There is a dearth of biomarkers in Idiopathic Inflammatory Myopathies (IIM) to recognize ongoing muscle inflammation and distinguish damage from activity. Since IIM is an autoantibody-mediated disease with tertiary lymphoid organogenesis reported in the diseased muscles, we aimed to study the peripheral blood T helper (Th) subset profiling as a plausible reflection of ongoing muscle inflammation.
MethodsFifty-six patients of IIM were compared with 21 healthy controls (HC) and 18 patients with sarcoidosis. Th1, Th17, Th17.1, and Treg cells were identified after stimulation assays (BD Biosciences). Myositis autoantibodies were tested by line immunoassay (Euroimmune, Germany).
ResultsAll Th subsets were elevated in IIM as compared with HC. As compared to HC, PM had elevated Th1 and Treg while Th17 and Th17.1 populations were higher in OM. Patients with sarcoidosis had higher Th1 and Treg but lower Th17 population as compared to IIM {Th1(69.1% vs 49.65%, p<0.0001), {Treg (12.05% vs 6.2%, p<0.0001), {Th17 (2.49% vs 4.4%, p<0.0001)}. Similar results were obtained when sarcoidosis ILD was compared with IIM ILD with a higher Th1 and Treg population but lower Th17 population in the former. No difference in T cell profile was observed after stratification for MSA positivity, type of MSA, clinical features of IIM and disease activity.
ConclusionTh subsets in IIM are distinct from sarcoidosis and HC with a TH17 predominant paradigm, creating a case of exploring Th17 pathway and IL-17 blockers for the treatment of IIM. However, cell profiling cannot distinguish active from inactive disease limiting its predictive potential as a biomarker of activity in IIM.
Hay una escasez de biomarcadores en las miopatías inflamatorias idiopáticas (MII) para reconocer la inflamación muscular en curso y distinguir el daño de la actividad. Dado que la MII es una enfermedad mediada por autoanticuerpos con organogénesis linfoide terciaria informada en los músculos enfermos, nuestro objetivo fue estudiar el perfil del subconjunto de linfocitos T helpers (Th) de sangre periférica como un reflejo plausible de la inflamación muscular en curso.
MétodosSe compararon 56 pacientes de MII con 21 controles sanos (CS) y 18 pacientes con sarcoidosis. Las células Th1, Th17, Th17.1 y Treg se identificaron después de los ensayos de estimulación (BD Biosciences). Los autoanticuerpos de miositis se analizaron mediante inmunoanálisis en línea (Euroimmune, Alemania).
ResultadosTodos los subconjuntos de Th estaban elevados en las MII en comparación con los CS. En comparación con los CS, PM tenía Th1 y Treg elevados, mientras que las poblaciones de Th17 y Th17.1 eran más altas en OM. Los pacientes con sarcoidosis tenían una población Th1 y Treg más alta pero una población Th17 más baja en comparación con MII {Th1 (69,1% frente a 49,65%, p<0,0001), {Treg (12,05% frente a 6,2%, p<0,0001), {Th17 (2,49% frente a 4,4%, p<0,0001)}. Se obtuvieron resultados similares cuando se comparó la EPI de sarcoidosis con la EPI de las MII con una mayor población Th1 y Treg pero menor población Th17 en la primera. No se observaron diferencias en el perfil de células T después de la estratificación por positividad de MSA, tipo de MSA, características clínicas de las MII y actividad de la enfermedad.
ConclusiónLos subconjuntos de Th en las MII son distintos de la sarcoidosis y los CS con un paradigma predominante TH17, lo que crea un caso de exploración de la vía Th17 y los bloqueadores de IL-17 para el tratamiento de las MII. Sin embargo, el perfil celular no puede distinguir la enfermedad activa de la inactiva, lo que limita su potencial predictivo como biomarcador de actividad en las MII.
Idiopathic Inflammatory Myopathies (IIM) are heterogeneous and often result in morbidity and at times even mortality. The various subsets of IIM, namely polymyositis (PM), dermatomyositis (DM), overlap myositis (OM), and anti-synthetase syndrome (ASS) have varied pathogenic origins with common involvement of the muscle and often the pulmonary tissue. While innate immune activation in response to environmental influences is usually the first breach in tolerance that initiates autoimmunity, consistent adaptive immune activation is essential for a sustained inflammatory response in autoimmune diseases including IIM. Recent literature suggests pathogenic origins of certain subsets of IIM such as ASSD in the lung, particularly in smokers with the ancestral haplotype, which later triggers systemic autoimmunity. The presence of tertiary lymphoid organogenesis (TLO) has been demonstrated in the lungs and muscle tissues of patients with DM and ASSD respectively.1–3 Furthermore, the oligoclonal expansion of T cells suggests common antigenic drivers of autoimmunity.4 To the same effect, maternal microchimeric antigens are known to persist in muscle tissue of male children with jDM, as a reminder of antigenic drivers.5 Thus, it seems plausible that the various T helper (Th) cell subsets in the peripheral blood may be reflective of the ongoing events and mechanisms of autoimmunity in the various subsets of IIM.
