Findings of specific antibodies and histopathology data are essential for the diagnosis of Sjögren syndrome (SS). Although the minor salivary gland biopsy (MSGB) is technically simple, it needs to be performed in a medical institution to avoid complications.
ObjectiveTo determine the frequency of complications and the usefulness of this technique.
Materials and methodsPatients who underwent a minor salivary gland biopsy for a possible diagnosis of SS at Rivadavia Hospital between October 2007 and May 2010 where included. The patients were seen a week and a month after the procedure for follow-up.
ResultsFrequency of acute complications (n=186): 15 patients; 8.1%, 95% CI: 4.7–13.2 (Bleeding 7.5%, syncope 3.2%, hematoma 2.7%. No accidents occurred). Medium term complications (n=164): 16 patients: 9.75%, 95% CI: 5.9–15.6 (pain 7.32%, inflammation 3.66%, sensitivity disorders 3.05%, granuloma 1.22%). No infections or suture dehiscence occurred. Microscopic results: 154 biopsy reports were received: glandular 90.9%, 95% CI: 85–95 (typical, sialadenitis, grade III and IV infiltration).
ConclusionsMSGB has very low frequency of medium term and acute complications and it has high usefulness.
El hallazgo de anticuerpos específicos y datos histopatológicos son indispensables para llegar al diagnóstico de síndrome de Sjögren (SS). La biopsia de glándulas salivales menores (BGSM), si bien es un procedimiento sencillo, debe ser realizada en una institución a fin de evitar complicaciones.
ObjetivoEstimar la frecuencia de complicaciones mediatas e inmediatas y el rédito de la técnica.
Materiales y métodosSe incluyeron los pacientes derivados al Hospital Rivadavia para realización de biopsia, entre octubre del 2007 y mayo del 2010. Los pacientes fueron citados a la semana y al mes del procedimiento para control de la lesión.
ResultadosFrecuencia de complicaciones inmediatas (n=186): 15 pacientes: 8,1%, IC del 95%, 4,7–13,2 (sangrado 7,5%, lipotimia 3,2%, hematomas 2,7%; no hubo accidentes). Complicaciones mediatas (n=164): 16 pacientes: 9,75%, IC del 95%, 5,9–15,6 (dolor 7,32%, signos inflamatorios 3,66%, trastornos de sensibilidad 3,05%, granuloma 1,22%). No hubo casos de infecciones, ni dehiscencia del punto de sutura. Rédito microscópico: total 154 biopsias: se obtuvo tejido glandular en el 90,9%, IC del 95%, 85–95 (típica, sialoadenitis, infiltrado grado III y IV).
ConclusionesLa BGSM presenta una baja frecuencia de complicaciones mediatas e inmediatas y un alto rédito en el estudio anatomo-patológico.
Sjögren's syndrome (SS) is a chronic systemic disease of autoimmune origin of the exocrine glands, manifested by symptoms that result from hyposecretion. SS may occur alone (primary SS [pSS]) or associated with other autoimmune diseases (secondary SS).1
The histopathology of the salivary glands as a part of diagnosis was proposed first in 1970 by Waterhouse, Chisholm, and Mason, the latter established a score based on inflammatory2 cell aggregates.
According to the European-American3 criteria for diagnosis of pSS, the presence of a SGB compatible with the disease and/or the presence of antibodies anti-Ro/anti-La is needed. The MSGB is also a method for diagnosing diseases such as infiltrative amiloidosis4 and sarcoidosis.5
Numerous surgical techniques have been described. They vary according to size from 1.5mm to 30mm, the shape of the incision (elliptical, horizontal, vertical, and wedge), and localization.6 While this is an invasive procedure, it is easy to perform and has a low frequency complications.7
Caporali et al. described transient adverse effects in 64 (12.7%) cases in their series of 502 procedures: paresthesias (57), hematoma (8), local swelling (27), and others (5).8 In other studies sensory disturbances (anesthesia, paresthesia) are described as the complications encountered with greater frequency, being transient in most cases. However, other authors have reported no complications.6
As for the sample collected, 99% is needed to be considered as the material is useful for study.8
Despite the simplicity of the technique, MSGB is not incorporated in daily practice in all centers. Although other authors have reported complication rates of the different techniques and usefulness of the procedure, we consider important to evaluate the safety and yield (obtaining suitable material for pathology) of the method in our center.
In this paper we estimate the percentage of mediate and immediate complications of MSGB and frequency of obtaining suitable material for pathology.
