Celiac disease is an autoimmune systemic disease having among its clinical manifestations frequent symptoms common to rheumatologic diseases such as musculoskeletal pain, asthenia, and cognitive fatigue. It is associated with other autoimmune diseases like Sjögren disease. It is a well-characterized disease with specific diagnostic tests.
Non-celiac gluten sensitivity is an emerging entity with symptoms similar to celiac disease, but without specific diagnostic tests. The concept of non-celiac gluten sensitivity and its diagnostic problems are reviewed, and the hypothesis of its association with fibromyalgia, spondyloarthritis, and autoimmune conditions is proposed. Clinical observations supporting the hypothesis are described, highlighting the benefit of treating non-celiac gluten sensitivity.
La enfermedad celíaca es una enfermedad autoinmune sistémica que tiene entre sus manifestaciones clínicas síntomas frecuentes en las enfermedades reumatológicas, como dolor musculoesquelético crónico, astenia y fatiga mental. Se asocia a otras enfermedades autoinmunes, como la enfermedad de Sjögren. Es una enfermedad bien caracterizada con pruebas diagnósticas específicas.
La sensibilidad al gluten no celíaca es una entidad emergente, con sintomatología similar a la de la enfermedad celíaca, pero sin pruebas diagnósticas específicas. Se revisan el concepto y los problemas diagnósticos de la sensibilidad al gluten no celíaca y se propone como hipótesis la asociación de la sensibilidad al gluten no celíaca a la fibromialgia, las espondiloartropatías y las enfermedades autoinmunes. Se describen observaciones clínicas que apoyan esta hipótesis, destacando el beneficio clínico del tratamiento de la sensibilidad al gluten.
Celiac disease (CD) has traditionally been considered to be a pediatric gastrointestinal disease, characterized by malabsorption and failure to thrive; however, this perspective has changed substantially in recent years. It is now considered a common autoimmune disease that can present at any age, with both intestinal and extraintestinal manifestations.1–6 Although the objective of this article is not to review CD, we think it necessary to point out certain aspects that rheumatologists should take into account: (a) CD can be present in the absence of gastrointestinal symptoms; in fact, nearly half of the CD patients diagnosed in adulthood do not have relevant gastrointestinal symptoms; (b) in addition to the classic iron-deficiency anemia, diarrhea and osteoporosis, CD is the cause of symptoms such as asthenia, mental fatigue and chronic musculoskeletal pain, which accompany many systemic diseases7; in fact, it has been referred to as the great imposter8; and (c) CD is known to be associated with other autoimmune diseases, most frequently, autoimmune thyroid disease and Sjögren's syndrome. Table 1 shows the diseases associated with CD.9 The presence of the rheumatic diseases associated with CD is reason enough to consider performing serologic testing for CD in rheumatic patients with asthenia, anemia, chronic musculoskeletal pain or systemic diseases. In a study carried out in 211 patients with unexplained signs of joint disease, the rate of positive CD serology was much higher than that of the control population. Positivity for immunoglobulin A (IgA) endomysial antibodies (the most specific serologic test for CD) was detected in 2.3% of the patients and in only 0.28% of blood donors used as the control group.10
Groups at Risk for Celiac Disease.
| First-degree relatives |
| Patients with associated diseases |
| Autoimmune disease |
| Type 1 diabetes mellitus |
| Autoimmune thyroiditis |
| Selective IgA deficiency |
| Inflammatory bowel disease |
| Sjögren's syndrome |
| Systemic lupus erythematous |
| Addison's disease |
| IgA nephropathy |
| Chronic autoimmune hepatitis |
| Primary biliary cirrhosis |
| Rheumatoid arthritis |
| Psoriasis, vitiligo and alopecia areata |
| Neurological and psychiatric disorders |
| Progressive encephalopathy |
| Cerebellar syndromes |
| Dementia with brain atrophy |
| Leukoencephalopathy |
| Epilepsy and calcifications |
| Schizophrenia |
| Other associations |
| Down syndrome |
| Williams syndrome |
| Turner syndrome |
| Cystic fibrosis |
| Hartnup disease |
| Cystinuria |
| Microscopic colitis |
| Cardiomyopathy |
| Fibromyalgia |
| Chronic fatigues syndrome |
Taken from Diagnóstico precoz de la enfermedad celíaca. Spanish Ministry of Health and Consumer Affairs, 2008.9
Celiac disease is considered to be an autoimmune enteropathy caused by exposure to gluten in genetically predisposed individuals. Those who are positive for human leukocyte antigen (HLA) DQ2.5 (DQA1*05-DQB1*02) or DQ8 (DQA1*0301-DQB1*0302) may have an adaptive immune response to gluten, with production of antibodies (to tissue transglutaminase [tTG] and endomysium) and infiltration of the intestinal epithelium by CD3+ lymphocytes (intraepithelial lymphocytosis), which, when severe, leads to the atrophy of the intestinal villi observed in the duodenal biopsy (Marsh type 3 lesion). The genetic susceptibility triad consisting of HLA DQ2 or DQ8, specific antibodies (to tTG and endomysium) and intestinal villous atrophy on duodenal biopsy is what characterizes and defines CD.