Inflammation in the lung may be granulomatous, as seen in sarcoidosis, wherein a Th1 response is dominant and central to Interferon-gamma (IFN-γ) production and Th cell and macrophage activation. Th subset plasticity occurs along a continuum, with a variable switch of Th1 subsets to Th17 phenotype in the appropriate milieu. The Th17.1 cells are plastic variants demonstrating fluidic shifts in an inflamed microenvironment. Understanding the Th1 and Th17 balance assumes a larger role in the era of precision medicine wherein biotherapeutics with specific targets are available. Since the Th cell subsets are well characterized in sarcoidosis,6,7 and lung inflammation and consequent Interstitial Lung Disease (ILD) in IIM continues to be a challenge, exploring the T helper subset profiles of these individuals in comparison with ILD in sarcoidosis may provide greater insight.
Previously proportions of various T cell subsets have lent researchers a greater understanding of the pathogenesis in other autoantibody-driven diseases, such as lupus, rheumatoid arthritis, and ANCA associated vasculitis. The utility of T helper subsets as biomarkers has also been explored. There is a dearth of biomarkers in IIM to identify ongoing inflammation in the muscle and distinguish it from inactivity or damage. Since myositis is an autoantibody-mediated disease and TLO is reported in the diseased muscles we had previously focussed on B cell associated biomarkers. We now looked for peripheral blood T helper subset profiling as a reflection of ongoing muscle inflammation. We further compare the T helper subset ratios in various clinical and antibody defined subsets of IIM and compare and contrast the picture in myositis ILD with that in sarcoidosis, where the disease is less heterogeneous, and pathogenesis is better understood.
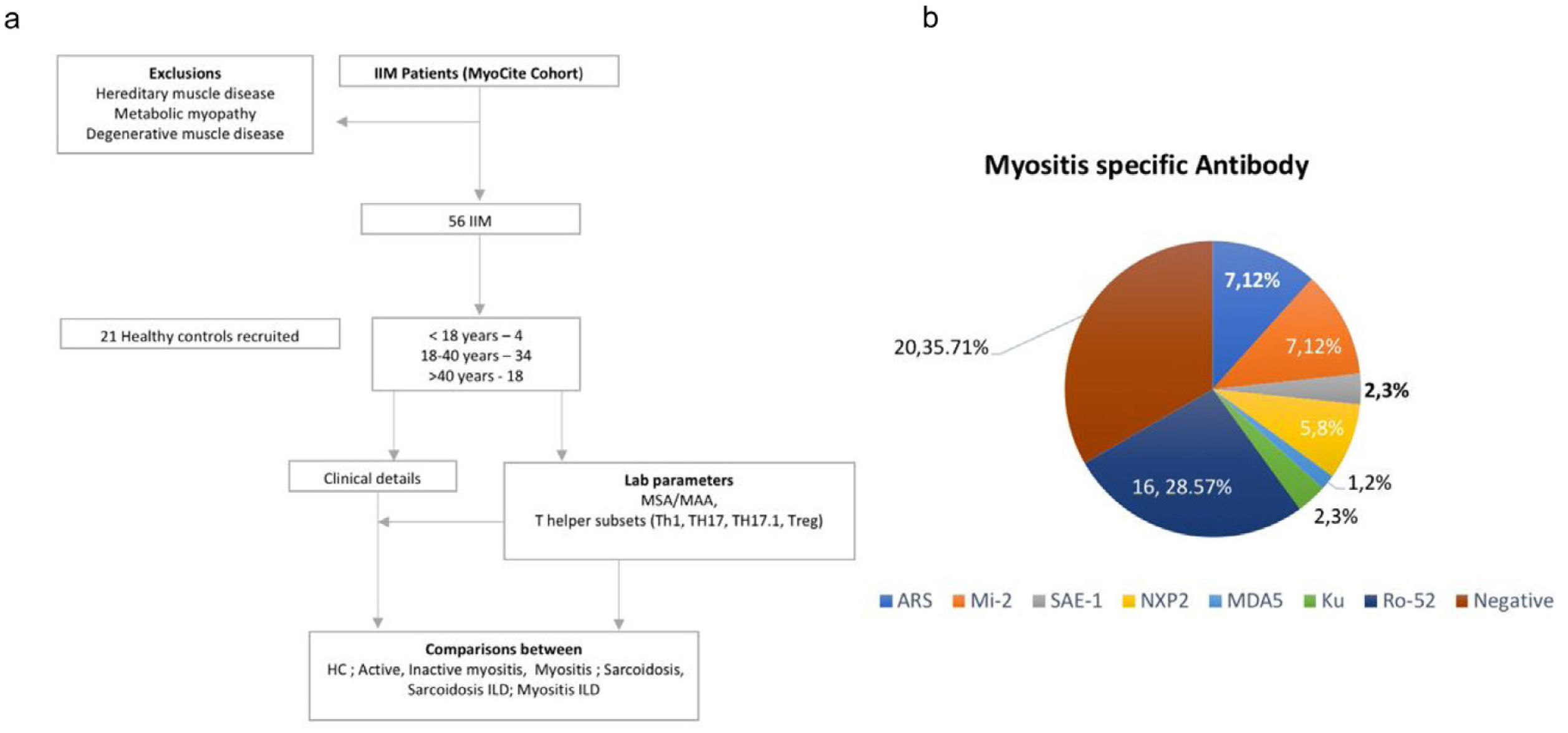
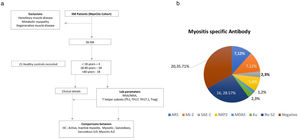
MethodsFifty-six patients with probable or possible IIM by 2017 ACR-EULAR classification criteria8 were prospectively evaluated with the assessment of clinical features and laboratory data (including autoantibodies) in an institutional review board certified study [2017-41-IP-76]. They were screened to identify cases without active ongoing infection, pregnancy, acute renal dysfunction, or chronic kidney disease. Those with metabolic, degenerative, inherited, or other forms of myopathies were excluded (Fig. 1). Further sub Classification into Polymyositis (PM), DM, Juvenile IIM (JIIM), Overlap Myositis (OM – in association with another CTD) was done. Those with two or more rashes suggestive of DM but without gottron's or heliotrope rashes were also classified as DM.9 Twenty-one healthy controls and 18 patients with sarcoidosis were included as diseased controls for comparison.
