Paraguay and Uruguay in the same platform: each country participates independently, with a common coordinator for both. Patients are included in the registry as they start the target treatment and remain in the registry while they follow it. Since it is not known whether one-time exposure to biologics has long-term effects, patients are considered to be exposed indefinitely, and therefore the included patient is followed even after the BT is stopped. Patients who enter the registry are evaluated at least once a year, unless there is notified change in treatment (e.g., discontinuation, drug changes) or occurrence of AE. Within the registry, there is a group of patients with autoimmune inflammatory rheumatic diseases treated traditionally and without BT who make up the control group.
This registry includes all patients from the first phase of the project who have the updated data required by the new platform and any patient starting a new BT
Registration takes place on an electronic platform (https://biobadaguay.ser.es) which is accessed by a User ID and password for each participating centre. The admitted person is given a code number to maintain their anonymity and preserve the confidentiality of the data. The registry has 3 sections: (a) patient data, including demographic data (age, sex, etc.), clinical data (diagnosis, data of diagnosis and disease specifications), comorbidities, Charlson comorbidity index9; (b) treatment, including the biological drug (dose and administration route) or, if the patient is in the control group, the date treatment began, tuberculosis study, disease activity at onset (DAS28 or BASDAI/ASDAS or SLEDAI), concomitant treatment, date of treatment discontinuation and reason. This section records the annual follow-up visit (drug, dose and administration route), disease activity and concomitant treatment. The biological drug appears with the trade name to distinguish it from the biosimilar; (c) AE, where the type of AE, outcome and its severity are recorded. The MedDRA (Medical Dictionary for Drug Regulatory Activities) nomenclature is used to classify each AE (www.meddra.org). Naranjo’s algorithm is used for the causality study of AE.10
For quality control of the data, 100% of the cases are monitored online. The monitor contacts the manager of each centre through online messages to correct errors or make updates.
Statistical analysisFor the AE, we calculated the incidence rate (IR) per 1000 patients/year with the 95% confidence intervals (95% CI) based on the Poisson distribution. For the comparative study of incidence of AE, Poisson regression was used, obtaining the incidence rate ratio (IRR), its 95% CI and P value of statistical significance (nominal significance value .05). The mean survival of BT was determined with the Kaplan–Meier estimator. The Cox regression model was used to evaluate the effect of the predictor variables, which obtained the value of statistical significance and risk ratio. Patients who were still receiving treatment at the time of study closure were registered on the right. When independently analysing survival with respect to each reason for discontinuation, the remaining reasons were also registered on the right side at the time of discontinuation. R software v. 3.4.0 (https://www.r-project.org/) was used for the statistical analysis.
ResultsIn Biobadaguay, the Hospital Central del Instituto de Previsión Social and the Hospital de Clínicas de la Universidad Nacional de Asunción participated in Paraguay. In Uruguay, the National Institute of Rheumatology, the Hospital Pereira Rosell and the Centro Asistencial Médico de Soriano participated. For the present study, all patients registered from October 1, 2008 to May 31, 2017 were included.
Clinical characteristics of the patients and treatmentsFrom November 2016 to May 2017, 826 registrations were made, of which 700 belonged to the previous phase of registration (data registered since 2008 and updated to the current platform). Of these, 176 corresponded to controls and 650 to BT. Women accounted for 586 (70.9%), the mean age at the start of treatment was 46.6 ± 14.4 years and the mean disease duration at the start of treatment was 11 ± 8.8 years. The most frequent comorbidity observed was arterial hypertension in 197 patients (23.8%). The most frequent diagnosis was rheumatoid arthritis (RA) in 63.2% of patients, followed by juvenile idiopathic arthritis (JIA) in 11.3%. The main clinical-epidemiological characteristics of the population are presented in Table 1 and the distribution of the population in terms of diagnosis in Table 2.