Non-celiac gluten sensitivity (NCGS) is an emerging entity characterized by gluten-related intestinal and extraintestinal symptoms in patients with negative CD tests who, thus, are not considered to be celiac patients.11–14 Clinical observations of patients who responded to a gluten-free diet (GFD) in whom CD could not be confirmed date back at least to 1978. In recent years, this entity is gaining increasing prominence and is no longer regarded as a rare condition. Non-celiac gluten sensitivity is considered to be more prevalent than CD, which affects 1% of the population. Although there are no systematic epidemiological studies, due to the fact that there is no diagnostic marker that enables them to be performed, NCGS is estimated to affect around 5% of the population.
The most prevalent concept, according to a consensus reached at an expert meeting held in Oslo, is that CD and NCGS are 2 different conditions within the spectrum of gluten-related disorders, which also includes wheat allergy.15 As there is no diagnostic test for NCGS, the diagnosis is based on the exclusion of CD (absence of anti-tTG antibodies and of intestinal villous atrophy on duodenal biopsy) and of wheat allergy in patients with gluten-related symptoms. With regard to the symptoms, CD and NCGS are indistinguishable, although NCGS is considered to have no relationship to systemic diseases. Table 2 shows the symptoms observed in 347 cases. With respect to HLA, only about half the patients with NCGS carry DQ2.5 or DQ8. In CD, the adaptive immune system predominates, responding with production of anti-endomysial and anti-tTG antibodies, which leads to an increase in intestinal permeability and to intestinal villous atrophy. Antibodies to deamidated gliadin peptide, which are nearly as specific for CD as anti-tTG antibodies, are also present. In NCGS, the innate immune system is considered to predominate. IgG antibodies against native gliadin, which have a low specificity for intestinal villous atrophy and are not useful in the diagnosis of CD, do prove to be of use in detecting NCGS, although the sensitivity is limited.
Symptoms of Non-celiac Gluten Sensitivity.
| Intestinal | |
| Abdominal pain | 68% |
| Diarrhea | 33% |
| Nausea | |
| Weight loss | |
| Bloating, flatulence | |
| Cutaneous | 40% |
| Erythema | |
| Eczema | |
| General | |
| Headache | 35% |
| Bone and joint pain | 11% |
| Muscle contractures | 34% |
| Numbness of hands and feet | 20% |
| Chronic tiredness | 33% |
| Behavioral | |
| Attention deficit | |
| Depression | 22% |
| Hyperactivity | |
| Oral | |
| Chronic ulcerative stomatitis | |
Taken from Czaja-Bulsa.11
However, the dichotomous working approach of considering CD and NCGS as different entities does not depict the complexity of a disease that is probably the expression of a biological continuum. There are many examples of patients who, following strict criteria, cannot be considered celiacs, but whose profile overlaps substantially with CD. The clearest examples are the patients with gluten-sensitive enteropathy without villous atrophy. In healthy individuals, there are few intraepithelial lymphocytes and they are distributed predominantly at the base of the villus. In gluten-sensitive enteropathy, there may be no villous atrophy, only an increase in the number of intraepithelial lymphocytes all along the intestinal villus, over 25 CD3+ lymphocytes per 100 enterocytes, characteristically present at the tip of the villus.16 There are patients with CD-like symptoms, negative tests for specific antibodies and no villous atrophy, but they have HLA susceptibility and intraepithelial lymphocytosis on duodenal biopsy and respond to the GFD. It is considered that these patients have gluten-sensitive enteropathy despite the absence of villous atrophy, and that the serological markers of CD are sensitive in the detection of villous atrophy, but have a low sensitivity for non-atrophic gluten-sensitive enteropathy. This gluten-sensitive enteropathy, which corresponds to Marsh type 1 lesions and, if strict criteria are applied, is “non-celiac”, has been demonstrated in several clinical conditions, as well as in relatives of celiacs and patients with irritable bowel syndrome.17–20 It is important to point out that intraepithelial lymphocytosis