Clinical detailsA standardized case record form (CRF) was used to record clinical and laboratory variables. Clinical data and sera of patients and healthy controls were retrieved from the database and biobank respectively.10–12 Case details were supplanted with standard outcome measures as described by the International Myositis Assessment & Clinical Studies Group (IMACS). Active disease was defined by Myositis Disease Activity Assessment Tool (MDAAT) score of greater than or equal to one. STROBE's checklist was followed for reporting results.
ControlsHealthy controls: Twenty-one healthy adults without any comorbidities were included as healthy controls (HC) (Table 1).
Baseline characteristics of patients with inflammatory myositis.
| Characteristics | Demographic details (n, % or median, IQR) | Healthy control (median, IQR) | Sarcoidosis (n, % or median, IQR) |
|---|---|---|---|
| Age | 36 (25–45) | 32 (24.5–41) | 42 (33.75–53.75) |
| Gender (M:F) | 1:4 | 2:1 | 1.7:1 |
| Diagnosis* | |||
| PM | 17 (30.35%) | ||
| DM | 31 (55.35%) | ||
| OM | 5 (8.93%) | ||
| JDM | 3 (5.35%) | ||
| Disease course | |||
| Monocyclic | 8 (14.28%) | ||
| Polycyclic | 10 (17.85%) | ||
| Chronic continuous | 1 (1.79%) | ||
| Undefined | 14 (25%) | ||
| Clinical profile | |||
| Myositis | 4 (15.3%) | - | |
| ILD# | 14 (25%) | 8 | |
| Rash | 22 (39.28%) | 9 | |
| Arthritis | 25 (44.64%) | ||
| Other | 16 (23.69) | ||
| Disease duration (years) | 3 (2–8) | 2±3.3 (Mean±SD) | |
| Disease activity | |||
| Active | 30 (53.57%) | ||
| Inactive | 26 (46.43%) | ||
| Antinuclear antibodies | |||
| Positive | |||
| Nuclear | |||
| Speckled | 21 (37.5%) | ||
| Homogenous | 7 (12.5%) | ||
| Nucleolar | 4 (7.14%) | ||
| Other | 11 (19.64%) | ||
| Cytoplasmic | 6 (10.71%) | ||
| Negative | 10 (17.86%) | ||
| Myositis specific antibodies* | |||
| Positive | |||
| ARS | 7 (12.5%) | ||
| Mi-2 | 7 (12.5%) | ||
| SAE-1 | 2 (3.57%) | ||
| NXP2 | 5 (8.93%) | ||
| MDA5 | 1 (1.79%) | ||
| MAA | |||
| Ku | 2 (3.57%) | ||
| Pm-Scl | 0 | ||
| ds-DNA | 0 | ||
| Ro52 | 16 (28.57%) | ||
| Negative | 20 (35.71%) | ||
*Abbreviations: ARS, antibodies to amino acyl t RNA synthetase, NXP, nuclear matrix protein; ILD, Interstitial Lung Disease; SAE-1-Anti, small ubiquitin-like modifier-1 activating enzyme; MDA, melanoma differentiation-associated protein; dsDNA-Anti, double standard DNA antibody; Ku-anti, Ku antibodies; Mi-2, antibodies to nucleosome remodeling deacetylase (NuRD) complex; PM, polymyositis; DM, dermatomyositis; OM, overlap myositis.
#Of the 14 cases, all had chronic ILD except one which had a rapidly progressive ILD.
Disease controls: Eighteen patients of sarcoidosis with a male to female ratio of 1.4:1 were included as disease controls (Table 1).
Laboratory assaysSera from all the patients were tested for Myositis Specific/Myositis Associated Antibodies (MSA/MAA) by the Line immunoassay (G4 panel, Euro-Immune, Lubeck, Germany) and Anti-Nuclear antibodies (ANA) using the immunofluorescence assay (IFA, Nova-lite, Inova, CA, USA) according to the manufacturer's instructions. A cut-off of ++ was taken as positive for the former and 1:80 dilution was used for the analysis of ANA. Samples were diluted to 1 in 100 for the latter.