Clinical-demographic characteristics of patients at the start of treatment.
| Variables | Overall | Controls | BT |
|---|---|---|---|
| Patients (n) | 826 | 176 | 650 |
| Age (mean ± SD) | 46.6 ± 14.4 | 49.4 ± 14.1 | 45.7 ± 14.3 |
| Women (%) | 70.9 | 77.0 | 68.9 |
| ANA+ (%) | 13.1 | 14.7 | 12.7 |
| FR (%) | 46.3 | 58.5 | 43.2 |
| Anti-CCP (%) | 42.7 | 66.3 | 36 |
| DAS28 (mean ± SD) | 5.2 ± 1.2 | 4.5 ± 1.5 | 5.4 ± .9 |
| BASDAI (mean ± SD) | 6.4 ± 2.04 | 5.8 ± 1.49 | 6.4 ± 2.1 |
| Comorbidities n (%) | |||
| Arterial hypertension | 197 (23.8) | 58 (32.95) | 141 (21.7) |
| Ischaemic heart disease | 8 (1.0) | 3 (1.7) | 5 (.8) |
| Heart failure | 8 (1.0) | 3 (1.7) | 5 (.8) |
| Diabetes | 36 (4.4) | 11 (6.3) | 25 (3.8) |
| Dyslipidaemia | 53 (6.4) | 16 (9.1) | 37 (5.7) |
| Osteoporosis | 29 (3.5) | 11 (6.3) | 18 (2.8) |
| Kidney failure | 2 (.2) | 0 (.0) | 2 (.3) |
| Chronic obstructive pulmonary disease | 10 (1.2) | 3 (1.7) | 7 (1.1) |
| Smoking | 111 (13.4) | 15 (8.5) | 96 (14.8) |
| Cancer | 9 (1.1) | 7 (3.9) | 2 (.3) |
| Charlson Index (mean ± SD) | .29 ± .6 | .31 ± .59 | .29 ± .55 |
ANA: antinuclear antibodies; anti−CCP: anti-cyclic citrullinated peptide antibodies; BASDAI: bath ankylosing spondylitis disease activity index; BT: biological therapies DAS28: disease activity score; RF: rheumatoid factor; SD: standard deviation; N: number.
Distribution of patients according to diagnosis.
| Diagnosis | Overall (n = 826) n (%) | Controls (n = 176) n (%) | BT (n = 650) n (%) |
|---|---|---|---|
| Rheumatoid arthritis | 522 (63.2) | 144 (81.8) | 378 (58.2) |
| Juvenile idiopathic arthritis | 93 (11.3) | 1 (.6) | 92 (14.2) |
| Ankylosing spondylitis | 87 (19.5) | 6 (3.4) | 81 (12.5) |
| Psoriatic arthritis | 58 (7.0) | 6 (3.4) | 52 (8.0) |
| Systemic lupus erythematosus | 18 (2.2) | 16 (9.1) | 2 (.3) |
| Undifferentiated spondyloarthopathy | 10 (1.2) | 1 (.6) | 9 (1.4) |
| Enteropathic arthritis | 8 (.9) | 0 (0) | 8 (1.2) |
| Seronegative polyarthritis | 6 (.8) | 2 (1.1) | 4 (.6) |
| Juvenile undifferentiated spondyloarthropathy | 4 (.5) | 0 (0) | 4 (.6) |
| Uveitis without rheumatic disease | 4 (.5) | 0 (0) | 4 (.6) |
| Polymyositis | 3 (.4) | 0 (0) | 3(.5) |
| Juvenile Ankylosing Spondylitis | 3 (.4) | 0 (0) | 3 (.5) |
| Non-radiological axial spondylitis | 3 (.4) | 0 (0) | 3 (.5) |
| Vasculitis | 3 (.4) | 0 (0) | 3 (.5) |
| Overlap syndrome | 1 (.1) | 0 (0) | 1 (.2) |
| Sarcoidosis | 1 (.1) | 0 (0) | 1 (.2) |
| Reactive arthritis | 1 (.1) | 0 (0) | 1 (.2) |
| Mixed connective tissue disease | 1 (.1) | 0 (0) | 1 (.2) |
BT: biological therapies.
