To review the clinical evidence on subcutaneous (SC) abatacept and to formulate recommendations in order to clear up points related to its use in rheumatology.
MethodAn expert panel of rheumatologists objectively summarized the evidence on the mechanism of action, practicality, effectiveness, and safety of abatacept sc and formulated recommendations after a literature review.
ResultsThe efficacy and safety of abatacept sc were studied in 7 clinical trials, 3 double-blind, 3 open, and one mixed, with the following endpoints: comparison against abatacept iv, impact on immunogenicity, effect of replacing iv by sc, abatacept sc in monotherapy, and non-inferiority to adalimumab. No significant differences were found between sc and iv abatacept on efficacy or safety. The development of sc abatacept has allowed a complementary study to the iv, formulation, thus making the abatacept profile better defined.
ConclusionsThis is a practical document to supplement the summary of product characteristics. In summary, abatacept sc is presented as an effective and safe drug and, therefore, as an alternative to use within the broad armamentarium the rheumatologist has to treat RA. It also has the advantage of being the only biological agent that can be administered iv and sc which can facilitate its use in certain patients.
Revisar la evidencia clínica sobre abatacept subcutáneo (sc) y emitir recomendaciones con objeto de aclarar su uso en reumatología.
MétodoUn panel de expertos reumatólogos resumió de forma objetiva las pruebas existentes sobre el mecanismo de acción, el modo de uso, la eficacia y la seguridad de abatacept sc y desarrolló un documento sobre el uso de este fármaco en situaciones concretas, previa revisión de la bibliografía.
ResultadosEl abatacept sc sustenta su eficacia y seguridad en 7 ensayos clínicos, 3 doble ciego, 3 abiertos y uno mixto, en los que se compara la administración sc frente a la iv de abatacept, se estudia el posible impacto sobre la inmunogenicidad, el efecto de sustituir la vía iv por la sc en pacientes que previamente venían recibiendo abatacept iv, la monoterapia y la no inferioridad frente a adalimumab. No se han encontrado diferencias significativas frente a abatacept iv ni en cuanto a la eficacia ni en cuanto a la seguridad. El desarrollo de abatacept sc ha permitido un estudio complementario al del iv, con lo que el perfil del mismo queda más definido.
ConclusionesSe trata de un documento práctico como complemento a la información en ficha técnica. En resumen, el abatacept sc se presenta como un fármaco eficaz y seguro y, por lo tanto, como una alternativa más para utilizar entre los múltiples tratamientos con que cuenta hoy en día el reumatólogo. Además, cuenta con la ventaja de ser el único agente biológico que se puede administrar por vía iv y sc, lo cual puede facilitar su uso en determinados pacientes.
The efficacy and safety of a biological agent are key elements when it comes to their selection for the treatment of rheumatoid arthritis (RA), but other factors also play an important role, including the route of administration. Many patients prefer the autonomy the ability to inject the drug subcutaneously (SC) provides versus having to go to a day hospital or intravenous infusion (IV) unit. A significant number of doctors prefer the SC route, considering its efficacy and safety, due to, among other factors, the fact is that it has less organizational complexities. Hence the interest in developing SC administrable formulations for drugs available for IV use still persists.
The change of the IV administration route for the SC one in the case of a protein derived drug is not a matter of simple substitution at all. SC administration poses significant differences compared to IV, both from the point of view of efficacy and safety, requiring studies and independent development. Some aspects are particularly relevant. The first is the dosage, as the SC route carries pharmacokinetic differences that result in different patterns of administration, dosages and different intervals than IV. Another key aspect is immunogenicity. Parenteral administration of proteinaceous drugs is associated, at least theoretically, with the possibility of developing antibodies against the drug (ADA). The route of administration and the dosage are factors that may influence this phenomenon, because, among other things, differences in antigen presentation1; in addition, the different composition of the excipients for both formulations may also contribute to differences in immunogenicity and hence the enormous importance of analyzing the immunogenicity in the process of developing an IV biologic drug for SC administration. In addition, factors such as drug temporary interruption and subsequent reintroduction, the change in the same patient from IV to SC of the use of the drug alone or association with disease modifying drugs (DMARDs), may modify the immunogenic properties of a protein product.2 Another safety aspect that deserves special analysis is the possible occurrence of reactions at the site of SC injection.