T-cell stainingFresh venous peripheral blood samples were collected from the study patients and controls by venipuncture using sterile lithium heparin-treated tubes. The blood samples were diluted in a ratio of 1:1 with incomplete RPMI-1640 culture media (Sigma–Aldrich, St. Louis) and stimulated with phorbol 12-myristate 13-acetate (PMA, 20ng/ml; Sigma–Aldrich, St. Louis), ionomycin (1μg/ml; Sigma–Aldrich, St. Louis) and monensin (2μM; bd biosciences, San Diego, Ca) for 5h at 37°C in culture incubator. The surface staining was performed using an appropriate surface markers CD4+IFN-γ+, CD4+ IL-17+, CD4+IFN-γ+IL-17+, and CD4+CD25+ FOXP3+ for Th1, th17, Th17. 1 and Treg cells shown in Supplementary Table I and Supplementary Fig. 4. After that, red blood cells were lysed using BD FACS™ lysing solution buffer. Cells were washed with 1X PBS, fixed and permeabilized using BD Cytofix/Cytoperm™ (BD Pharmingen) followed by washing with cytoperm wash buffer as per the provided instructions. The stained cells were acquired on the BD Facs Canto II Flowcytometry machine. All the results were analyzed using BD Facs diva software version (6.1.3), considering at least 10,000cells for the lymphocyte gate.
Statistical analysisData were represented as median±IQR or %. Unpaired t tests or Mann–Whitney U tests were used for intergroup comparisons. Kruskal–allis test was used for multi-group comparisons. p-Values of <0.05 were considered statistically significant. GraphPad Prism software, version 8.2.1 was used for statistical analysis.
ResultsBaseline characteristic56 patients with IIM [31 DM (55.35%), 17 PM (30.35%), 5 OM (8.93%), 3 JDM (5.35%)] of median age 36 (25–45) years (4.1:1 F:M) and disease duration of 3(2–8) years were studied. Twenty-one HC (14 Male and 7 Female) with median age 32 (24.5–41) and 18 patients with sarcoidosis of median age 42 (33.75–53.75) {ILD (n=8) or without ILD (n=10)} were included as controls (Table 1).
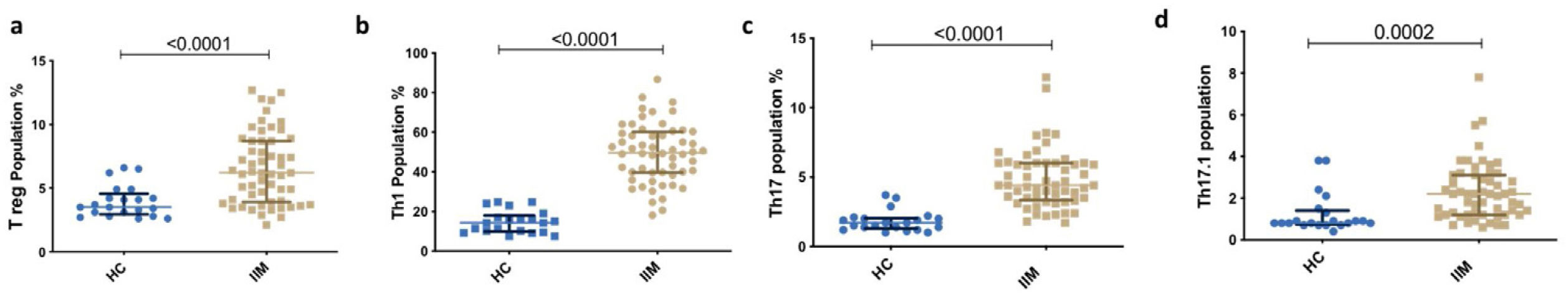
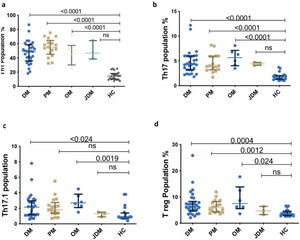
Th subsets in IIM versus healthy controlsAll Th subsets were elevated in IIM than HC [Th1 (49.65% vs 14.32%, p<0.0001); Th17 (4.4% vs. 1.7%, p<0.0001); Th17.1 (2.2% vs. 0.8%, p=0.0002); Treg (6.2% vs. 3.5%, p<0.0001)] (Figs. 2 and 3).
