Ankylosing spondylitis is a chronic inflammatory disease that is associated with adverse cardiovascular events. This study aimed to determine the relationship between ankylosing spondylitis and the risk of stroke.
MethodsA systematic literature search in PubMed/MEDLINE, Scopus, and Web of Science were conducted from inception to December 2021 to identify relevant articles investigating the risk of stroke in patients with ankylosing spondylitis. A random-effects model (DerSimonian and Laird) was used to estimate a pooled hazard ratio (HR) and 95% confidence intervals (CI). Meta-regression based on the length of follow-up and subgroup analysis based on the type of stroke, study location, and year of publication to investigate the source of heterogeneity.
ResultsA total of eleven studies comprising 1.7 million participants were included in this study. Pooled analysis showed a significantly increased stroke risk (56%) among patients with ankylosing spondylitis (HR: 1.56, 95% CI 1.33–1.79). Subgroup analysis revealed a higher risk of ischemic stroke among patients with ankylosing spondylitis (HR: 1.46, 95% CI: 1.23–1.68). However, meta-regression analysis showed no association between the duration of ankylosing spondylitis and stroke incidence (Coef=−0.0010, P=0.951).
ConclusionThis study reveals that ankylosing spondylitis was associated with an increased risk of suffering a stroke. Management of cerebrovascular risk factors and the control of systemic inflammation should be considered in patients with ankylosing spondylitis.
La espondilitis anquilosante es una enfermedad inflamatoria crónica que se asocia con eventos cardiovasculares adversos. Este estudio tuvo como objetivo determinar la relación entre la espondilitis anquilosante y el riesgo de accidente cerebrovascular.
MétodosSe realizó una búsqueda sistemática de la literatura en PubMed/Medline, Scopus y Web of Science a partir de diciembre de 2021 para identificar los artículos relevantes que investigan el riesgo de accidente cerebrovascular en pacientes con espondilitis anquilosante. Se usó un modelo de efectos aleatorios (Dersimonian y Laird) para estimar una relación de peligro agrupada (HR) e intervalos de confianza (IC) del 95%. Meta-regresión basada en la duración del seguimiento y análisis de subgrupos basados en el tipo de accidente cerebrovascular, la ubicación de estudio y año de publicación para investigar la fuente de heterogeneidad.
ResultadosUn total de 11 estudios que comprenden 1,7 millones de participantes, se incluyeron en este estudio. El análisis agrupado mostró un riesgo de accidente cerebrovascular significativamente aumentado (56%) entre los pacientes con espondilitis anquilosante (HR: 1,56; IC 95%: 1,33-1,79). El análisis de los subgrupos reveló un mayor riesgo de accidente cerebrovascular isquémico entre los pacientes con espondilitis anquilosante (HR: 1,46; IC 95%: 1,23-1,68). Sin embargo, el análisis de meta-regresión no mostró ninguna asociación entre la duración de la espondilitis anquilosante y la incidencia de accidentes cerebrovasculares (coef=−0,0010; P=0,951).
ConclusionesEste estudio revela que la espondilitis anquilosante se asocia a un mayor riesgo de sufrir un accidente cerebrovascular. La gestión de los factores de riesgo cerebrovasculares y el control de la inflamación sistémica deben considerarse en pacientes con espondilitis anquilosante.
Ankylosing spondylitis (AS) is a chronic inflammatory arthritis disease and one of the most common spondyloarthropathies.1,2 It typically occurs in the third decade of life, affecting males with an incidence rate approximately two to three times more likely to develop it than females.3 The condition mainly affects axial vertebrae of the spine, the sacroiliac joints, and surrounding joints, affecting extra-articular organs to a much lesser extent.4 The clinical presentation of AS includes chronic pain, impaired physical mobility, and various functional disabilities.5 Additionally, specific diseases have been identified to be associated with AS, including uveitis, aortic and valve disease, and IgA nephropathy.1,6,7 In general, AS results in severe impairment of spinal mobility, physical function, and thereby the quality of life.