Abatacept is a selective proteinaceous biological modulator of T cell costimulation, approved for treatment of RA. IV use has demonstrated efficacy with an adequate safety profile in different populations of patients with this disease, including patients who had never previously received methotrexate (MTX), patients with inadequate response to synthetic disease-modifying drugs (DMARDs) and anti-TNF biological.3–5
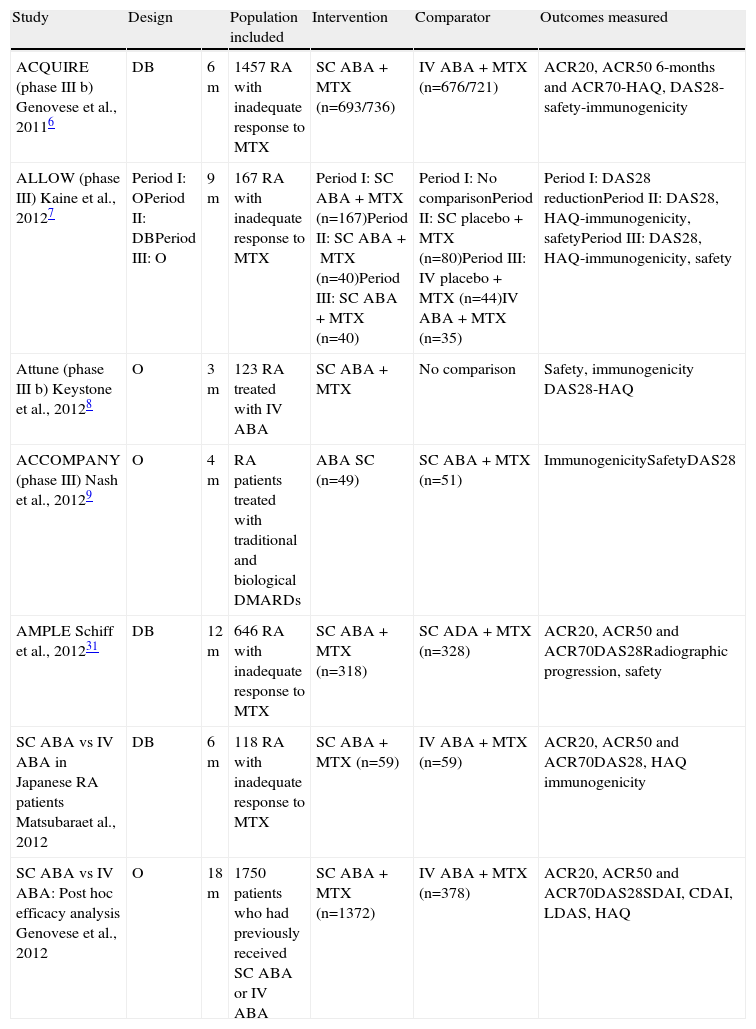
In addition to the IV formulation, in recent years a new way to use abatacept has been developed subcutaneously. Table 1 shows the summary of the major clinical trials. The ACQUIRE study is the main trial, with a larger number of patients, which compared, from the point of view of efficacy and safety, compared SC to IV administration of abatacept.6 The ALLOW study specifically analyzes the possible impact on immunogenicity of the suspension and subsequent drug reintroduction.7 The ATTUNE trial studied the effect of replacing IV abatacept administration with SC in patients who previously had been receiving IV.8 In the ACCOMPANY trial, the effect of SC administration of abatacept in monotherapy versus combination with MTX is investigated mainly from the standpoint of immunogenicity.9 In the AMPLE study, the efficacy and safety of two biologic drugs, abatacept and adalimumab are compared in combination with MTX.10
Description of Abatacept Clinical Trials.
| Study | Design | Population included | Intervention | Comparator | Outcomes measured | |
| ACQUIRE (phase III b) Genovese et al., 20116 | DB | 6m | 1457 RA with inadequate response to MTX | SC ABA+MTX (n=693/736) | IV ABA+MTX (n=676/721) | ACR20, ACR50 6-months and ACR70-HAQ, DAS28-safety-immunogenicity |
| ALLOW (phase III) Kaine et al., 20127 | Period I: OPeriod II: DBPeriod III: O | 9m | 167 RA with inadequate response to MTX | Period I:SC ABA+MTX (n=167)Period II:SC ABA+MTX (n=40)Period III:SC ABA+MTX (n=40) | Period I:No comparisonPeriod II:SC placebo+MTX (n=80)Period III:IV placebo+MTX (n=44)IV ABA+MTX (n=35) | Period I:DAS28 reductionPeriod II:DAS28, HAQ-immunogenicity, safetyPeriod III:DAS28, HAQ-immunogenicity, safety |
| Attune (phase III b) Keystone et al., 20128 | O | 3m | 123 RA treated with IV ABA | SC ABA+MTX | No comparison | Safety, immunogenicity DAS28-HAQ |
| ACCOMPANY (phase III) Nash et al., 20129 | O | 4m | RA patients treated with traditional and biological DMARDs | ABA SC (n=49) | SC ABA+MTX (n=51) | ImmunogenicitySafetyDAS28 |
| AMPLE Schiff et al., 201231 | DB | 12m | 646 RA with inadequate response to MTX | SC ABA+MTX (n=318) | SC ADA+MTX (n=328) | ACR20, ACR50 and ACR70DAS28Radiographic progression, safety |
| SC ABA vs IV ABA in Japanese RA patients Matsubaraet al., 2012 | DB | 6m | 118 RA with inadequate response to MTX | SC ABA+MTX (n=59) | IV ABA+MTX (n=59) | ACR20, ACR50 and ACR70DAS28, HAQ immunogenicity |
| SC ABA vs IV ABA: Post hoc efficacy analysis Genovese et al., 2012 | O | 18m | 1750 patients who had previously received SC ABA or IV ABA | SC ABA+MTX (n=1372) | IV ABA+MTX (n=378) | ACR20, ACR50 and ACR70DAS28SDAI, CDAI, LDAS, HAQ |
O: open, RA: rheumatoid arthritis; ABA: abatacept, ADA: adalimumab, DB: double-blind, MTX: methotrexate; PBO: placebo, m: months.