Material and MethodsWe designed a prospective, observational, descriptive, and longitudinal study.
We included patients referred with suspected SS and who underwent MSGB. Patients should have no evidence of coagulation disorders and a platelet count within normal within 1 month before the procedure.
Exclusion criteria were: use of aspirin or other NSAIDs 1 week before the study, clinical signs of local infection, patients on anticoagulants, and those who refused inclusion in the protocol.
The study was approved by the Ethics Committee of the hospital. All patients signed an informed consent.
We prospectively studied MSGB conducted between October 2007 and May 2010, at the Rheumatology Service of Hospital Bernardino Rivadavia City of Buenos Aires, Argentina.
All patients underwent minor salivary gland biopsy with the surgical technique described by Caporali et al.8
The material obtained was preserved in 10% formalin and sent for analysis to pathology.
We analyzed the characteristics of the incision and any complications during the procedure and after it. The patient was followed up a week and a month after the procedure. The yield was evaluated through the results of the pathology.
Measurements: immediate complications: bleeding that obstructs the proceedings, fainting, accidents due to health staff while performing the procedure. Mediate complications: surgical wound infection, suture dehiscence, sensory disturbances (dysesthesia, anesthesia, paresthesia), defective wound healing, as defined by the presence of granuloma or keloid scarring at the site.
Statistical AnalysisComplications were studied with a frequency analysis and calculation of confidence intervals of 95%.
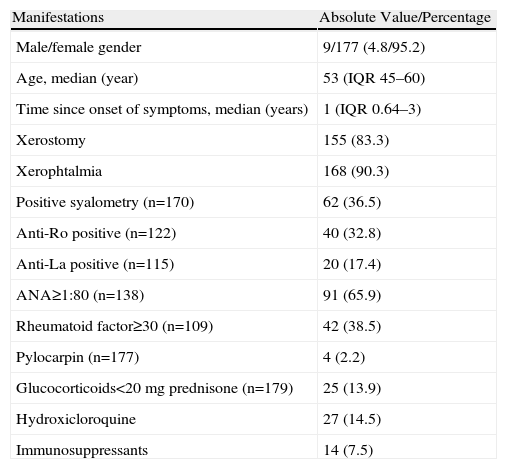
ResultsWe included 186 procedures performed between October 2007 and May 2010, of which 29 were performed to confirm the diagnosis of secondary SS and the rest of pSS. Among patients with suspected secondary SS, 21 had a diagnosis of rheumatoid arthritis (RA), 3 systemic lupus erythematosus (SLE), 3 primary biliary cirrhosis (PBC), 1 mixed connective tissue disease (MCTD) and 1 of cryoglobulinemic vasculitis. The clinical and serological features are described in Table 1.
Clinical and Serologic Characteristics of Patients Evaluated.
| Manifestations | Absolute Value/Percentage |
| Male/female gender | 9/177 (4.8/95.2) |
| Age, median (year) | 53 (IQR 45–60) |
| Time since onset of symptoms, median (years) | 1 (IQR 0.64–3) |
| Xerostomy | 155 (83.3) |
| Xerophtalmia | 168 (90.3) |
| Positive syalometry (n=170) | 62 (36.5) |
| Anti-Ro positive (n=122) | 40 (32.8) |
| Anti-La positive (n=115) | 20 (17.4) |
| ANA≥1:80 (n=138) | 91 (65.9) |
| Rheumatoid factor≥30 (n=109) | 42 (38.5) |
| Pylocarpin (n=177) | 4 (2.2) |
| Glucocorticoids<20mg prednisone (n=179) | 25 (13.9) |
| Hydroxicloroquine | 27 (14.5) |
| Immunosuppressants | 14 (7.5) |
IQR: interquartile range.
Of the 14 (7.5%) patients receiving immunosuppressive therapy at the time of the procedure, 11 received methotrexate (9 of them had a diagnosis of RA), 1 leflunomide and 1 azathioprine.
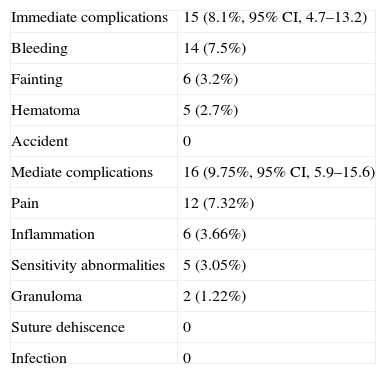
All patients tolerated the procedure and no serious adverse events were recorded. 15 patients had postoperative complications (8.1%, 95% CI, 4.7–13.2). Complications included: bleeding 7.5%, fainting 3.2%, hematoma 2.7%. There were no accidents (Table 2).