on duodenal biopsy can be underestimated. A good evaluation requires immunohistochemical staining and the biopsies should be analyzed by a pathologist with experience in this disease. On the other hand, the increase in intraepithelial lymphocytes is a nonspecific findings that can be observed in other diseases, such as Helicobacter infection or lactose intolerance. Moreover, some of the patients with NCGS that clearly overlaps with CD have a sensitivity to gluten that evidently falls outside the CD spectrum, as they do not carry either DQ2.5 or DQ8, but have a clinical picture similar to that of CD and respond to the GFD, with or without changes in the duodenal biopsy.
Diagnosing NCGS is complicated because there is no diagnostic test. HLA typing and duodenal biopsy are merely suggestive of the direction to take. It is the whole series of clinical data, HLA typing and intraepithelial lymphocytosis on duodenal biopsy that point to NCGS, which is confirmed by the response to the GFD and, ideally, by a worsening following double-blind gluten challenge. To complicate things more, in our experience, often the response to the GFD is not immediate and can take months, as occurs in CD.
The clinical approach to suspected CD and NCGS should take into account this complexity. If the question is: “Does the patient have celiac disease?”, arriving at the response is relatively simple when based on the tests that confirm or rule out CD: anti-tTG antibodies and duodenal biopsy if that first test is positive. If there is a strong clinical suspicion of CD and HLA susceptibility, a duodenal biopsy is justified even if the test for anti-tTG antibody is negative. However, if the question is: “Is the patient sensitive to gluten?”, the problem is much more complex, as there are no tests to confirm or rule out NCGS. It must be taken into account that the serology and biopsy should be performed prior to starting a test GFD.
The idea that has guided the clinical development dealt with in this article is that NCGS occurs frequently and is the cause of a number of rheumatic complaints. The following sections provide case reports that support this hypothesis and describe the development of the model that relates gluten sensitivity to rheumatic conditions.
Non-celiac Gluten Sensitivity and FibromyalgiaThe first clinical situation in which we looked for gluten sensitivity was in patients with refractory fibromyalgia (FM). Tiredness, chronic musculoskeletal pain, irritable bowel syndrome and mental fatigue are all characteristic of FM. This approach seemed reasonable, given that these symptoms are also associated with CD and that the diagnosis of FM is merely a descriptive, syndromic diagnosis. The concept of NCGS had not yet been coined, but even then, it was known that there were celiac patients with negative anti-tTG antibody tests, and the March type 1 gluten-sensitive enteropathy, which did not meet the criteria for CD, had been characterized. At the beginning, it was frustrating; CD serology was positive in very few cases, an experience that coincides with that of another group that found no increase in the incidence of CD in patients with FM who underwent CD serology.21 With the authorization of the Clinical Research Ethics Committee and the informed consent from each of the patients, gastroscopy and duodenal biopsy were performed despite the negative CD serology. Once again, the findings were frustrating, as villous atrophy was hardly observed in any of the patients in our study population. Despite this fact, a strict GFD was recommended with the aid of the Asociación de Celíacos de Madrid (Association of Celiac Patients of Madrid), which, since then, has come to be called the Asociación de Celíacos y Sensibles al Gluten de Madrid (Association of Celiac and Gluten-Sensitive Patients of Madrid). Other recommendations were the elimination from the diet of dairy products and lactose if there was any clinical suspicion of intolerance to either and the incorporation of vitamin and mineral supplements, both of which are frequently suggested to celiac patients. The use of anti-inflammatory agents, proton pump inhibitors and psychotropic drugs was also minimized because of their secondary effects on the small intestine and on the central nervous system.