Nine hundred and seventy-three treatment cycles were registered, 778 corresponded to BT and 195 to controls. Of the 778 BT treatments, 440 (56.6%) were adalimumab, 184 (23.70%) etanercept, 75 (9.6%) tocilizumab, 44 (5.70%) rituximab, 27 (3.50%) infliximab, 4 (.51%) golimumab, 3 (.38%) were infliximab biosimilars and one (.13%) abatacept. Concomitant treatments recorded at the start of BT in order of frequency were methotrexate 71.9% (n = 559), glucocorticoids 47.6% (n = 370), leflunomide 26.7% (n = 208), hydroxychloroquine 16.6% (n = 197), sulfasalazine 5.5% (n = 50) and less than 5%: azathioprine, cyclosporine, cyclophosphamide, mesalazine and mycophenolate. In relation to the controls, the treatments registered were: methotrexate 68.7% (n = 134), glucocorticoids 44.1% (n = 86), hydroxychloroquine 34.9% (n = 68), leflunomide 29.7% (n = 58), azathioprine 6.7% (n = 139) and in less than 5%: cyclophosphamide, sulfasalazine and mycophenolate. There were 254 (26.10%) discontinuations from total treatments, 26 in controls and 228 in BT. The reasons for stratified treatment discontinuation are shown in Table 3.
Stratified discontinuation by treatment.
| Treatment | n discontinuation | Inefficacy % | AE % | Remission % | Loss % | Other % |
|---|---|---|---|---|---|---|
| Control (n = 195) | 26 | 23.1 | 26.9 | 15.4 | 15.4 | 19.2 |
| BT (n = 778) | 228 | 42.1 | 29.4 | 7.5 | 7 | 14 |
| Adalimumab (n = 440) | 139 | 44.6 | 27.3 | 7.2 | 5.8 | 15.1 |
| Etanercept (n = 184) | 51 | 45.1 | 25.5 | 11.8 | 9.8 | 7.84 |
| Tocilizumab (n = 75) | 23 | 21.7 | 39.1 | 4.4 | 8.7 | 26.1 |
| Rituximab (n = 44) | 8 | 50.0 | 25.0 | .0 | 12.5 | 12.5 |
| Infliximab (n = 27) | 7 | 57.1 | 42.9 | .0 | .0 | .0 |
| Other* (n = 8) | 0 | .0 | .0 | .0 | .0 | .0 |
AE: adverse events; n discontinuation: number of discontinuations; BT: biological therapy.
We identified 358 AE, 330 (92.2%) in the BT group and 28 (7.8%) in the control group. There were 279 (77.9%) mild and 79 (22.01%) severe AE. The percentage of severe AE in the control group was 17.86% and in the BT group, 22.4%. The global IR of AE in BT patients was 143 (95% CI: 128.8–160.8). When stratified by severity, IR of 32.6 (95% CI: 25.3–40.5) and 111.6 (95% CI: 98.4–126.2) were observed for severe and mild AE, respectively. In the control group, the IR was 38.7 (95% CI 25.7–58.9), with 31.8 (95% CI 20.2–47.7) for mild AE and 6.92 (95% CI 2.3–16.1) for severe AE.
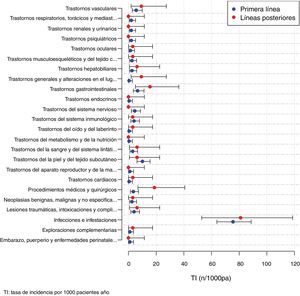
The AE observed in patients treated with BT according to the MedDRA classification are presented in Table 4. Of the 330 AE in patients with BT, infection was the most frequent AE (n = 175; 53% of total AE with BT): mild 136 (77.7%) and severe 39 (22.3%). Likewise, of the total 74 serious BT AE, infection was the most frequent (50.7%; n = 39/74). Among the 39 severe infection AE, respiratory tract infection was the most frequent (43.6%). Among the severe infection AE, 5 cases of BT were recorded. The overall incidence of infections per 1000 patients/year with BT was 76.3 (95% CI: 65.4–88.5): 59.3 (49.76–70.2) in mild infections and 17 (12.1–23.3%) in severe infections. Six neoplasms were observed, of which three were breast, one lung, one colon and one gastrointestinal. Six deaths were recorded: one from pneumonia, one from pseudomonas sepsis, one from cerebrovascular accident, one from acute myocardial infarction, one from gastrointestinal cancer and one death due to heart surgery.