Immediate and Mediate Complications.
| Immediate complications | 15 (8.1%, 95% CI, 4.7–13.2) |
| Bleeding | 14 (7.5%) |
| Fainting | 6 (3.2%) |
| Hematoma | 5 (2.7%) |
| Accident | 0 |
| Mediate complications | 16 (9.75%, 95% CI, 5.9–15.6) |
| Pain | 12 (7.32%) |
| Inflammation | 6 (3.66%) |
| Sensitivity abnormalities | 5 (3.05%) |
| Granuloma | 2 (1.22%) |
| Suture dehiscence | 0 |
| Infection | 0 |
There were 164 patients who attended follow-up visits a week and a month after the procedure. Mediate complications were found in 16 patients (9.75%, 95% CI, 5.9–15.6), distributed as follows: pain 7.32%, inflammatory signs 3.66%, sensory loss 3.05%, and granuloma 1.22%. Sensitivity disorders were transient. No cases of infections or dehiscence of the suture were seen.
At the time of analysis, we had the pathology report of 154 biopsies and in 140 (90.9%, 95% CI, 85–95) glandular material was obtained (typically minor salivary glands, lipomatosis, chronic nonspecific sialadenitis, grade III and IV infiltrates), and in other cases fibromuscular tissue and fat or little material was obtained.
DiscussionEstablishing the diagnosis of SS has some difficulty due to the low specificity and sensitivity of serological markers and the lack of specific symptoms of the syndrome. To date, different classification criteria have been proposed, which are based on a combination of clinical, serological, and histologic data.3,9–13 There are some differences between the San Francisco9 (which is based on the histopathology) and San Diego10 criteria, where the MSGB is essential to reach the diagnosis of SS, as well as the Copenhague,11 the Greek,12 Japanese,13 and European9 community criteria, which do not require MSGB or the presence of antibodies, or the American-European criteria 2002,3 which require the presence of histological or serological criteria. Minor salivary gland biopsy is therefore essential for diagnosis in patients with negative suspected SS autoantibodies.
The importance of MSGB is stressed for differential diagnosis with other pathologies and it is currently proposed that the lymphoid organization be a marker for the development of Hodgkin's14 lymphoma.
MSGB is a technically simple procedure and complications are rare. Furthermore, with the technique presented, 90.9% of the material obtained was useful for pathology, somewhat lower than that described by Caporali et al.8 (99.2%) and Teppo–Revonta15 (98.4%).
This study describes a high prevalence of immediate complications of 8.1% and 9.75% mediate complications, showing the reliability of both health personnel and patients, since there were no serious or medium or long term complications during the procedure. Within a week, 12 patients reported pain and 6 had inflammatory signs during the first days, 5 had mild sensory loss, which disappeared within a month, and in 2 of them we found the formation of granuloma.
Several observations analyzed MSGB with a technique similar to that used in this study and obtained comparable results; the complications were uncommon, and the most prevalent were paresthesias at the site of incision, transient in nature, in most the patients.6 Richards et al.16 reported 2 cases of disorders of sensation at the incision site, which persisted beyond 1 year, with mild features and no objective evidence of neurological disease, while Berquin et al.17 described anesthesia in 1 case and Marx et al.18 reported 3 cases of which one was persistent after 2 years of follow-up. Caporali et al.8 reported transient adverse events in 64 of the 502 patients, of whom 57 had transient paresthesias, 8 hematoma, 27 local inflammatory signs, and 5 had others (granuloma, bleeding, and internal scar). Pijpe et al.19 reported 4 cases of hypoesthesia of the incision site, 2 of which persisted for more than 12 months and 11 cases of pain lasting than 1 month. Moreover, Teppo and Revonta reported a case of pyogenic granuloma.15
Patients who underwent MSGB were referred for the study of sicca syndrome by professionals of both our institution and other rheumatology departments who did not have the means for carrying it out. This explains the lack of some data for analysis.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
DisclosureThe authors have no disclosures to make.
To Dr. Nora Castiglia for her scientific assessment.
Please cite this article as: Lida Santiago M, et al. Frecuencia de complicaciones y rédito de la biopsia de glándula salival menor. Reumatol Clin. 2012. http://dx.doi.org/10.1016/j.reuma.2012.03.006.