The first clinical observations were surprisingly favorable, surpassing our expectations. A report on a selection of 20 of the first patients with a clear clinical response and Marsh type 1 lesions has been published.22 Relevant clinical improvement was defined as achieving at least one of the following objectives: remission of FM, return to work or to normal life, or the discontinuation of treatment with opioids. The mean follow-up time was 16 months (range: 5–31 months). Only 11 patients carried DQ2.5 or DQ8. Follow-up data are available for 246 FM patients in whom this GFD-based strategy was applied, and relevant clinical improvement has been observed in 90 (36%). We consider it quite probable that patients who carry HLA DQ2 or DQ8 and have intraepithelial lymphocytosis on duodenal biopsy will respond to a GFD, although a lack of response does not exclude them. On the basis of the clinical data, the findings that we consider most suggestive of NCGS are: having a relative with CD, recurrent aphthous stomatitis, diarrhea-predominant irritable bowel syndrome and iron-deficiency anemia.
Non-celiac Gluten Sensitivity and SpondyloarthritisNext, we considered the presence of NCGS in patients with spondyloarthritis. Symptom overlap between spondyloarthritis and FM has been widely reported as the chronic low back and polyenthesitic pain associated with spondyloarthritis can be similar to the symptoms of FM. There is no analytical test to confirm or rule out either of the two diseases, and, in our experience, it is not uncommon to examine FM patients for preradiographic spondyloarthritis and even administer a test treatment. In a study of 30 patients with ankylosing spondylitis, 11 had anti-gliadin antibodies, whereas none of the subjects in the control group did. However, CD was confirmed in only one patient.23 As the intestinal etiology and pathogenesis are important in spondyloarthritides, and sacroiliitis has been associated with CD in some reports,24,25 it seems reasonable to postulate that NCGS may be a cause of enteropathic spondyloarthritis.
We describe a number of clinical observations that support this hypothesis. The cases involve 4 patients with axial spondyloarthritis, 2 of them with ankylosing spondylitis and 1 with psoriatic spondyloarthritis. Celiac disease was ruled out in all 4 patients, and a clear response to the GFD was observed, with resolution of their chronic inflammatory low back pain, which recurred after gluten intake. Three patients underwent duodenal biopsy, which revealed intraepithelial lymphocytosis.
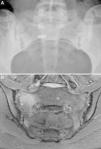
Case no. 1: a 28-year-old woman with a 10-year history of chronic inflammatory low back pain and asthenia. Plain radiography revealed evident bilateral sacroiliitis. Magnetic resonance images (MRI) of the sacroiliac joints showed the following signs of sacroiliitis: sclerosis, erosions and marked bilateral periarticular bone marrow edema (Fig. 1). As she was HLA-B27-positive, a diagnosis of ankylosing spondylitis was established. She had a sister with CD. She had no associated gastrointestinal symptoms. Serological tests for CD, consisting of screening for anti-tTG and anti-deamidated gliadin peptide antibodies, both IgG and IgA, was negative. HLA typing demonstrated that she was homozygous for DQ7 and the absence of DQ2 and DQ8. The duodenal biopsy revealed intraepithelial lymphocytosis with 37 CD3+ lymphocytes per 100 enterocytes, without villous atrophy. There was a clear improvement in low back pain and asthenia 3 months after initiating the GFD. At 10 months, her chronic low back pain had resolved, but recurred after inadvertent gluten ingestion.
Case no. 1. (A) Radiography of sacroiliac joints showing widening, erosions and bilateral sclerosis, which corresponds to bilateral grade 3 sacroiliitis. (B) Magnetic resonance image of sacroiliac joints showing sclerosis, erosions and marked bone marrow edema, all signs of active sacroiliitis.