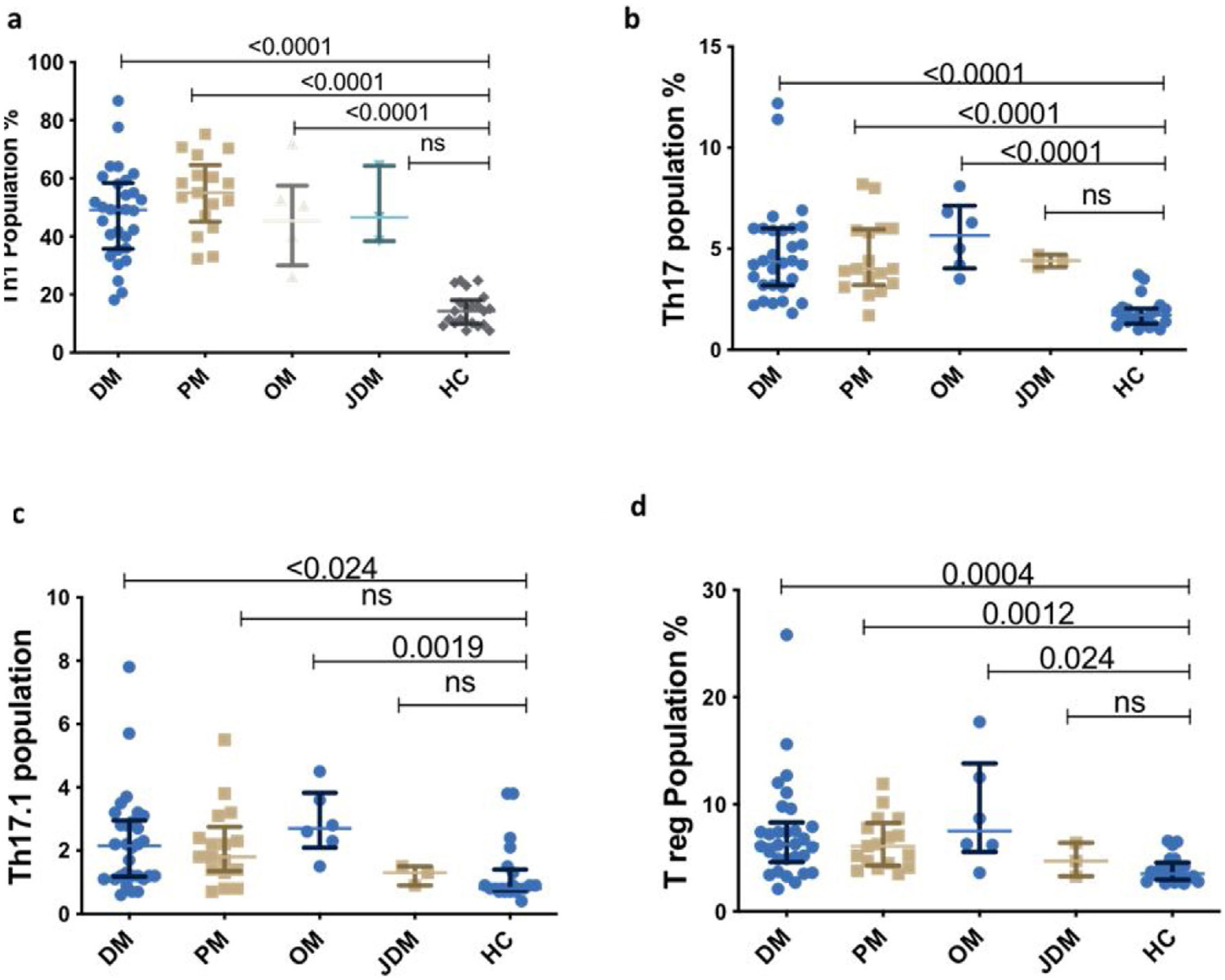
Representative dot plot comparing percentage of T cell subsets in various subsets of myositis (Dermatomyositis – DM, Polymyositis – PM, Overlap Myositis – OM, juvenile dermatomyositis – JDM with healthy controls (HC). (a) Th1 (b) Th17 (c) Th17.1 (d) T reg. Statistical test used is Kruskal–Wallis test.
All Th subsets were elevated in the three subtypes of IIM–DM, PM and OM than HC [Th1 (55%, p<0.0001; 49.15%, p<0.0001; 45.35%, p=0.024); Th17 (4.0%, p<0.0001; 4.3%, p<0.0001; 5.6%, p<0.0001) Th17.1 (1.8%, p=0.0078; 2.2%, p=0.024; 2.7%, p=0.0019;) and Treg cells (6.1%, p=0.0012; 6.3%, p=0.004; 7.5%, p=0.024, Fig. 3)] respectively except in JDM.
No difference was observed in the Th subsets after subcategorization based on MSAs (ARS, Mi-2, SAE-1, NXP-2, MAA, Negative) (Supplementary Fig. 1I).