Adverse events in patients with biological therapies.
| Adverse events BT: 330 | |||
|---|---|---|---|
| Type of AE | N (%) | Mild N (%) | Severe N (%) |
| Infections and infestations | 175 (53.03) | 136 (77.7) | 39 (22.3) |
| Skin and subcutaneous tissue disorders | 22 (6.67) | 22 (100) | 0 (0) |
| Gastrointestinal disorders | 16 (4.84) | 14 (87.5) | 2(12.5) |
| Vascular disorders | 14 (4.24) | 8 (57.1) | (42.9) |
| Medical-surgical procedures | 13 (3.94) | 6 (46.1) | 7 (53.9) |
| Traumatic injuries, poisonings and complications of therapeutic procedures | 10 (3.03) | 7 (70.0) | 3 (30.0) |
| Immune system disorders | 9 (2.73) | 7 (77.8) | 2 (22.2) |
| Nervous system disorders | 9 (2.73) | 8 (88.9) | 1 (11.1) |
| Disorders of the blood and lymphatic system | 8 (2.42) | 8(100) | 0 (0) |
| Benign and malignant and unspecified neoplasms | 8 (2.42) | 2 (25.0) | 6 (75.0) |
| Hepatobiliary disorders | 7 (2.12) | 6 (85.7) | 1 (14.3) |
| Musculoskeletal and connective tissue disorders | 6 (1.82) | 5 (83.3) | 1 (16.7) |
| General and administration site-related disorders | 4 (1.21) | 3 (75.0) | 1 (25.0) |
| Eye disorders | 4 (1.21) | 3 (75.0) | 1 (25.0) |
| Psychiatric disorders | 4 (1.21) | 4 (100) | 0 (0) |
| Kidney and urinary disorders | 4 (1.21) | 4 (100) | 0 (0) |
| Respiratory, thoracic and mediastinal disorders | 4 (1.21) | 1 (25.0) | 3 (75.0) |
| Complementary explorations | 3 (.91) | 3 (100) | 0 (0) |
| Pregnancy, puerperium and perinatal diseases | 2 (.61) | 2 (100) | 0 (0) |
| Cardiac disorders | 2 (.61) | 1 (50.0) | 1 (50.0) |
| Reproductive system disorders | 2 (.61) | 2 (100) | 0 (0) |
| Disorders of the ear and labyrinth | 2 (.61) | 2 (100) | 0 (0) |
| Metabolic and nutritional disorders | 1 (.30) | 1 (100) | 0 (0) |
| Endocrine disorders | 1 (.30) | 1 (100) | 0 (0) |
AE: adverse event; BT: biological therapy.
In the first part of the comparative study, the IR of AE was analysed in patients diagnosed with RA in the control group with the BT treatment group. It was observed that exposure to BT is very significantly associated with an increased risk of developing an AE of any kind (IRR = 4.14; 95% CI: 2.37–7.24; P = 6.18 × 10−7). The increased risk observed in patients with BT compared to controls was also much higher in severe AE (IRR = 12.05; 95% CI: 2.98–48.74) than in mild AE (IRR = 3.21; 95% CI: 1.74–5.91).