Abatacept for SC use is presented in prefilled glass syringes with 125mg of active ingredient in 1ml volume. The SC formula contains no maltose, unlike the IV form 11. Phase I and phase II studies have concluded that a weekly dose of 125mg SC provides therapeutic levels of abatacept.12
The availability of a new formulation of abatacept for SC use expands options for the treatment of RA. The objective of this paper is to review the clinical evidence on SC abatacept and discuss potential benefits that its use may incur.
MethodsThe document was based on a meeting in which the available evidence on SC abatacept was discussed and decisions about the issues that were most important for clinical practice were taken. Each panelist carried out a review of the relevant item that was assigned based on searches in PubMed, ACR and EULAR meetings and drug inserts. The final document was agreed upon by all the panelists. The level of evidence was graduated with the Oxford scale.13
ResultsPharmacokineticsSeveral studies have shown that the trough concentration at steady state of abatacept provides optimal inhibition of T cells and thus leads to an adequate clinical response is ≥10μg/ml.14 These concentrations are achieved with the approved IV abatacept dose and in 90% of patients treated with SC abatacept.11 To demonstrate the efficacy and safety of SC abatacept compared to the classical IV form used so far, studies in animals15,16 and humans have been performed.6,11,17,18 To this end, clinical trials were designed with and without an IV loading dose where the impact of the dose on clinical efficacy, pharmacokinetics and immunogenicity of abatacept SC was evaluated. In clinical trials of SC abatacept (including the essential ACQUIRE) an IV loading dose of abatacept is included on day one to rapidly achieve therapeutic concentrations and then compare whether the efficiency of the SC administration is similar to the IV administration.6,11,17,18 In these studies, a similar profile regarding efficacy and safety for the 2 routes of administration is demonstrated, and the vast majority of patients receiving SC abatacept reach a stable concentration of abatacept in the valley μ≥10g/mL, with less variation between peak and trough concentration than with IV administration.18
Although with some reservations, due to the different study designs (ALLOW and ACCOMPANY), we can deduce that the clinical efficacy of SC abatacept, followed or not by an IV loading dose, is similar at 3 months of starting treatment, with abatacept attaining therapeutic levels at 2 weeks in the majority of patients (88%) in which no loading dose was used.17,19 Thus, in those patients who are scheduled to start SC abatacept, it does not seem necessary to administer a loading dose (LE 2b).
Several studies have determined the level of abatacept in serum, and the presence of antiabatacept antibodies by ELISA, showing that SC abatacept is well tolerated and has a safety profile similar to IV abatacept regarding immunogenicity.6,9,11,20 It has been similarly shown, in healthy volunteers, that both IV administration and SC abatacept produce a similar receptor occupancy (75%) (information provided by Bristol–Myers Squibb).
SC abatacept exhibits linear pharmacokinetics and has fewer variations in the valley than IV administration.12 The bioavailability of SC administration compared to IV administration is 78.6%.18 The estimated systemic clearance (0.28mL/(hkg)), volume of distribution (0.11L/kg) and elimination half-life (14.3 days) media are comparable between SC and the IV administration. As occurs intravenously, the pharmacokinetics analysis showed that with the SC form, drug clearance increases with increasing weight. Age-and sex–adjusted for body weight–did not affect drug clearance. Concomitant medications such as MTX, corticosteroids and NSAIDs, apparently do not influence abatacept clearance12,21 (LE 2b).
EfficacyEfficacy in the Short and Long Term (Level of Evidence 1b)The clinical development of SC abatacept is based on a phase II study of safety and 4 dose-finding phase III studies (Table 1).