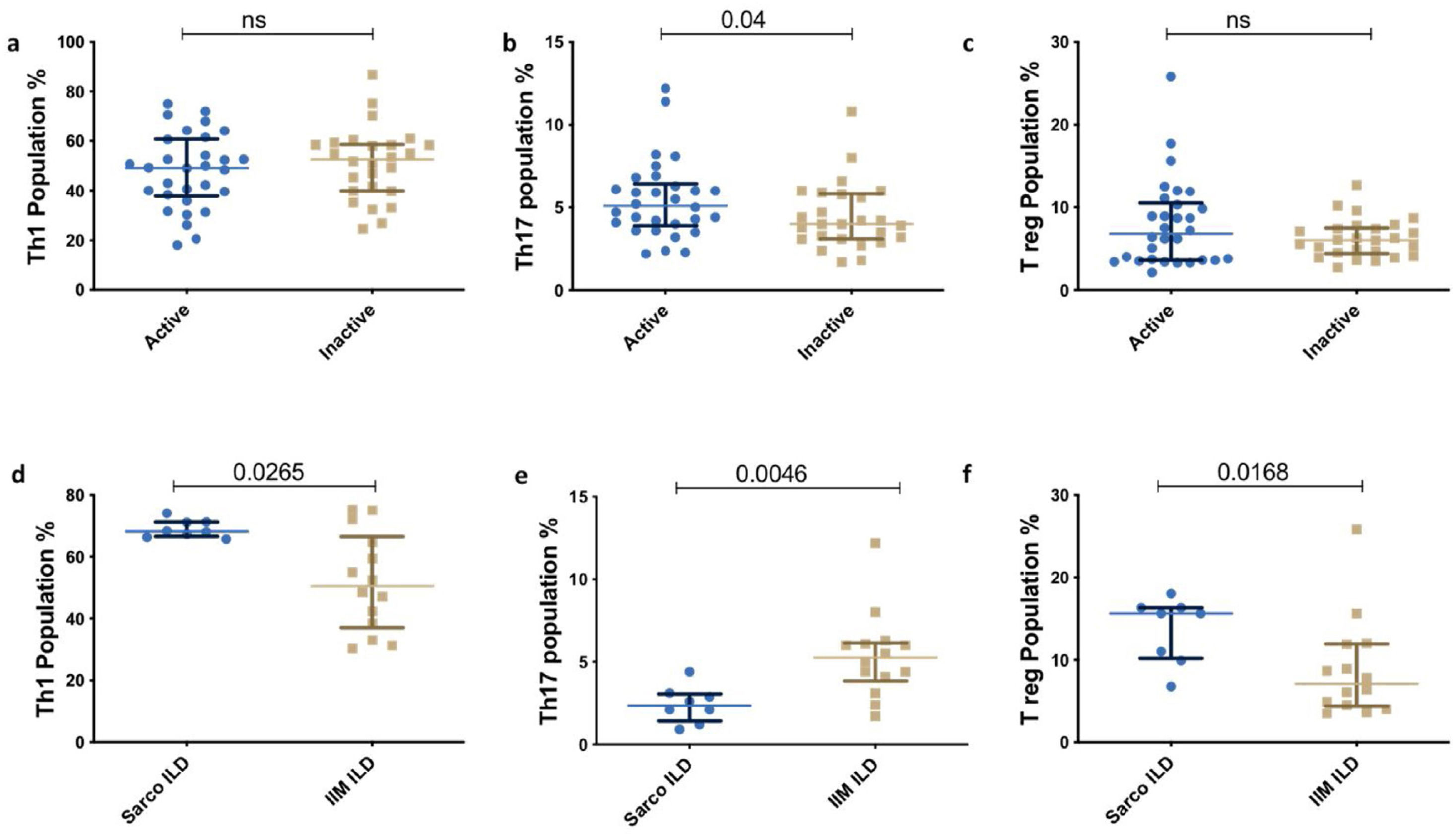
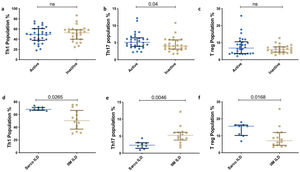
Th subsets in IIM versus sarcoidosisTh1 and Treg were significantly elevated in Sarcoidosis as compared with IIM. However, Th17 population was lower in sarcoidosis compared to IIM. [Th1(69.1% vs 49.65%, p<0.0001], [Treg (12.05% vs 6.2%, p<0.0001)], [Th17 (2.49% vs 4.4%, p<0.0001)] respectively. Similarly, the distribution of the Th subsets was similar in the subpopulation with ILD between both the groups[Th1 (68.15% vs 50.40, p=0.0265), Treg (15.61% vs 7.1%, p=0.0168), Th17 (2.35% vs 5.25%, p=0.0046)] (Fig. 4).
T helper subsets based on clinical manifestation and disease activityThere was no significant difference in between the various phenotypic subsets (arthritis present vs. absent, Fig. 3b) and active vs. inactive disease groups (Supplementary Figs. II and III).
DiscussionThe peripheral blood T cell signature is heterogeneous in patients with IIM. We evaluated the T cell profile in IIM and compared it with those of HC and sarcoidosis – a multisystem disease with chronic lung involvement akin to IIM with a well characterized pathogenesis. We observed an elevated peripheral blood Th1, Th17, Th17.1, and Treg population in IIM as compared to HC irrespective of the subtype of IIM, MSA specificity, and disease activity. Patients with IIM exhibited a stronger Th17 and lower Th1 and Treg signature as compared with sarcoidosis. Patients with OM showed a dominant Th17.1 signature, possibly suggesting the dominance of a plastic phenotype of Th cells.