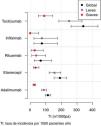
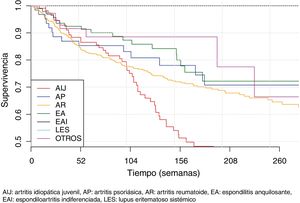
The second part of the comparative study looked at the incidence of AE in patients with BT depending on diagnosis, type of BT, line of treatment and gender. On analysing the incidence rate of AE with BT according to diagnosis (Table 5), it was observed that diagnosis of JIA was significantly associated with a higher number of overall AE when compared with the other diagnoses (IRR = 2.3; 95% CI: 1.6–3.4; P = 4.27 × 10−6). On the other hand, diagnosis of RA was significantly associated with a greater number of severe AE when compared to the other diagnoses (IRR = 2.20; 95% CI 1.2–4.1; P = 1.17 × 10−2). The incidence of AE according to diagnosis and severity of AE is shown in Fig. 1.
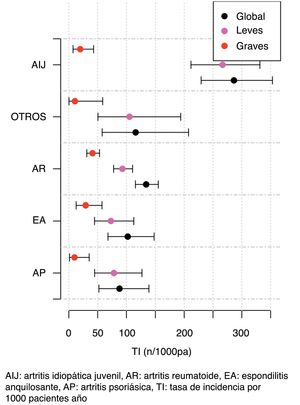
Incidence rates and incidence rate ratios of adverse events (1000 patients/year).
| Variable | Incidence | Overall IRR | P |
|---|---|---|---|
| Psoriatic arthritis | 88 (52.2−139.1) | .59 (.33−1.04) | 7.03 × 10−2 |
| Ankylosing spondylitis | 102.3 (68.0−147-8) | .68 (.40−1.17) | 1.63 × 10−1 |
| Rheumatoid arthritis | 134.2 (115-6–155-0) | .85 (.62−1.15) | 2.81 × 10−1 |
| Juvenile idiopathic arthritis | 286.4 (229.4−353.2) | 2.34 (1.63−3.37) | 4.27 × 10−6 |
| Adalimumab | 113.5 (96.9−132.1) | .57 (.43−.77) | 1.86 × 10−4 |
| Etanercept | 190.4 (152.7−234.6) | 1.44 (1.03−2.02) | 3.30 × 10−2 |
| Rituximab | 66.9 (26.9−137.8) | .45 (.18−1.13) | 8.93 × 10−2 |
| Infliximab | 75.0 (24.3−175.0) | .51 (.21−1.23) | 1.35 × 10−1 |
| Tocilizumab | 340.0 (261.8−434.2) | 2.69 (1.90−3.82) | 3.13 × 10−8 |
IRR: incidence rate ratio.
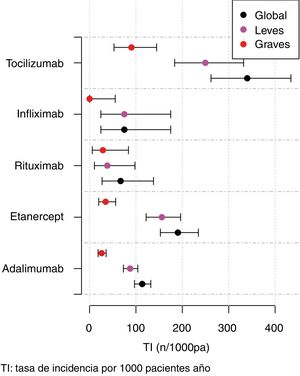
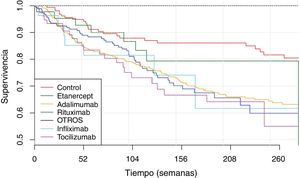
The IR was then analysed according to the type of BT and the severity of the AE (Fig. 2, Table 5). IR was also analysed according to gender and line of treatment. No significant differences were obtained according to the line of treatment when comparing the overall incidence considering the 1st line of BT with subsequent lines. The incidence of each type of AE according to treatment line is shown in Fig. 3. In reference to gender, an overall incidence of AE was significantly higher (IRR = 1.48; 95% CI: 1.05–2.08; P = 2.54 × 10−2) in women (IR = 159.7; 95% CI: 140.6–180.6) than in men (IR = 108.1; 95% CI: 85.2–135.4).
Survival of biological therapiesThe mean BT survival considering diagnosis was 322 ± 18 weeks for ankylosing spondylitis, 315 ± 22.3 weeks for psoriatic arthritis, 289 ± 8.5 weeks for RA and 233 ± 16.7 weeks for JIA (Fig. 4). Mean survival considering the biological agent was 271 ± 29.8; 265 ± 7.1; 261 ± 33.9; 258 ± 12.6 and 250 ± 20.1 for rituximab, adalimumab, infliximab, etanercept and tocilizumab, respectively (Fig. 5). The effect of certain variables on BT survival is presented in Table 6.