AS cases are at increased risk of dying from cardiovascular disease (CVD).8 Given this association, the European Alliance of Associations for Rheumatology (EULAR) has recommended annual CVD risk assessment and risk management for patients with AS.9 Findings from previous systematic reviews and meta-analyses have demonstrated that AS patients have a higher incidence of myocardial infarction than controls, which could be due to low high-density lipoprotein (HDL) cholesterol levels or systemic inflammation.10 Additionally, this study reports a significant risk of stroke linked to AS compared to control. A recent study by Trömmer et al.,11 showed that AS was tendentially associated with stroke and indicated that this increased risk was primarily within older populations (>60 years). Recently, two updated reviews showed a significant risk of both myocardial infarction and stroke related to AS.12,13 Following these reviews, several new studies have emerged concerning adverse cardiovascular events and stroke; however, findings from individual studies provided variable results on the association between AS and cerebrovascular events. Therefore, a comprehensive review and meta-analysis was conducted to determine the relationship between AS and the risk of stroke.
MethodsSearch strategyThis systematic review and meta-analysis were conducted following PRISMA guidelines.14 A literature search was conducted using MeSH and key terms in PubMed/MEDLINE, Scopus, and Web of Science to identify relevant articles published in the English language from inception to December 2021. References from the relevant articles were also screened for any additional studies. The detailed search strategy is reported in Supplementary Table 1.
Inclusion criteriaTo be included, studies must: (1) evaluate the risk of stroke among patients with ankylosing spondylitis; (2) report results with appropriate statistical parameters (odds ratio (ORs), Risk ratio (RRs), or Hazard ratio (HRs)); (3) use an appropriate design to conduct the study (case–control, controlled trial, or cohort).
Studies that did not include data on the association between AS and the risk of stroke were not considered. This meta-analysis also excluded review articles, in vitro research, editorials, commentaries, case reports/series, letters and studies with preliminary or insufficient data.
Data extraction and quality assessmentStudies were screened in steps: (1) title and abstract screening, and (2) the full-text screening, using predesigned extraction forms. Two reviewers extracted the data, with subsequent double-checking by an additional reviewer. A third author was consulted when discrepancies occurred. The Newcastle-Ottawa Scale (NOS) for nonrandomized studies was used to assess the quality of included studies.15
Statistical analysisIn each included study, fully adjusted models were used as corresponding estimates. The pooled results are presented as HR with corresponding 95% confidence intervals (CIs). Heterogeneity among the results was evaluated with Cochran's Q test and I2 statistics; where the I2 showed no evidence of heterogeneity (I2<25%), analyses were conducted using the inverse-variance fixed effects model for pooling the studies, whereas when I2>25%, the DerSimonian and Laird random-effects model was used.16 Subgroup analyses and meta-regression analyses were performed to identify the source of heterogeneity, and sensitivity analysis was performed to investigate the effect of each study on pooled estimates. The publication bias was determined by visual inspection of funnel plots, Egger's regression test, and Begg's correlation test. A P-value less than 0.05 was considered statistically significant. All the statistical tests were conducted using STATA 14.0 (StataCorp LP, College Station, TX, USA).
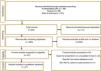
ResultsIn total, 341 articles were retrieved in the initial search. Of these 111 duplicate records were removed, and 203 articles were excluded following title and abstract screening. 21 studies were screened in the full-text evaluation following the inclusion criteria; 16 irrelevant records were excluded for various reasons (details presented in Fig. 1). Finally, 11 studies met the inclusion criteria and were included in the systematic review and meta-analysis.5,11,17–25
Study characteristics and quality assessmentThose studies that met the inclusion criteria are listed in Table 1, along with their study characteristics. These studies were published from 2006 to 2021 with 1,741,532 participants. One study from Germany,11 Korea,17 UK,23 Canada,24 USA,25 two studies from Sweden,18,22 and four from Taiwan.5,19–21 Eight studies followed a cohort design,11,17–20,22–24 with the remaining being case–control studies.5,21,25 All studies contained both men and women and had an average follow-up length of 11 years. Supplementary Table 2 provides the quality assessment of included studies, with most exhibiting good quality (NOS score ≥7).
