To determine the concentrations of sCD40L in patients with PAPS, and establish its association with the number of thrombosis.
Patients and methodsWe included patients with PAPS and healthy controls of the same age and sex. For analysis, patients with PAPS were divided into 2 groups: (1) patients with 1 thrombosis, and (2) patients with >1 thrombosis. Soluble CD40L concentrations were determined by ELISA method.
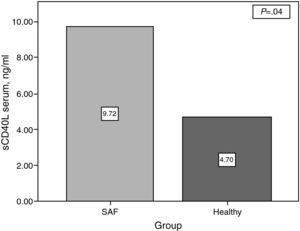
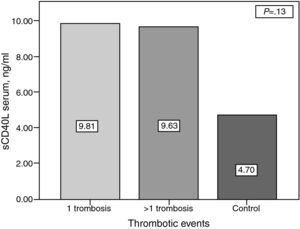
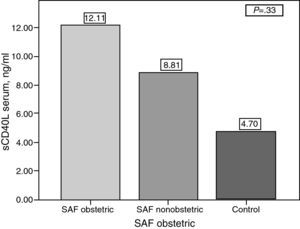
ResultssCD40L concentrations were significantly higher in patients with PAPS compared with the controls (9.72±11.23ng/ml vs 4.69±4.04ng/ml) (P=.04) There was no association between serum levels of sCD40L and the number of thrombosis (1 thrombosis: 9.81±9.87ng/ml vs 9.63±12.75ng/ml in ≥1 thrombosis (P=.13). In women with pregnancy and abortions (13 patients), concentrations of sCD40L were higher than in those patients without a history of abortion (26 patients) but without statically significant difference (12.11±16.46ng/ml vs 8.80±8.61ng/ml) (P=.33). There was no correlation between levels of sCD40L and the total number of thrombosis.
ConclusionsPatients with PAPS have higher concentrations of sCD40L compared with healthy subjects, although this is not associated with a greater number of thrombosis. Among patients with PAPS, there is a tendency to higher concentrations of sCD40L in women with pregnancy and history of abortion. Since the platelet is the main cellular source of sCD40L, is possible that this pathway plays a pathogenic role in patients with PAPS.
Determinar las concentraciones de sCD40L en pacientes con SAFP, y su asociación con el número de trombosis.
Pacientes y métodosSe incluyó a pacientes con SAFP y controles sanos de la misma edad y sexo. Para su análisis, los pacientes con SAFP se dividieron en 2 grupos: a) pacientes con 1 trombosis, y b) pacientes >1 trombosis. Se determinaron las concentraciones de sCD40L por método de ELISA en cada uno de ellos.
ResultadosLas concentraciones de sCD40L fueron significativamente mayores en los pacientes con SAFP en comparación con el grupo control: 9,72±11,23ng/ml vs 4,69±40,04ng/ml (P=0,04). No hubo asociación entre las concentraciones séricas de sCD40L y el número de trombosis (9,81±9,67ng/ml en pacientes con una trombosis vs 9,6±12,75ng/ml en ≥1 trombosis) (P=0,13) En mujeres con embarazo y aborto del primer trimestre (13 pacientes), las concentraciones de sCD40L fueron mayores que en aquellas pacientes sin antecedente de aborto (26 pacientes), pero sin una diferencia estadística significativa (12,11±16,46ng/ml vs 8,80±8,61ng/ml; P=0,33). No se encontró correlación entre las concentraciones de sCD40L y el número total de trombosis.
ConclusionesLos pacientes con SAFP tienen concentraciones mayores de sCD40L en comparación con sujetos sanos, sin que esto se asocie a un mayor número de trombosis. Entre las pacientes con SAFP, existe una tendencia a mayores concentraciones de sCD40L en aquellas con embarazo e historia de aborto. Al ser la plaqueta la principal fuente celular de sCD40L, es posible que esta vía desempeñe un papel patogénico en la enfermedad.