The ACQUIRE study6 compared the efficacy of 24 weeks of abatacept 150mg SC weekly versus traditional IV administration. The study included 1457 patients with active RA despite treatment with MTX. The SC group received one IV loading dose on day one. Some patients with a biological DMARD failure (3.3% in the SC and 4.5% in the IV group) were allowed for inclusion. The primary objective was to demonstrate non-inferiority of the SC formulation against IV by ACR20 at 24 weeks. The characteristics of the patients were similar in both groups and showed significant clinical activity, since patients would be required to have a number of painful joints and swollen over 10 and a DAS28 exceeding 6 in both groups. The primary endpoint showed no significant differences, since the SC abatacept group had an ACR20 response of 76% and IV treated patients, 75.8%. Other secondary objectives, such as ACR50 and 70, were similar. There were no differences when stratifying patients by weight. Disability measured using the Health Assessment Questionnaire (HAQ) improved in both groups. HAQ response was seen in 68.2% versus 63.8% in SC and IV patients, respectively. The reduction in DAS28 was similar in both groups: 2.57 (SC) and 2.55 (IV). In both groups, similar percentages of patients with low activity were seen at 6 months (DAS28<3.2: 39.5 SC and 41.3 IV) and remission: 24.2 and 24.8 for SC and IV, respectively. There were also no differences in patient assessment of pain and disease activity.
Comparison of ACQUIRE With AIMData from the open-label extension of the AIM and ACQUIRE4 trials6 serve as comparison of IV and SC abatacept administration.22 The analysis included 1372 patients from ACQUIRE, 378 from AIM in its open-label extension phase. The patients in both studies had high rates of activity, with swollen joint count at baseline 31 for AIM and 29.6 in ACQUIRE and DAS28 of 6.4 and 6.2, respectively. For comparison of both treatment group, the beginning of the days until the last visit was considered as 253, on day 897. The values of ACR20, 50, and 70 were similar in both studies, both at baseline and at the end of follow-up. An ACR20 efficacy of 81.8% and 80.1% at the start of the IV and SC groups, which remained to the end of the period of observation period (83.6% and 83.4%) was observed. The behavior was similar in the case of ACR50, 50.8% (IV) and 54.9% (SC) at the start of the comparison and 58.6% (IV) and 59.9% (SC) at the end. The ACR70 was 27.1% compared with 32% and 34.7% and 38.1% for IV and SC treatment at the beginning and end of treatment, respectively. The disease activity dropped to DAS 2.72 (IV) and 3.01 (SC) from baseline at the start of the comparison in ACQUIRE, with virtually identical values in AIM. The percentage of patients with low activity measured by DAS28 <3.2 and remission <2.6 was similar in both treatment groups. The low activity in AIM went from 40% at baseline to 50.2% at the end and a remission rate of 20.5 and 29.7. In ACQUIRE, there was low activity in 44% and 53.8%, remission in 27.3% at baseline and 35% at the end. In short, the results of SC abatacept are similar to those obtained with IV after comparing patients included in the AIM study, with similar populations, patients with very active disease, refractory to MTX. The degree of improvement and the percentages of ACR improvement and patients in remission or low activity were maintained during the observation period for two and a half years.
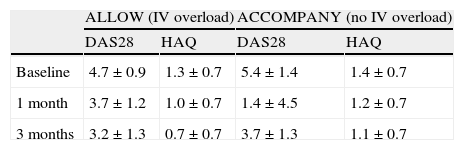
Loading Dose (Level of Evidence 1b)Some studies included an IV loading dose to rapidly achieve therapeutic concentrations of the drug. At the start of the ALLOW open trial, 167 patients received abatacept SC with an initial IV loading dose (about 10mg/kg body weight). In the long-term extension of ACCOMPANY, patients were stratified to SC abatacept, 125mg weekly without initial loading dose. In both studies, patients were refractory to DMARD or biologicals and similar in other demographics, although the severity of the disease was lower in patients who received an IV loading dose compared to than those who did not.
A total of 167 patients participated in ALLOW, receiving SC abatacept plus an IV initial loading dose and 100 patients entered the ACCOMPANY study and received SC abatacept with or without MTX, without a loading dose. Improvements in DAS28 and HAQ were similar in both groups with or without the IV loading dose (Table 2). Although the data should be used with caution by people with different baseline severity, the magnitude of improvement was similar in both studies, regardless of whether an initial IV loading dose was administered.23
Comparison of the Efficacy Results of the ACCOMPANY and ALLOW Studies.
| ALLOW (IV overload) | ACCOMPANY (no IV overload) | |||
| DAS28 | HAQ | DAS28 | HAQ | |
| Baseline | 4.7±0.9 | 1.3±0.7 | 5.4±1.4 | 1.4±0.7 |
| 1 month | 3.7±1.2 | 1.0±0.7 | 1.4±4.5 | 1.2±0.7 |
| 3 months | 3.2±1.3 | 0.7±0.7 | 3.7±1.3 | 1.1±0.7 |
Results are expressed as mean ± standard deviation.