Characteristics of included studies.
| Study | Year | Country | Design | Study name or data | Age | Participants (n) | Case (n) | Follow-up (year) | Stroke type (ischemic/hemorrhagic) |
|---|---|---|---|---|---|---|---|---|---|
| Trömmer K. et al. | 2021 | Germany | Cohort | IQVID (2000–2015) | 54.8 | 58,212 | 154 | 15 | Both |
| Lee, D. H. et al. | 2018 | Korea | Cohort | Taiwan National Health Insurance (NHIS) (2010–2014) | 40 | 12,988 | 323 | 6 | Ischemic |
| Eriksson, J. K. et al. | 2017 | Sweden | Cohort | Swedish National Patient Register (NPR) and one general population (GP) cohort (2006–2012) | 48 | 30,364 | 250 | 5 | Both |
| Lin, C. W. et al. | 2014 | Taiwan | Cohort | National Health Insurance (NHI) (2000–2003) | 31 | 27,372 | 84 | 2 | Ischemic |
| Keller, J. J. et al. | 2014 | Taiwan | Cohort | NHI (2001–2005) | 42 | 14,475 | 83 | 5 | Ischemic |
| Chou, C. H. et al. | 2014 | Taiwan | Case–control | National Health Insurance Research Database (NHIRD) (2000–2009) | >65 | 31,310 | 3409 | – | Ischemic |
| Zoller, B. et al. | 2012 | Sweden | Cohort | Dates of hospital admissions (1987–2008) | >29 | 216,291 | 111 | 22 | Ischemic |
| 42 | Hemorrhagic | ||||||||
| Brophy, S. et al. | 2012 | UK | Cohort | Routine data (1999–2010) | 35 | 1,208,307 | 20,252 | 12 | Ischemic |
| Szabo, S. M. et al. | 2011 | Canada | Cohort | Population-based administrative data from Quebec | 42 | 59,315 | – | 10 | Ischemic |
| Kang, J.H. et al. | 2010 | Taiwan | Case–control | NHIRD (2005–2007) | 70,206 | 1349 | – | Ischemic | |
| Han, C. et al. | 2006 | USA | Case–control | PharMetrics Patient-Centric Database (2001–2002) | 47 | 9215 | 242 | – | Ischemic |
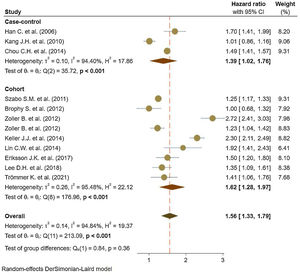
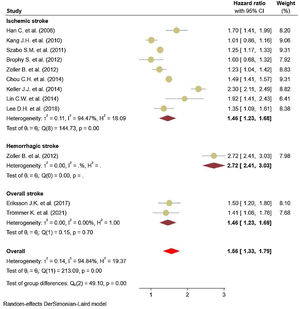
Eleven studies5,11,17–25 comprised 1.74 million subjects, reported around 26,000 cases of stroke. The pooled analyses of case–control and cohort studies showed that the overall risk of stroke is significantly higher (56%) in patients with ankylosing spondylitis (HR: 1.56, 95% CI: 1.33–1.79, P<0.001; I2=94.8%) versus controls (Fig. 2). Moreover, when studies were assessed based on the type of stroke, results indicated that the risk of ischemic stroke was 46% in patients with ankylosing spondylitis (HR: 1.46, 95% CI: 1.23–1.68) (Fig. 3) with only one study21 reporting a higher risk of hemorrhagic stroke in patients with ankylosing spondylitis (HR: 2.72, 95% CI: 2.41–3.03).
Meta-regression based on length of follow-up showed no relation with stroke (Coef=−0.0010, P=0.951) (Supplemental Figure 1). Subgroup analysis based on study location showed no difference in reported risk of stroke among ankylosing spondylitis in studies conducted in North America (2 studies): 1.45 (95% CI: 1.01–1.89), Asia (5 studies): 1.60 (95% CI: 1.18–2.01), and Europe (5 studies): 1.57 (95% CI: 1.02–2.12), more details in Supplemental Figure 2. Furthermore, studies stratified based on year of publication indicated studies conducted between 2006 and 2012 had a pooled HR of 1.47 (95% CI: 1.13–1.81) versus studies conducted after 2012 (HR: 1.66, 95% CI: 1.33–1.99) (Supplemental Figure 3).