The primary antiphospholipidic syndrome (PAPS), first described in 1986 by Hughes, Gharavi and Harris,1 is an autoimmune disease characterized by antibodies against a variety of phospholipids and phospholipid binding proteins. The cardinal feature of this syndrome are thrombotic complications. Multiple mechanisms have been proposed to explain the involvement of antiphospholipid antibodies in the pathogenesis of the entity, including: induction of tissue factor expression by endothelial cells, inhibition of anticoagulant proteins, complement activation and increased platelet activation.2–5
Platelet activation through the CD40/CD40L pathway has been described as a key player in inflammatory phenomena, including atherosclerosis. Atherosclerosis is now considered an inflammatory condition associated with PAPS.6–8 CD40 is a membrane glycoprotein belonging to the family of receptors of tumor necrosis factor and is expressed in numerous cell types, including platelets, endothelial cells and macrophages.9,10 Following cellular activation triggered by CD40–CD40L interaction, the soluble ligand fraction is released into the circulation (sCD40L). Platelets are by far the largest producers of the soluble form of CD40 ligand.10
CD40/CD40L interaction produces fundamental inflammatory changes in the atherosclerotic plaque progression, such as the expression of cell adhesion molecules on endothelial cells and increased Th1 response on sites of atherosclerosis, as well as the production of inflammatory cytokines, such as interleukin-1 and interferon gamma INF-γ.11–14 Current clinical evidence demonstrates a strong association between the presence of thrombotic events and increased sCD40L concentrations.15,16 The incidence of cerebrovascular and coronary events as well as the rate of restenosis in patients undergoing angioplasty, increases in direct proportion to the concentrations of sCD40L.17
The role of CD40/CD40L pathway platelet activation in PAPS is still unknown. Some studies have found increased sCD40L concentrations in patients with secondary APS.18 Few studies have been conducted in patients with PAPS and the results are inconclusive.18,19 The objective of this study was to determine the mean serum concentration of sCD40L in patients with PAPS and its association with the number of thrombotic events.
Patients and MethodsWe conducted a descriptive study in a tertiary medical care center. The study included patients with an established diagnosis of PAPS according to the 2006 revised criteria,20 18 years of age or older, and with at least one documented episode of thrombosis. We excluded patients with a history of coronary heart disease or thrombosis unrelated to APS as well as diabetic patients with a history of major surgery or thrombotic events documented in the 3 months prior to the study. We included 22 healthy controls with similar demographic characteristics to those of patients.
For analysis the subjects were divided into three groups: the first (control group) consisted of healthy health personnel from the same hospital with similar demographic characteristics to the other study groups. The second group was composed of patients with PAPS and documented thrombosis. The third group was made up of patients with PAPS and 2 or more thromboses at the time of inclusion. In patients with PAPS, we collected information on date of onset of the disease, the number of thrombotic complications, arterial or venous events, antiphospholipid antibody titers at the onset of the disease and at the time of the study and the presence of concomitant thrombotic manifestations and comorbidities. All patients signed a letter of informed consent and the study was approved by the local ethics and health research committee.
Laboratory MethodsEach patient provided a sample of 5ml of blood in a dry tube. The sample was centrifuged to separate the serum from cellular components. Serum sCD40L concentrations were determined by the ELISA method; we used a commercial kit and specifications were followed Edor Prove (Invitrogen #KHS4001); reading was performed using Biotek_ELx equipment. The concentrations of sCD40L are expressed in ng/ml.
Statistical AnalysisWe conducted a descriptive analysis for demographic variables and a comparison of means of the serum concentrations of sCD40L in the different study groups by ANOVA. Moreover, patients were grouped into quintiles of serum sCD40 and then retested through a one-way ANOVA to compare mean episodes of thrombosis among each of the groups.