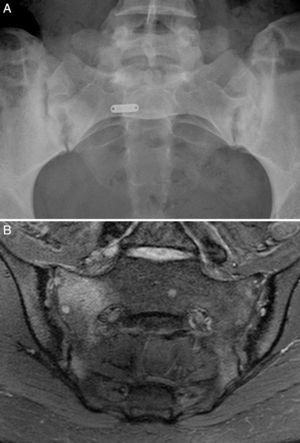
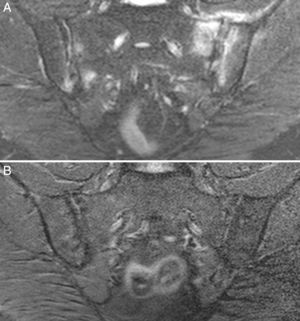
Case no. 2: a 28-year-old woman with a 1-year history of chronic low back pain with inflammatory characteristics and generalized pain that met the criteria for FM. She tested negative for HLA-B27 and positive for antinuclear antibodies (ANA), with a low titer of 1/80, which showed no specificity. Plain radiography revealed sclerosis of both sacroiliac joints, with a difficult differential diagnosis between condensing osteitis and sacroiliitis. On MRI, sclerosis, erosions and bone marrow edema were observed, demonstrating sacroiliitis (Fig. 2). Thus, the patient met the Assessment of SpondyloArthritis International Society (ASAS) criteria for axial spondyloarthritis. Moreover, she had gastrointestinal symptoms associated with dyspepsia, nausea and vomiting. Serological screening for CD was negative. HLA typing revealed the presence of DQ7 and DQ6 and absence of DQ2 and DQ8. The duodenal biopsy revealed intraepithelial lymphocytosis with 44 CD3+ lymphocytes per 100 enterocytes, without villous atrophy. After 7 months of a GFD, there was remission of both low back pain and generalized pain, as well as the gastrointestinal symptoms. After 20 months of follow-up, clinical remission was maintained, and she reported the recurrence of the pain and gastrointestinal symptoms after having ingested gluten.
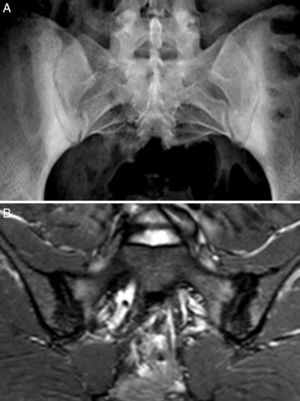
Case no. 3: a 50-year-old woman who had been diagnosed with ankylosing spondylitis 3 years earlier, with bilateral sacroiliitis on plain radiography and HLA-B27 positivity. She had severe refractory, disabling, inflammatory low back pain, with an insufficient response to nonsteroidal anti-inflammatory drugs, and difficult management due to the coexistence of obesity, spondyloarthrosis, hypertension, chronic elevations of transaminase levels, diabetes mellitus, autoimmune hypothyroidism and diarrhea. The pain confined her to a life of crutches and wheelchairs, with dependence on others for her personal care. Ileocolonoscopy with biopsies of ileum and colon was normal. Magnetic resonance imaging of sacroiliac joints revealed marked bone marrow edema, demonstrating sacroiliitis. Serological screening for CD was negative; HLA typing revealed homozygosity and absence of DQ2 and DQ8. Duodenal biopsy was proposed to her, but she opted for trying the GFD without duodenal biopsy. Her response to the GFD and vitamin D supplements was very good, with remission of the disabling low back pain and diarrhea after 7 months. Mechanical low back pain and asthenia persisted. Milk and dairy products were eliminated from her diet and the asthenia improved. Magnetic resonance imaging of the sacroiliac joints performed after 17 months of follow-up showed remission of bone marrow edema (Fig. 3). She had returned to a normal active life and to work. She did not ingest gluten again.
Case no. 4: a 39-year-old man, with a history of over 15 years of chronic low back pain. He had been diagnosed with psoriatic spondyloarthritis on the basis of sacroiliitis, with bone marrow edema on MRI of the sacroiliac joints, and psoriasis. His conditions were refractory and treatment with tumor necrosis factor inhibitors was proposed. He had a son with CD. He did not have associated gastrointestinal symptoms. Serology for CD was negative and HLA typing revealed the presence of DQ2.2 (DQA1*02 DQB1*02). The duodenal biopsy revealed intraepithelial lymphocytosis with 60 CD3+ lymphocytes per 100 enterocytes, without villous atrophy. The low back improved notably after around 1 month on a GFD. After 1 year of follow-up, his low back pain was in remission, but recurred if he ingested gluten. His psoriasis did not improve.