The autoimmune basis of IIM is substantiated with CD4 and CD8 T cell infiltration in muscle biopsy specimens. In recent years, the discovery of MSA and MAA further supports a direct role of B cells in the pathogenesis of IIM.13 B cell and autoantibody production, in turn, require help from T cells. The known susceptibility of IIM to certain HLAs (predominantly HLA-DR) and demonstration of restricted TCR repertoire in muscle tissues, point towards an indisputable role of T cells in the pathogenesis of IIM.4
IFN-γ, the signature cytokine produced by Th1 cells is elevated in PM/DM muscle biopsy samples. Furthermore, IFN-γ cytokine levels along with Tumor Necrosis Factor alpha (TNF-α) are elevated in muscle specimens from Inclusion Body Myositis (IBM).14 Th1 response through IFN-γ activates proinflammatory M1 macrophages resulting in further inflammation and tissue damage. Traditionally, the Th1 response was considered to be the predominant inflammatory phenotype responsible for the pathogenesis in IIM until the discovery of Th17 cells. Analysis of the peripheral blood for Th1 cells has shown a lower frequency in active DM as compared to HC with elevated Th2/Th1 ratios in DM15,16 (Table 2). However, a clear Th1 predominant pattern is seen in the peripheral blood in patients with IBM.21 Although we observed elevated Th1 levels in IIM as compared to HC, the levels were lower when compared with patients with Sarcoidosis. This can be explained by the heterogeneity of the subtypes of IIM, the differences in duration of disease and the possible influence of treatment. Moreover, the Th cell subsets cannot be strictly defined at all times given considerable plasticity based on the inflammatory milieu.
Peripheral blood T cell profile in IIM reported in literature.
| Our study | Oretga et al.23 | Wilkinsin et al.22 | Antiga et al.28 | Banica et al.30 | Shimojima et al.29 | Aleksza et al.15 | Ishii et al.16 | |
|---|---|---|---|---|---|---|---|---|
| Number | ||||||||
| IIM | 56 | 30 | 44 | 15 | 11 | 25 | 99 | 21 |
| HC | 21 | 30 | 15 | 14 | 18 | 11 | 32 | 10 |
| Subtype | – | |||||||
| PM | 17 | 10 | 9 | – | 10 | 50 | 8 | |
| DM | 31 | 20 | 23 | 15 | 15 | 49 | 13 | |
| OM | 5 | – | 12 | – | – | – | – | |
| jDM | 3 | – | – | – | – | – | – | |
| M:F, IIM | 1:4 | – | 1:3.4 | 1:3.67 | – | 1:2.1 | 1:2.5 | 1:4 |
| Mean age * Median | 36* (25–45) | 41 | 39* (31–49) | 53.6 | – | 50.5 | 45.5 | 52.5 |
| As compared to HC | ||||||||
| Th17 | Elevated | Elevated | Elevated | – | – | Decreased | – | – |
| Tregs | Decreased | Decreased | Elevated | Decreased | Decreased | – | – | – |
| Th1 | Elevated | – | (DM) | – | – | Increased | Decreased | Decreased |
IIM, idiopathic Inflammatory m/myopathy; HC, healthy control; M, male; F, female; PM, polymyositis; DM, dermatomyositis; OM, overlap myositis, jDM, juvenile dermatomyositis.
Since the discovery of Th17 cells, their role has been explored in the pathogenesis of various autoimmune rheumatic diseases, and myositis is no exception. An increased expression of IL-17 and IL-23 mRNA in muscle specimens from patients with IIM compared to HC suggests activation of the IL-17/IL-23 pathway.17 IL-17 along with IL-1 stimulated IL-6 production regulates the survival and differentiation of B cells.18 IL-17 induced Nuclear factor kappa B expression in myoblasts enhances inflammatory injury, inhibits myocyte differentiation, and myocyte migration through induction of TNF-like weak inducer of apoptosis (TWEAK). A predominant Th17 mediated inflammation is proposed in a subcategory of patients responding to IVIG.19 Moreover, IL-17 may play an important role in TLO genesis and sustenance. Elevated IL-17A has been found in patients with ASSD and Connective tissue disease associated ILD as compared to HC.20,21 Thus, IL-17 plays a central role in enhancing inflammation, induction of antibodies, and impairing muscle regeneration which is characteristic of the pathogenesis of IIM. In our present work, we observed increased Th17 numbers in the peripheral blood as compared to HC as previously depicted in the work by Ortega and Wilkinson et al.22,23 (Table 2).
Th cells exhibit considerable plasticity and in presence of IL-12 and IL-23 without Transforming Growth Factor (TGF-β), Th17 cells can evolve into IFN producing Th17.1 cells. The role of Th17.1 cells is explored in cancer biology, inflammatory bowel disease, and rheumatoid arthritis and defined phenotypically as an aggressive phenotype that exhibits resistance to glucocorticoids by expression of P-glycoprotein/multidrug resistance. Ours was a novel attempt at characterizing Th17.1 signature in the peripheral blood in patients with IIM and it was significantly elevated as compared to HC. Further, DM and OM had a trend towards greater Th17 and Th17.1 signature as compared to PM in the peripheral blood. This can be explained based on a greater extent of systemic inflammation and multiorgan involvement in the latter two. Although this seems to be a plausible explanation, the findings need to be verified in a larger cohort.