ResultsBetween April 2009 and January 2010, a total of 47 patients with PAPS and 22 healthy controls were included. Of the patients with PAPS, 23 subjects had one thrombosis, while 24 patients had 2 or more thrombotic complications (thrombosis mean 3.2 per patient). The demographic characteristics of the 3 groups were similar (Table 1). For pregnant patients, the percentage of fetal loss was similar in the groups with PAPS (30.4% vs 20.8%) (Table 1).
Demographic Characteristics of Patients.
| Variable | SAFP <1 thrombosis | SAFP >1 thrombosis | Control group | P |
| (No.=23) | (No.=24) | (No.=22) | ||
| Female, % | 19 (82) | 20 (83) | 17 (77) | .85 |
| Gender: male | 4 (18) | 4 (17) | 5 (23) | .85 |
| Age, mean | 41.83 | 40.83 | 40.05 | .87 |
| BMI | 27.75 | 27.17 | 27.45 | .65 |
| Abortions | 0.78 | 0.37 | – | .19 |
| ACL IgG titers at diagnosis | 50.25 | 34.38 | – | .43 |
| ACL IgM titers at diagnosis | 44.5 | 15.17 | – | .82 |
| ACL IgG titers at time of study | 47.28 | 45.95 | – | .94 |
| ACL IgM titers at time of study | 29.44 | 17.54 | – | .38 |
| Thrombotic manifestations, % | 17 (73.9) | 13 (54.1) | – | .15 |
In patients with PAPS, non thrombotic manifestations occurred with a frequency of 73.9% in those with one thrombosis vs 54.1% of patients with 2 or more thrombosis (Table 1). In these patients, valvulopathy was the non thrombotic manifestation observed more frequently, with varying degrees of valvular regurgitation being the most prevalent. Other non thrombotic manifestations included thrombotic APS nephropathy, skin ulcers and livedo reticularis and thrombocytopenia. Most of the patients had more than one non thrombotic manifestation (Table 2).
The platelet serum sCD40L concentration was higher in patients with PAPS compared with controls (9.72±11.23 vs 4.69±4.04, P=.04) (Fig. 1). However, there was no difference between groups with PAPS (9.81ng/ml in patients with <1 thrombosis vs 9.63 in patients with >1 thrombosis, P=.13) (Fig. 2).
To assess the correlation between serum sCD40L and the number of thrombotic episodes in patients with PAPS, sCD40L serum concentrations were distributed in percentiles and the amount of thrombotic complications in each compared. However, we failed to establish an association between sCD40L concentrations and the number of thrombosis.
Of the patients with PAPS, 13 had a history of one or more abortions in the first trimester of pregnancy (mean 2.1 per patient).
SCD40L comparing the group of women with complications in pregnancy and other women, there was a tendency to have higher concentrations of sCD40L in those with complications, however, we failed to establish a significant difference (12, 1±16.46ng/ml vs 8.81±8.61ng/ml, P=.33) (Fig. 3).
DiscussionIn the present study we documented that patients with PAPS have higher sCD40L concentrations compared with healthy controls. However, we failed to establish an association with the number or type of thrombosis, or with non thrombotic manifestations of the disease.