Factors associated with biological therapy survival.
| Variables | Cox regression model | |
|---|---|---|
| P | Hazard-ratio (95% CI) | |
| Age | 9.9 × 10−2 | .99 (.99−1.00) |
| Sex | 1.5 × 10−1 | 1.25 (.93−1.68) |
| NBT | 3.3 × 10−2 | 1.43 (1.03−1.98) |
| JIA | 2.3 × 10−4 | 1.80 (1.32−2.46) |
| PA | 2.3 × 10−1 | .72 (.42−1.23) |
| RA | 8.5 × 10−1 | 1.03 (.79−1.34) |
| AS | 7.6 × 10−2 | .65 (.41−1.05) |
| Etanercept | 7.9 × 10−1 | 1.04 (.76−1.43) |
| Adalimumab | 9.4 × 10−1 | .99 (.76−1.30) |
| Rituximab | 3.1 × 10−1 | .69 (.34−1.41) |
| Infliximab | 9.9 × 10−1 | 1.00 (.47−2.13) |
| Tocilizumab | 4.4 × 10−1 | 1.19 (.77−1.83) |
| Corticosteroids | 1.5 × 10−2 | 1.38 (1.06−1.79) |
| Methotrexate | 1.5 × 10−1 | 1.25 (.92−1.69) |
| Leflunomide | 5.2 × 10−1 | .91 (.67−1.23) |
| Sulfasalazine | 3.8 × 10−1 | 1.28 (.74−2.19) |
| Azathioprine | 6.2 × 10−2 | .27 (.07−1.07) |
| Chloroquine | 8.0 × 10−1 | 1.05 (.74−1.48) |
JIA: juvenile idiopathic arthritis; PA: psoriatic arthritis; RA: rheumatoid arthritis; AS: ankylosing spondylitis; NBT: number of previous biological therapies.
When analysing BT survival considering discontinuation due to inefficacy, we found that concomitant treatment with steroids (HR = 2.02; 95% CI: 1.33–3.06; P = 9.48 × 10−4), female gender (HR = 1.66; 95% CI: 1.01–2.72; P = 4.36 × 10−2) and number of previous BTs (HR = 1.66; 95% CI: 1.07–2.78; P = 2.56 × 10−2) are significantly associated with more discontinuation due to inefficacy. When we analysed BT survival in the group of treatments where therapy was discontinued due an AE we found that the diagnosis of RA (HR = 1.83; 95% CI: 1.07–3.15; P = 02.80 × 10−2), number of previous BT (HR = 1.76; 95% CI: 1.00–3.09; P = 4.83 × 10−2) and advanced age (HR = 1.03; 95% CI: 1.02–1.05; P = 7.14 × 10−5) were significant associated with more discontinuation. When analysing BT survival considering remission, it was observed that the diagnosis of JIA (HR = 30.58; 95% CI: 8.77–106.71; P = 7.93 × 10−8) and male sex (HR = 1.66; 95% CI: 1.01–2.72; P = 4.36 × 10−2) were the variables associated with more discontinuation. Age (HR = .83; 95% CI: .76–.90; P = 7.83 × 10−6) is associated with les discontinuation due to remission.
DiscussionThe long-term effects as well as the safety of BT have been reported in numerous studies and in various patient registries, mostly in developed countries, and most confirm the efficacy and safety of these agents.5,11–14 This is the first report since the union of the Paraguayan and Uruguayan registries under Biobadaguay (the Uruguayan-Paraguayan registry of AE with BT and biosimilars).