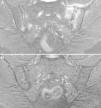
These 4 patients have axial spondyloarthritis, 2 of them with ankylosing spondylitis and 1 with psoriatic spondyloarthritis. They are sensitive to gluten, with a clear clinical response to a GFD, despite the fact that CD had been ruled out. In 2 cases, the patient has a first-degree relative with CD. The improvement in chronic low back pain has been highly relevant, to the extent that the clinical symptoms resolved. In 1 case, after a long follow-up period, MRI of the sacroiliac joints demonstrated remission of the edema. Moreover, in the 3 patients who had been exposed to gluten, exposure was followed by clinical recurrence. It is improbable that a clinical improvement like that described here be incidental or due to a placebo effect. The observation of intraepithelial lymphocytosis together with the clinical course supports the concept of enteropathic spondyloarthritis secondary to NCGS. Fig. 4 shows the duodenal biopsies from cases 1, 2 and 4, demonstrating the increased lymphocytes in the intestinal epithelium.
Duodenal biopsies after immunohistochemical staining for CD3+ lymphocytes showing an increase in the number of intraepithelial lymphocytes, without villous shortening or crypt hyperplasia. (A) Increase in the number of intraepithelial lymphocytes all along the intestinal villus (20×), case no. 1. (B) A close view of the increase in intraepithelial lymphocytes (40×), case no. 2. (C) A close view of the cluster of intraepithelial lymphocytes at the tip of the intestinal villus (40×), case no. 4, courtesy of Dr. Fernando Casco, HistoCitoMed.
The third clinical situation in which we investigated the possible presence of NCGS is in autoimmune diseases. The persistence of severe asthenia and arthralgia without arthritis is common in patients with autoimmune diseases, despite the achievement of a good control of the inflammatory markers with corticosteroid or immunosuppressive therapy. It seems reasonable to think that, like CD, NCGS is also associated with autoimmunity.
We reported a case of FM in which a relative with CD was discovered in connection with the patient, who had NCGS,26 and we have observed the same situation in a patient with NCGS and polyarthritis with diagnostic features of psoriatic arthritis, who tested positive for anti-cyclic citrullinated peptide (CCP) and anti-Ro antibodies.27 Here we present 5 case reports that support the existence of an association between NCGS and autoimmune diseases.
Case no. 5: a 51-year-old woman, who was being followed at another center for anti-CCP-positive erosive rheumatoid arthritis. Her clinical situation was one of poor control, despite treatment with chemical and biological agents. Her disease was resistant to treatment with anti-TNF and she had been included in clinical trials involving new biological agents, like ocrelizumab. She was being treated with methotrexate, rituximab, corticosteroids, indomethacin, proton pump inhibitors and sertraline. Although joint inflammation was reasonably controlled with immunosuppressive therapy, she was experiencing pain, severe asthenia, diarrhea, bloating and oral aphthae. The pain and severe asthenia confined her to a life of dependence on others. Serology for CD was negative. It was decided that she try a GFD, without undergoing either HLA typing or duodenal biopsy. The GFD was followed by a clear improvement in all her clinical symptoms in 6 months. After 4 years of adherence to the GFD, she remained in excellent clinical condition, was able to lead an independent life, with excursions to the countryside, and she could dance the zumba. She continued treatment with oral methotrexate (15mg a week) and prednisone (2.5mg daily). If she ingested gluten, she would experience recurrence of diarrhea, asthenia and arthritis, thus corroborating her gluten sensitivity.
Case no. 6: a 39-year-old woman, referred for symmetric polyarthritis, including a 6-year history of metacarpophalangeal joint and proximal interphalangeal joint involvement. She tested negative for rheumatoid factor (RF), anti-CCP antibodies, ANA and HLA-B27. She had been treated with salazopyrin and corticosteroids, with partial improvement. Marked asthenia, oral aphthae and a history of iron-deficiency anemia were associated. She had had a previous diagnosis of irritable bowel syndrome. Serological tests for CD were negative. HLA typing revealed the presence of HLA-DQ8 and the duodenal biopsy revealed 34 CD3+ lymphocytes per 100 enterocytes. Nine months after initiating a GFD, all of her symptoms had resolved. As she was asymptomatic, she continued to take only 1.5g of salazopyrin daily, and her previously diagnosed anemia had resolved. Two years after initiating the GFD, she remained asymptomatic without medication. The decision was made not to perform a gluten challenge test.