Multiple mechanisms have been evoked to explain the development of thrombotic complications in patients with APS, and platelet activation is one of them. As mentioned, the CD40/CD40L pathway was recently described as a pathogenic mechanism in the phenomenon of atherosclerosis and its influence on coronary and cerebral vascular events is more than tested.15–17
Our work was based on the determination of sCD40L, whose production is carried out mostly at the platelet level, and to determine whether this ligands’ concentrations were associated with a greater number of thrombosis. Previous studies have found increased concentrations of sCD40L in patients with secondary APS or PAPS compared with healthy controls.18 Our study was aimed at patients with PAPS, as its “per se” cardiovascular risk is known to involves the coexistence of another disease such as lupus erythematosus. It is also the first study that seeks to establish a correlation between sCD40L concentrations and the number of thrombosis in these patients. In general, our study showed that concentrations of sCD40L were significantly higher in subjects with PAPS vs healthy controls, but no association was established with the amount of thrombotic phenomena. The reasons for this conclusion may be several: first, when a patient is diagnosed with PAPS, anticoagulant treatment is started immediately. Many of our patients are diagnosed after the first episode of thrombosis, treated with anticoagulant therapy from the time of diagnosis, contributing to the non-recurrence of thrombotic complications. Furthermore, it is well known that APS pathogenic mechanisms are numerous and often coexist, and it is possible that different mechanisms of platelet activation via CD40/CD40L predominate and directly favor thrombosis. Finally, it is probable that sCD40L concentrations are increased in this group of patients for accelerated atherosclerosis is frequently observed therein. In fact, a high prevalence of metabolic syndrome has been reported in our population of patients with PAPS.19 Numerous mechanisms have been described to explain the high frequency of accelerated atherosclerosis in patients with APS, among them: increased oxidative stress,20 the presence of autoantibodies against oxidized LDL particles,21 increased uptake of LDL in macrophages induced by anti β 2GP,22 etc. It is possible that in these patients platelet activation through the CD40/CD40L pathway is an additional mechanism that contributes to accelerated atherosclerosis, and while it is true that its activation does not explain the presence of thrombosis in a direct way, it is possible that it does so indirectly through mechanisms which favor the progression of atherosclerotic lesions, such as endothelial activation and increased expression of cellular adhesion molecules and inflammatory and thrombogenic cytokines. In this regard, Gresele et al. found higher concentrations of sCD40L in23 patients with PAPS and associated cardiovascular risk factors compared to patients without them.
One finding that should be emphasized is the presence of higher concentrations of sCD40L in those patients with PAPS and abortions compared with patients without obstetric morbidity. It probably did not reach statistical significance due to the small number of patients. This finding is interesting because placental thrombosis is not a consistent finding in women with aPL and obstetric morbidity. To explain the miscarriages other mechanisms have been described, such as activation of the classical complement pathway or resistance to proteins such as annexin.24 Our results suggest that platelet activation and, specifically that triggered by activation of the CD40/CD40L pathway may have a pathogenic role in APS induced abortions.
Our research has some limitations. First, the small number of patients included, which may explain why many of our results did not reach a statistically significance. Secondly, being a cross-sectional study it was performed measuring sCD40L upon study entry. Therefore, it is possible that the association between concentrations of ligand and the presence thrombosis is underestimated, since the timing between the first and last thrombotic event is highly variable in our patients. This justifies conducting a longitudinal prospective study of sCD40L determination according to the time of presentation of thrombotic complications. On the other hand, we included only a small number of women with fetal losses, making it difficult to draw conclusions about the impact of sCD40L concentrations in this subgroup of patients. Also of note is the high frequency of non thrombotic events found in our cohort of patients with PAPS. Microangiopathhic APS is a recently described entity which refers to the presence of unrelated events with large vessel thrombosis. In these manifestations, microangiopathy, microthrombosis and endothelial dysfunction are the common denominator.25 Given its recent description, its pathogenic mechanisms have not been fully characterized. However, the sample size does not help establish, for the moment, any conclusion on the matter, but would question whether platelet activation occurs by this means, or otherwise, playing an important role in such manifestations.
Ethical ResponsibilitiesProtection of people and animals. The authors declare that procedures conformed to the ethical standards of the committee responsible for human experimentation and were in accordance with the World Medical Association Declaration of Helsinki.Data confidentiality. The authors state that no patient data apppears in this article.Right to privacy and informed consent. The authors have obtained informed consent from patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
DisclosuresThe authors have no disclosures to make.
Please, cite this article as: Galicia López A, et al. Incremento en las concentraciones de ligando CD40 soluble plaquetario en pacientes con síndrome antifosfolípido primario. Reumatol Clin. 2013;9:216–20.