The pharmacokinetic data obtained in these studies (discussed at length in this paper) also indicate that the IV loading dose is not necessary, since SC abatacept reaches therapeutic concentrations by the time of the second weekly dose.17
Fixed Subcutaneous Dose of Abatacept Regardless of Weight (Level of Evidence 1b)Both the study of multiple dose phase II11 trial of weight-dependent compared with fixed doses of 125mg weekly of abatacept, as in ACQUIRE,6 pharmacokinetic results showed that a weekly dose of 125mg of SC abatacept provided serum concentrations within the therapeutic range, regardless of patient weight.
In the ACQUIRE trial, patients with active RA, stratified by body weight (<60kg, 60–100kg, >100kg) were randomized to receive abatacept IV 10mg/kg body weight every 4 weeks or SC abatacept 125mg. The SC abatacept group received, on day 1, an IV infusion (10mg/kg) 1h before the first SC dose. Pharmacokinetic and immunogenic studies showed that a fixed dose of 125mg/week were well tolerated, did not induce immunogenicity against CTLA-4 and produced concentrations within the therapeutic range. The effectiveness, measured as ACR20/50/70 response, DAS28, low disease activity and remission was similar in the IV and SC abatacept groups. Subgroup analysis according to body weight did not show differences in the ACR20 response that depended on the route of administration.
Replacing IV Abatacept With SC Abatacept (Level of Evidence 2b)In the ATTUNE study,8 an open single treatment arm trial, 71 patients from the AIM extension study (abatacept in patients with failure to MTX4) and 52 from ATTAIN (abatacept in patients with anti-TNF failure5) were included in a 12-week study extended to a year, to assess the safety of changing the formulation of abatacept from IV to SC (both in combination with MTX). The percentage of patients with baseline remission or low disease activity remained stable and physical function was assessed by HAQ during the study period and at one year follow up. This occurred in all patients and when both populations, AIMs and ATTAINs, were analyzed separately.
Efficacy After Stopping and Restarting Treatment (Level of Evidence 2b)In the ALLOW study,7 167 patients were treated with SC abatacept+MTX. Of these, 120 patients had reduction in DAS28 greater than 0.6 at 12 weeks and were randomized to continue SC abatacept+MTX or placebo+MTX until week 24. At the end of this period, all patients entered an extension study of treatment with SC abatacept along with 37 patients who had no response at week 12. The reintroduction of abatacept in the group of patients who had temporarily suspended recovered the efficacy rate and induced a clinical response of the same magnitude as the abatacept group that remained with uninterrupted treatment.
Monotherapy (Level of Evidence 2b)In a 4-month study, a group of 100 patients were stratified to receive SC abatacept+MTX (n=51) or SC abatacept monotherapy (n=49). Of these, 50 and 46, respectively, entered the extension study. Both treatment groups were similar, except that the monotherapy group had higher activity than the combination group (baseline DAS28 5.6 vs 5.0). After 4 months, 55.3% (SC abatacept+MTX) and 44.2% (abatacept monotherapy) patients had low disease activity. At the end of an observation period of 18 months, this situation was present in 57.5% of the combined group and 69.4% in the monotherapy arm. After 4 months, the DAS28 remission occurred in 29.8% of the combined-therapy group and 32.6% of those who received monotherapy. In the extension phase at 18 months, remission rates of 42.5% (combination therapy) and 58.3% (monotherapy) were attained. Dropout rates were also equivalents.
Functional Capacity (Level of Evidence 2b)SC abatacept improves the functional capacity of RA patients equally than IV Abatacept. Functional capacity improvement is also maintained when patients receive SC abatacept after a period of withdrawal, or they receive it as a continuation of IV abatacept IV. Variations in physical function were also measured by HAQ with SC abatacept evaluated against IV abatacept in patients with inadequate response to MTX (ACQUIRE study),6 in adults with active RA (ALLOW study)7 and in patients switching treatment from IV to SC (ATTUNE study).8
The percentage of patients achieving an improvement in the HAQ in the ACQUIRE trial was 68.2% (95% CI [95% CI], 64.8–71.6) and 63.8% (95% CI, 60.3–67.3) for SC and IV abatacept groups, respectively (estimate of difference [4.5%, 95% CI, −0.4 to 9.4]). The change in adjusted mean±standard deviation from baseline to month 6 for SC abatacept was 0.69±0.02 and 0.70±0.02 for IV abatacept. The primary endpoint, non-inferiority of SC abatacept versus IV abatacept, based on ACR20 responses at 6 months was fulfilled.