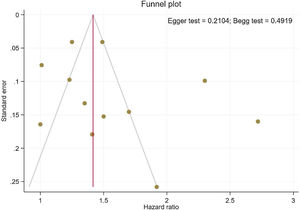
Publication bias and sensitivity analysisThe funnel plots provided in Fig. 4 indicate no asymmetry between the included studies and the Begg's P=0.210 and Egger's test P=0.491 confirmed a low risk of publication bias among the included studies. Sensitivity analysis is provided in Supplemental Figure 4, and shows no differences beyond of the 95% CI limitation for calculated combined results.
DiscussionA significant body of literature suggests that chronic systemic inflammation promotes clot formation by interfering with physiological hemostasis and inducing hypercoagulability, which can result in cerebrovascular events.1,4,6,7 In this context, epidemiological studies have reported an increased risk of stroke in patients with ankylosing spondylitis (AS),18,20,24 while other investigations did not identify any increased risk of stroke in patients with AS.5,22 This study sought to elucidate the association between AS and stroke development by combining the data of over 1.6 million participants from epidemiological studies, to provide an important update on the risk of stroke among patients with AS. Our data support the hypothesis that AS is a significant risk factor for stroke with an HR of 1.56 (95% CI: 1.33–1.79) relative to controls. The increased risk of stroke among AS patients could be due to a number of underlying risk factors, such as hypertension and traditional use of anti-inflammatory drugs as first-line therapies, which may increase the risk of cerebrovascular events.26–29 Moreover, AS patients typically have a worse cardiovascular (CV) profile than controls, a higher incidence of metabolic syndrome – itself a risk factor for CVD30–32 – and are more likely to receive a diagnosis of diabetes and hypertension.29,33,34
A limited body of literature exists on the risk of developing hemorrhagic stroke in AS populations, although a couple of reported results exist.18,22 On the other hand, the hazards of ischemic stroke for AS support an association with an elevated risk. Studies stratified based on study design showed a consistent 62% increase in the risk of stroke events reported in cohort studies and 39% in case–control studies.35,36
Exposure to higher does of non-steroidal anti-inflammatory drugs (NSAIDs) may increase the risk of adverse cardiovascular events, with their use in AS patients being a potential confounder; however, it is difficult to separate the biological impact of NSAIDs in AS patients, and it remains controversial whether these agents, particularly COX-2 inhibitors, are associated with an increased risk of stroke. A meta-analysis of 280 trials on NSAIDs vs. placebo showed little evidence that NSAIDs significantly increased the risk of stroke in these patients.37 Therefore, the use of NSAIDs and risk of stroke requires further elucidation, preferably through longitudinal studies.
By including a range of studies from different patient populations, with varying risk factors and study methodologies, it is possible to estimate the risk of stroke observed in AS. Meta-regression analysis relies on variability in the magnitude of effect across studies, and due to modest differences between the study groups, did not detect the occurrence of stroke in AS patients with the duration of time. Thus, our results suggest an independent association of increased stroke risk with AS, although the underlying reasons for this discrepancy are not clear. In contrast, our sensitivity analysis illustrated that most of the studies are beyond the limit of 95% CI of the combined results for each of the included studies. Egger's and Begg's tests did not identify any significant publication bias and showed symmetric funnel plots.
This study has several limitations: First, only two studies reported the risk of hemorrhagic stroke in AS and it can lead to non-comprehensive results. Second, variations in the sample sizes of the included studies and their study designs affect their interpretation, thus caution should be taken when drawing conclusions from the combined results. Differences in the patient characteristics may result in higher heterogeneity, which may confound with age, sex, or other factors that increase the risk reported for AS patients. Lastly, the outcomes reported by each study may have a different definition which could affect the pool estimates in the meta-analysis. Despite these limitations, our comprehensive literature review provides robust evidence of the association between AS and stroke events by integrating results of 7 large cohorts,17,22–24 and 3 case–control studies.5,21,25 The mean follow-up duration in cohort studies was 11 years and reported an overall 2.02% of stroke cases in 1.74 million AS subjects.
ConclusionThis study suggests that ankylosing spondylitis significantly increases the risk of stroke. Management of cerebrovascular risk factors and control of systemic inflammation should be considered in patients with ankylosing spondylitis.
Data availabilityAvailable on request.
FundingNo fund.
Author contributionsAnalysis, JR; Writing, ASB, BLB, BW and TDD; Screening and Data extracting, JR and ASB; Idea and Manuscript revision, JR; Revision, ASB, BLB, and BW.
Conflict of interestThe authors declare no conflict of interest.