In this registry, clinical, epidemiological and treatment-related data from 650 patients undergoing BT treatment were analysed. In relation to gender, it was observed that similar to other South American registries (e.g., Biobadasar, Biobadamex and BiobadaBrasil), the female sex predominated and the most frequent diagnosis was RA.6–8 The most common comorbidity at the start of BT was arterial hypertension, similar to that reported by the Argentinian group.15
The BT with the highest number of records was adalimumab (56.60%), followed by etanercept (23.70%). Variable exposure to BT can be observed in the different registers. This data is probably related to the time of marketing and approval of each agent for use in a given country. In this regard, in the Argentinian registry Biobadasar, the most used drugs were etanercept (25.12%) and adalimumab (13.3%), while in BiobadaBrasil they were infliximab (39.0%) and adalimumab (28.0%) and in Biobadamex they were etanercept (25.6%) and infliximab (19.8%).6–8
The main cause of treatment discontinuation was inefficacy of the drug, followed by AE. These data are in line with previous studies in which both inefficacy and AE were found to be the most frequent reasons for discontinuation.7,8 The Biobadaser group analysed the patterns of discontinuation of BT over a period of 10 years, and their study showed different discontinuation patterns depending on the reason. Interestingly, they found that during the first year of treatment the main reason for discontinuation was loss of efficacy, while discontinuation due to AE remained stable over time.16
One of the main objectives of this study was to analyse BT survival over 9 years of registration under clinical practice conditions. Similar to what has been previously published, we found that the mean BT survival was higher for ankylosing spondylitis when compared to RA.17–19 Possible explanations for this difference between the two have been postulated. It has been suggested that the younger age of patients with ankylosing spondylitis, the lower number of comorbidities, as well as different availability of BT for the group of patients with ankylosing spondylitis could be determining factors in BT survival compared to RA.
When analysing the survival of anti-TNFα agents, although higher mean values were found for treatment with rituximab, followed by adalimumab, infliximab and etanercept, it should be noted that there were no significant differences when analysing survival between treatments. As with our results, Duclos et al. did not find significant differences in survival rate between adalimumab, etanercept and infliximab.20 It is worth mentioning that, in previous publications, differences in survival of 3 anti-TNFα agents have been observed. This data could relate to several aspects, including the different number of patients with a given biological agent, as well as the differences between health systems in each of the countries, which could determine one agent being used more in relation to another.21
In the present study, the factors significantly associated with BT survival were the number of previous BT cases (HR = 1.43; 95% CI: 1.03–1.89; P = 3.32 × 10−2), concomitant treatment with glucocorticoids (HR = 1.38; 95% CI: 1.06−1.79; P = 1.54 × 10−2) and diagnosis of JIA (HR = 1.80; 95% CI: 1.32−2.46; P = 2.26 × 10−4). Both the number of previous BT and concomitant treatment with glucocorticoids have been previously described as variables associated with reduced BT survival.22 Interestingly, in our study, we observed that a JIA diagnosis was associated with a higher risk of BT discontinuation. BT discontinuation in patients with JIA was most often associated with discontinuation due to remission.
When we analysed the influence of the reason for discontinuation on BT survival, we observed that there are certain variables associated with the reason for discontinuation. In this study, a higher number of previous BT was associated with a higher risk of discontinuation due to both inefficacy and AE. This data coincides with previous studies, in which it was observed that a greater number of previous BT decreases the survival of successive BT. On the other hand, when we analysed the subgroup of treatments where discontinuation was due to remission, we observed that JIA diagnosis and male sex were the variables significantly associated with a greater probability of discontinuation due to remission. The finding that a diagnosis of JIA is associated with an increased probability of discontinuation due to remission explains the previous finding that a diagnosis of JIA was associated with an increased risk of discontinuation of biological therapy.
In the Biobadaguay registry, 22.4% of severe AE were found in the group of patients with BT. This value is higher than that reported by other groups such as Biobadamex (8.84%) and Biobadasar (15.2%).6,8 However, this value is closer to that published by the Biobadaser group (19.1%) and the Colombian group (22.7%).11,23 When comparing the IR of AE among the group of patients with BT with the control group, it was observed that, significantly, the group of patients with BT presented 4 times more risk of presenting an AE. These data are similar to those observed in other registries,6–8 however, they should be confirmed in cohorts that include a greater number of controls.