In the ALLOW trial, the primary endpoint (immunogenicity) was fulfilled. Efficacy assessments were secondary objectives. At the end of the period I, all patients had similar reductions in the DAS28 score. The mean reductions in HAQ for patients who remained in the SC abatacept group were: in period I −0.74 (95% CI, −0.91 to −0.57) in period II −0.72 (95% CI, −0.95 to −0.50) and −0.86 in period III (95% CI, −1.04 to −0.67). For patients who suspended SC abatacept during period II, the mean reductions in HAQ were, in period I −0.63 (95% CI, −0.76 to −0.49), in period II of −0.50 (95% CI, −0.63 to −0.37) and −0.72 in period III (95% CI,−0.85 to 0.60).
The main objective of the ATTUNE study was to evaluate the safety of SC abatacept during the first 3 months after switching from IV abatacept. Changing from IV to SC abatacept was well tolerated, with a safety profile consistent with that observed during treatment with long-term IV abatacept in ATTAIN and AIM.4,5 At baseline, the mean±SD HAQ for patients with an inadequate response to MTX (n=71) was 0.91 (0.68) and for patients with inadequate response to anti-TNF (n=52) was 0.98 (0.71). The mean HAQ, based on an analysis as observed for patients with inadequate response to MTX at month 3 (n=71) was 0.82±0.69, and at month 12 (n=69) was 0.86±0.70. For patients with an inadequate response to anti-TNF, HAQ at 3 months (n=49) was 0.92±0.67, and at month 12 (n=45) was 0.97±0.66. It can be concluded that abatacept SC maintains the improvement in the HAQ previously obtained with IV abatacept.
Comparison With Adalimumab and Radiological Progression (Level of Evidence 1b)Inhibition of radiographic progression with SC abatacept was evaluated in the AMPLE trial.10 This study conducted a comparison of SC abatacept versus adalimumab in patients, both with standard doses, with inadequate response to MTX. The primary endpoint was non-inferiority in the ACR20 at 12 months of treatment, resulting in a clinical response and similar kinetics with both drugs. An abstract presented at the last ACR congress (Washington, 2012) also showed that other secondary variables, such as pain, HAQ and the various components of the SF36,24 reached similar values. In addition, an important secondary outcome measure was the absence of radiographic progression assessed using the van der Heijde modified total Sharp Score (MTSS). Matched radiographic images were available at one year for 1290 and 289 patients in the abatacept and adalimumab groups, respectively. In both treatment groups, a similar inhibition of radiographic damage is observed. After one year, the mean changes from baseline in the abatacept+MTX and adalimumab+MTX groups were: total score, 0.58 and 0.38, erosion score, 0.29 and −0.01, and narrowing of the joint space, 0.28 and 0.39, respectively. The rate of absence of radiographic progression (defined as the smallest detectable change ≤2.8) was observed in 84.8% and 88.6% of patients in the abatacept+MTX and the adalimumab+MTX groups, respectively. In conclusion, it can be said that SC abatacept is similar in efficacy to adalimumab for inhibition of radiographic progression in RA.
Immunogenicity (Level of Evidence 2a)All processed proteins, as biological agents, have the potential to develop an immune response, but their structure is one of the most important factors that determine the appearance of immunogenicity.25 Chimeric antibodies, such as infliximab, due to its murine part, are the most frequently associated to anti-drug antibodies, while protein compounds formed from the extracellular portion of a receptor coupled to an immunoglobulin are the least immunogenic, as is the case of etanercept and abatacept.
Abatacept is a fully human recombinant protein, comprising the extracellular domain of the CTLA4 molecule and a fragment of the Fc portion of human IgG1, with just a difference of four amino acids in the connection or hinge region, genetically modified to reduce binding to Fc receptors and prevent complement activation; except the binding region or hinge protein, it is fully human and needs not be recognized as foreign by the immune system. Additionally, it has also been proposed that abatacept can reduce ADA formation due to its mechanism of action.
The clinical consequences of immunogenicity depends on the intensity of the immune reaction and the affinity for the ligand and may range from no clinical consequence, if the amount of antibody is small and of low affinity, to a significant alteration in the drugs pharmacokinetics, efficacy or safety. Non-neutralizing anti-drug antibody formation leads to small immune complexes, which are slowly removed by the liver and spleen, but, theoretically, the drug still retains the ability to bind to the ligand and therefore does not completely lose its effectiveness. The formation of neutralizing antibodies will prevent the drug from binding to its therapeutic target, so its clinical efficacy will be directly affected. In the case of abatacept, for example, there is a theoretical risk for the development of neutralizing antibodies that can react with the endogenous CTLA4 molecule expressed on the T lymphocytes, neutralizing the activity of the endogenous protein and lead to an immunostimulatory state, which potentially may worsen the underlying disease or help develop new autoimmune diseases.
One of the most important aspects of immunogenicity is the development of methodology to determine it, and because there is still no standardization of methods, the results can not be compared or contrasted.25 At present, there is only abatacept immunogenicity data reported by Bristol–Myers Squibb and no independent groups has studied it. It is important to note that, often, the data provided by the companies in the registration studies and data arising in clinical practice are dissimilar, primarily due to patient selection, different methodologies and longer follow-up all patients in observational studies, whereas in the extension studies only those continuing earn the benefit of treatment, which are probably those who are not developing anti-drug antibodies.