Case no. 7: a 49-year-old woman, diagnosed with limited systemic sclerosis. She had severe Raynaud's phenomenon, with anti-centromere antibodies at a titer of 1/2560. The remaining analyses were normal, with the exception of the hemoglobin level (11.5g/dl). Gastroscopy revealed hypertensive gastropathy with gastric angiodysplasia (watermelon stomach). The study of cardiopulmonary involvement was negative. The clinical picture had a history of 4 years. She had severe asthenia, poor memory and concentration, bloating, intermittent diarrhea, gastroesophageal reflux and oral aphthae. She had been unable to tolerate corticosteroid therapy. Immunohistochemical staining of the duodenal biopsy specimen that had been considered normal in a previous examination showed 25 CD3+ lymphocytes per 100 enterocytes. She carried the HLA-DQ2.5 haplotype. Six months after initiating a GFD, her gastrointestinal symptoms, tiredness and Raynaud's phenomenon had improved. She had discontinued nifedipine, which she had been taking previously. She began to take multivitamin supplements with minerals, coenzyme Q10 and omega 3. After 18 months on the GFD, she was asymptomatic and the gastrointestinal symptoms, asthenia and mental fatigue had resolved. Raynaud's phenomenon reappeared occasionally. She tested positive for anti-centromere antibodies, with a titer of 1/160. Occasional gluten ingestion had been followed immediately by asthenia, oral aphthae and recurrence of the gastrointestinal symptoms.
Case no 8: a 46-year-old woman with a 10-year history of polyarthritis and sicca syndrome, with later development of Raynaud's phenomenon, telangiectasia and digital ulcers. Her analyses showed RF 556IU, antibodies to extractable nuclear antigens (ENA) SSA/Ro>600, (U)1 ribonucleoprotein (RNP) 140 and RNP-70 114. Capillaroscopy revealed changes compatible with systemic sclerosis. Salivary gland biopsy was compatible with Sjögren's syndrome. Treatment with corticosteroids, methotrexate, antimalarial drugs, azathioprine, leflunomide, statins, antiplatelet therapy, nonsteroidal anti-inflammatory drugs, proton pump inhibitors and antidepressants had been prescribed. She had experienced secondary effects of the medication and poor adherence to treatment. Severe asthenia, diarrhea and oral aphthae were associated. Serological test for CD was negative and the duodenal biopsy was normal. HLA typing revealed DQ2.2 (DQA1*02 DQB1*02) and DQ8. Four months after initiating a GFD, she had a clear improvement in the digestive symptoms, asthenia and oral aphthae, as well as improvement in the hand swelling and digital ulcers. If she ingested gluten, diarrhea recurred. Two years later, the patient feels well and continues the GFD, and in addition, is being treated with 10mg a day of leflunomide, 2.5mg a day of prednisone and vitamin and mineral supplements. She continues to test positive for autoimmune markers at similar titers.
Case no. 9: a 44-year-old woman, referred from another center for recurrent arthritis. She had shown variable positivity for ANA and had tested positive for IgG and IgM anticardiolipin antibodies at low titers. She also had diarrhea and marked asthenia. She had been diagnosed with irritable bowel syndrome, and had a daughter with CD. Celiac disease had been ruled out by a negative serological test and duodenal biopsy, despite which, she had been following a GFD diet for 3 years, although not strictly, and her symptoms had clearly improved, and worsened following exposure to gluten. She did not want to undergo gluten challenge. She tested positive for ANA (1/160) and negative for anticardiolipin antibodies. She carried the HLA-DQ2 haplotype, and a review of the previous duodenal biopsy revealed Marsh type 1 lesions, with 33 CD3+ lymphocytes per 100 enterocytes. It was recommended that she follow a strict GFD diet and eliminate milk and dairy products. Vitamin and mineral supplements and omega 3 were added. After 2 years of follow-up, her gastrointestinal symptoms, asthenia and joint symptoms had remitted. She tested positive for ANA at a titer of 1/80 and continued to test negative for anticardiolipin antibodies.