As published on other registries, the most frequently reported AE in the group of patients with BT were infectious processes, which were mostly of a mild nature.6,11 With regard to the IR of infections (76.3 per 1000 patients/year), the result obtained in the present registry is midway between that reported by the Biobadamex registry (114 per 1000 patients/year)6 and the Biobadaser registry (56/1000 patients/year).6,11 In Biobadaguay the most frequent infection was respiratory tract, similar to the data reported by the Biobadasar group,8 but different from the Colombian group, where the most frequent infections were of the urinary tract.24
The IR according to the type of BT was significantly associated with treatment with tocilizumab for both overall and severe AE; in contrast, treatment with adalimumab was associated with a lower rate of AE. These data differ from those presented in other studies. The Japanese REAL group found a significant increased risk of severe AE and severe infections when comparing tocilizumab and the TNFα inhibitors, however, adjusting the risk to clinical and epidemiological patient variables did not show an increased risk for severe AE, indicating an influence of the patients' clinical characteristics on the safety profile of BT.25 On the other hand, with regard to TNFα inhibitors in publications from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry, the risk of severe infections was significantly lower with etanercept compared to infliximab and adalimumab, without finding differences between adalimumab and infliximab;26 however, Singh et al. found no differences between etanercept, adalimumab and infliximab.14
The diagnosis of RA was significantly associated with an increased risk of developing a severe AE. In relation to this data, most of the information on the safety of BT comes from studies of patients with RA, with a limited number of studies comparing the incidence of AE among different diseases, which makes it difficult to analyse this result. When comparing the RA patient group with the spondyloarthropathy group, the British registry of ankylosing spondylitis (BSRBR-AS) found a higher number of AE in the RA patient group and presented as a possible explanation for this finding older age, number of comorbidities and concomitant treatments of RA patients when compared to the spondyloarthropathy group.27
The present report has certain limitations such as the number of controls, the inclusion of paediatric patients, the different number of biological agents and the registration of AE by the investigator. In this sense, control patients should be more in number, as no meaningful comparisons can be made. In addition, changes should be made to the data for determining activity in patients diagnosed with JIA, since different scales are used. Since the timing of marketing and availability of the different biological agents was different in both countries, the number of registrations is not uniform: it is higher for those first adopted in the different centres analysed, which may lead to biases in the analysis of the data. Another limiting factor is the possible bias in the information on AE not completed by the investigator. It is likely that AE are reported more frequently in BT patients, as they have a stricter follow-up, and it is possible that mild AE are reported less frequently. That is why Biobadaguay is working to obtain better notification, expand the number of control patients and the variety of treatments in order to improve data quality.
This is the first report from Biobadaguay to show the situation of our patients with rheumatic diseases treated with BT. As previously explained, the results are in line with those presented by other Latin American and European registries. It is the result of collaboration between Paraguayan and Uruguayan rheumatologists and is coordinated by the rheumatology societies of both countries. This registry aims to obtain information on the use of these therapies, which can be used at multiple levels, from medical decision-making to the institutional and social, to influence health policies.
Conflict of interestsThe authors have no conflict of interest to declare.
The authors would like to thank the physicians participating in Biobadaguay. Paraguay: Pedro Babak, Agustina Maidana, María del Carmen Martínez, Teresa Romero, Yanira Yinde (Hospital Central del Instituto de Previsión Social); Uruguay: Margarita Calegari, Inés Corbacho, Alicia Ramagli, Raquel Teijeiro (Instituto Nacional de Reumatología); Juan Cameto, Rosario Jurado (Hospital Materno Infantil Pereira Rosell), Gonzalo Batersaghi (centro asistencial médico de Soriano), Mariela Harguindeguy (Colonia centre) for their collaboration.
Please cite this article as: de Abreu P, Ávila-Pedretti G, Morel Z, Acosta MI, Cabrera-Villalba S, Melgarejo P, et al. Seguridad y supervivencia de las terapias biológicas: primer informe del registro paraguayo-uruguayo de acontecimientos adversos con terapias biológicas Biobadaguay. Reumatol Clin. 2020;16:396–404.