Two methods were used to study the immunogenicity of SC abatacept, based on the first tests used in the studies of IV abatacept, with some improvements that increase sensitivity. Initially, we developed an ELISA directed against the entire abatacept molecule, but to avoid false positives and the signal produced by the presence of rheumatoid factor and nonspecific anti-Fc antibodies, a more specific assay was developed using only the portion of the CTLA4 molecule used as a positive control and a pool of polyclonal antiabatacept antibodies obtained by inoculating a monkey.26 All ELISA are exposed to results interfered with by the presence of free drug, which can not detect anti-drug antibodies.27 To minimize this, all samples were collected just before administration of the next dose and have also been studied in patients who had skipped some infusions or after the treatment period.
The second method is based on another ELISA, called electroluminiscence (ELS), and has proven to be more sensitive and therefore does not suffer interference from circulating drug levels. Samples are first treated with acid to dissociate immune complexes and to minimize the effects of other nonspecific antibodies. This assay can detect antibodies against all regions of abatacept.8 All positive sera with anti-CTLA4 antibody in ESL and ELISA were studied to identify the presence of neutralizing antibodies through a bioassay for determining whether the serum of patients with anti-drug antibodies is capable of inhibiting the immunosuppressive effect of abatacept in lymphocyte culture (LE 5).
The results of immunogenicity studies with IV abatacept, both during blinded treatment as long-term extension, overall show very low immunogenicity. From a total of 2237 patients studied, 62 (2.8%) demonstrated anti-abatacept or CTLA4 antibodies. Using ESL, the figure increased to 3% and no protective effect of MTX (2.3% with MTX vs 1.4% without MTX) was demonstrated. Patients who discontinued treatment had slightly higher frequencies of anti-drug antibodies than those who continued treatment (7.4% vs 2.6%) and only 8 of 13 patients studied showed neutralizing activity. No clinical relevance was demonstrated in terms of loss of efficacy, a need to increase the dose, side effects or increased development of autoimmune diseases.26
Development of SC abatacept should achieve efficacy and safety results similar to those of the IV route, with similar immunogenicity levels, taking into account that the SC route of administration is more immunogenic than IV when forming aggregates at the injection site, process antigens differently and more efficiently and transports most of the volume administered by the lymphatic route to the bloodstream.1 The study was based on four general principles, a study comparing the two routes of administration, another after the suspension and the reintroduction of the drug, one that investigates the relationship with concomitant medication with MTX and finally the development of immunogenicity in patients moving from IV to SC route.
In the ACQUIRE study, few patients who developed antiabatacept antibodies or tested positive for them in the anti-CTLA4 trial, which failed to demonstrate any effect on the efficacy or safety in these patients.6 These results support the fact that immunogenicity is not increased when the SC route is used compared to IV. In a similar study in Japan,28 after 6 months of treatment no anti-drug antibodies were observed in any of the 59 SC patients versus one of 59 with IV treatment.
I the ALLOW7 study, the effect of drug suspension and rechallenge on immunogenicity in patients receiving MTX was investigated. In the first 3 months, all patients were treated with SC abatacept openly and end of the period, 2 (1.7%) of 118 patients developed anti-drug antibodies. In the open period, none of the patients who continued treatment developed immunogenicity while the other developed seven (9.6%) among the 73 who had stopped the drug for 3 months. In phase III of rechallenge, anti-drug antibodies were demonstrated in 1 (2.6%) of the 38 patients who remained on treatment throughout the period and in 2 (2.7%) of 73 patients who reintroduced treatment. These results may be due to a direct effect of the drug inhibiting the development of anti-drug antibodies during the administration or loss of interference of ELISA in the presence of the drug. Whatever the reasons, it is confirmed, as in the IV development, that the frequency of immunogenicity after discontinuing the drug and reintroducing it is very low.
In ACCOMPANY, the effect of concomitant treatment with MTX on immunogenicity was investigated when not using an IV loading dose.29 At 4 months of treatment, 3 (6.2%) of 48 patients receiving combination therapy showed anti-drug antibodies versus 2 (4.2%) of 47 in monotherapy. In the extension period, which reached 16 months, ADA were demonstrated in one (2.1%) patient treated in combination versus 0 in monotherapy. No effects were demonstrated in efficacy or safety.
Finally, in the ATTUNE trial8 investigating the development of immunogenicity in patients switching from IV treatment from AIM and ATTAIN to SC treatment, at 3 months of SC treatment, anti-drug antibodies were demonstrated in 8 (6.6%) of 122 patients in the ELISA while only 1 (0.8%) of the 122 patients were detected using the ESL assay, confirming the very low rates of immunogenicity of SC abatacept (LE 2b).