A tilted balance between pro-inflammatory Th1, Th17, and Treg in favour of the former has been seen in most autoimmune diseases. Most studies show lower peripheral blood Treg cells in IIM as compared to HC. However, the frequency of Treg is comparable between IIM and HC in muscle biopsy specimens.23 The lower numbers in the peripheral blood in patients with IIM could indicate sequestration to sites of inflammation in the affected muscles and other organs. But conversely, we demonstrated higher peripheral blood Treg frequency IIM than HC as in the study by Wilkinson et al.22,28,30
Sarcoidosis was traditionally believed to be a Th1 mediated disease with IFN-γ playing a central role in the granulomatous inflammation. Th2 mediated inflammation dominates the chronic phase of the disease with established fibrosis.6,7,24 However, recent studies have identified increased Th17 fractions in the bronchoalveolar fluid as well as the peripheral blood, with a correlation of Th17.1 cells with the Scadding stage, arthritis, and relapsing disease.25,26 A recent study identified Th17.1 cells as the predominant IFN-γ producing cells in the BAL fluid in patients with Sarcoidosis challenging the Th1 paradigm. An exaggerated Th1 profile was seen in Sarcoidosis as compared to IIM, with lower Th17 levels and similarly elevated Th17.1 values in peripheral blood. This difference may account for the characteristic IFN-γ driven granulomatous inflammation characteristic of Sarcoidosis steered by either Th1, or Th17.1 cells, depending on the phase of the disease.
The discovery of MSA in IIM with some showing a correlation with disease activity throws light on the significant role of B cells in the immunopathogenesis, and interaction between Th17 and B cell is reciprocatory. Th17 predominant inflammation in IIM enhances proliferation of B cells with subsequent autoantibody production possibly through IL-6 production. And, B-1 cells can in turn induce Th17 cell differentiation from naïve Th cells. This interaction is further supported by Th17 predominant inflammation in a subcategory of patients with IIM responding to IVIG. TLO is reported in new onset JDM with a poor response to standard therapy and a greater need for treatment with cyclosporine, IVIG, or Rituximab in those with well-defined follicular structures in the inflamed muscles. TLO is observed in Rheumatoid arthritis (in the synovium) and Sjogren's Syndrome (in the salivary glands) and represents an extra nodal site of immune activation with greater Dendritic cell-T-B cell interaction. It also bypasses the immune regulation that normally occurs in regional lymph nodes. We did not find any influence of MSA specificity on the T cell profile possibly due to a smaller sample size as positivity is expected in around 60% of patients.
Drugs targeting the IL-23-IL-17 axis are being considered to be potentially useful and clinical case reports of IL-6 inhibition in IIM are described. A clinical trial of Ustekinumab in PM/DM is underway and is in the stage of recruitment. The interplay between B cells and Th17 cells, with the known role of Th17 cells in the establishment and maintenance of TLO further supports the possibility of exploring Th17 inhibition in IIM in addition to B cell targeting therapy. Similarly, a Th1 predominant inflammation in the acute phase of Sarcoidosis with the chronic fibrotic stage induced by Th17 cells is proposed. Another evolving concept is the activation of a metabolic checkpoint kinase mechanistic target of rapamycin complex 1 (mTORC1) that drives the proliferation of macrophages and granuloma formation. Both these newer targets are being explored in Sarcoidosis with an ongoing trial of Ustekinumab and case reports of sirolimus use in chronic Sarcoidosis. The current observations of a Th17 paradigm in IIM open the case for exploring these pathways therapeutically in the disease.
Other than T and B cells, role of the innate immune system has been also implicated in various subtypes of myositis. Increased NK cell infiltration secondary to downregulation of NKp30 in muscle and lung in ASSD and decreased functioning in JDM has been demonstrated. NKG2D and NKp46 correlated with lung involvement and its severity in ASSD and DM in a recent study.27 The role of Innate lymphoid cells have been studied extensively in other autoimmune inflammatory diseases like rheumatoid arthritis, Systemic Lupus Erythematosus and Spondyloarthritis but their role in IIM is yet to be explored. IL-23 independent IL-17 producing cells like ILC-3 and NKT cells could be important players in IIM. Future projects integrating biomarkers of innate and adaptive immune systems will be able to shed light better on the differential role of these in IIM and its subtypes.
The distinct strengths of our paper include attempts at characterization of Th17.1 cells in the peripheral blood in IIM and comparing the T cell profile with Sarcoidosis, another multisystem disease with an established T cell mediated pathogenesis. The drawbacks include a cross-sectional evaluation of the T cell profile. T helper subsets are known to undergo fluidic shifts depending on the stage of the disease and activity. Therefore, a timed follow-up evaluation with an analysis of disease activity may provide greater insight into the changing T cell profile relevant to the pathogenesis of IIM. Furthermore, validating the findings in a larger subset may provide greater insight into the pathogenetically distinct subtypes of IIM. To further explore the role of T cells in TLO, evaluation of muscle and lung tissue for T cell subsets will be of relevance in the future.
In conclusion, Th axis was skewed towards a Th1, Th17, and Th17.1 profile in IIM compared to HC with a greater Th17 and lower Th1 fractions as compared with Sarcoidosis. It explains the diverse organ involvement seen across both the disease with the difference plausibly explaining the type of inflammation seen in the underlying involved organs.
Conflict of interestThe authors declare that they have no conflict of interest.