It has recently been reported that 14% of the patients with NCGS have an associated autoimmune disease, mainly autoimmune thyroiditis and psoriasis.14 These case reports include polyarthritis of unknown cause, polyarthritis associated with ANA and with anticardiolipin antibodies, refractory rheumatoid arthritis, systemic sclerosis and mixed connective tissue disease. They show that NCGS can be associated with a variety of autoimmune diseases. These patients, whose clinical condition was very poor, despite the intensification of immunosuppressive therapy, showed a striking improvement after following a GFD. As in FM and spondyloarthritis, it is improbable that this be due to a coincidence or a placebo effect and, thus, the question is to what extent can the GFD play an important role in the treatment of these patients. It is important to point out that the clinical improvement permitted the reduction or discontinuation of immunosuppressive therapy. The favorable clinical course was produced despite the persistence of positivity for markers of autoimmune disease, although there were cases in which they also improved. As in FM and spondyloarthritis, the findings that point to the presence of NCGS are severe asthenia, oral aphthae, associated gastrointestinal symptoms and having a relative with CD. These clinical observations contradict the study of Vives et al., who found very few cases of gluten-sensitive enteropathy among their patients with systemic diseases.28 This may be due to the fact that we identify NCGS on the basis of the clinical response, including both the forms nearest to CD, with disease-susceptible HLA and Marsh type 1 enteropathy, and the forms farthest from CD, even with no need to demonstrate the presence of enteropathy. In their study, Vives et al. attempt to detect gluten-sensitive enteropathy by performing duodenal biopsy in patients with positive CD serology or disease-susceptible HLA, or with symptoms indicative of CD, and the diagnosis of gluten-sensitive enteropathy requires the demonstration of enteropathy and, aside from the clinical response, an analytical or histological response.
These clinical observations of NCGS associated with systemic diseases, in which it may take months for the benefits of the diet to be noted, differ from the accepted ideas about NCGS, in which it is considered to be a nonautoimmune condition, in which the clinical response to the GFD can easily be observed in a matter of a few days or weeks.
ConclusionsCeliac disease is an autoimmune disease with a wide spectrum of rheumatic manifestations, which should be taken into account by rheumatologists. The incidence of NCGS is probably higher than that of CD, with similar symptoms, but it is difficult to diagnose due to the lack of specific diagnostic tests. In this article, we express the hypothesis, based on reasonable arguments and clinical observations, that NCGS is associated with a wide range of rheumatic manifestations that include FM, the spondyloarthritides and systemic autoimmune diseases. In our experience, the most important clinical data that indicate the presence of NCGS are severe asthenia of unknown etiology, oral aphthae, the associated gastrointestinal symptoms, iron-deficiency anemia and having a relative with CD. It seems that these clinical data should particularly be taken into account when a patient with a systemic disease has associated symptoms of FM. The favorable course following the GFD, observed in both FM-like manifestations and in arthritis and sacroiliitis, leads us to think that gluten sensitivity may play an etiological and pathogenic role that acts as a trigger in some patients with systemic autoimmune diseases. Of course, prospective studies and clinical trials with double-blind challenge will be necessary to establish the extent to which the occurrence of NCGS can be considered frequent, and its treatment relevant, in rheumatic diseases.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThe contribution of MSD Inmunología was decisive during the initial phases of this study, providing financial resources for the performance of immunohistochemistry in the duodenal biopsies. Genyca Innova contributed to the performance of HLA typing of the patients.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We acknowledge the authors of this report who are not among the signatories due to an editorial norm that limits the number of authors: Natalia Fernández Puga, digestive system specialist at Hospital Puerta de Hierro, Madrid; Isabel Colmenero and Fernando Casco, pathologists at Birmingham Children's Hospital, Birmingham, U.K. and at Labco, Madrid, respectively; María José Castro Panete, immunologist at Hospital Doce de Octubre, Madrid, and the Association of Celiac and Gluten Sensitive Patients of Madrid (la Asociación de Celíacos y Sensibles al Gluten de Madrid).
Please cite this article as: Isasi C, Tejerina E, Morán LM. Sensibilidad al gluten no celíaca y enfermedades reumatológicas. Reumatol Clin. 2016;12:4–10.