SafetyThe administration of a SC or IV biologic drug may be accompanied by differences in immunogenicity and pharmacokinetics. These differences may reflect individual differences in safety. The “a priori” assumption that the two administration forms will have a similar safety profile may be wrong. Therefore, the comparison is essential (Table 3).
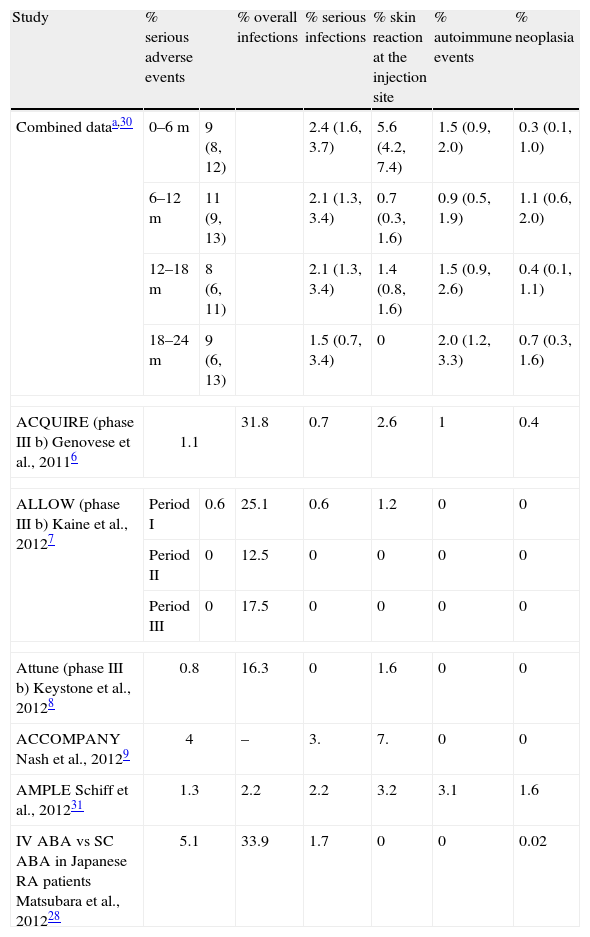
Safety of Subcutaneous Abatacept in Clinical Trials.
| Study | % serious adverse events | % overall infections | % serious infections | % skin reaction at the injection site | % autoimmune events | % neoplasia | |
| Combined dataa,30 | 0–6m | 9 (8, 12) | 2.4 (1.6, 3.7) | 5.6 (4.2, 7.4) | 1.5 (0.9, 2.0) | 0.3 (0.1, 1.0) | |
| 6–12m | 11 (9, 13) | 2.1 (1.3, 3.4) | 0.7 (0.3, 1.6) | 0.9 (0.5, 1.9) | 1.1 (0.6, 2.0) | ||
| 12–18m | 8 (6, 11) | 2.1 (1.3, 3.4) | 1.4 (0.8, 1.6) | 1.5 (0.9, 2.6) | 0.4 (0.1, 1.1) | ||
| 18–24m | 9 (6, 13) | 1.5 (0.7, 3.4) | 0 | 2.0 (1.2, 3.3) | 0.7 (0.3, 1.6) | ||
| ACQUIRE (phase III b) Genovese et al., 20116 | 1.1 | 31.8 | 0.7 | 2.6 | 1 | 0.4 | |
| ALLOW (phase III b) Kaine et al., 20127 | Period I | 0.6 | 25.1 | 0.6 | 1.2 | 0 | 0 |
| Period II | 0 | 12.5 | 0 | 0 | 0 | 0 | |
| Period III | 0 | 17.5 | 0 | 0 | 0 | 0 | |
| Attune (phase III b) Keystone et al., 20128 | 0.8 | 16.3 | 0 | 1.6 | 0 | 0 | |
| ACCOMPANY Nash et al., 20129 | 4 | – | 3. | 7. | 0 | 0 | |
| AMPLE Schiff et al., 201231 | 1.3 | 2.2 | 2.2 | 3.2 | 3.1 | 1.6 | |
| IV ABA vs SC ABA in Japanese RA patients Matsubara et al., 201228 | 5.1 | 33.9 | 1.7 | 0 | 0 | 0.02 | |
Serious adverse event: event that involves withdrawal.
In the pooled safety analysis of30 Phase II trials for dose-finding, ACCOMPANY9 ACQUIRE 6, ALLLOW7 and ATTUNE8 with an exhibition of 4214 patient-years for 1879 patients, the incidence rates of adverse events serious per 100 patient-years for SC abatacept were 9.25 (95% CI, 7.46–11.48), 10.75 (95% CI, 8.72–13.24), 8.21 (95% CI, 6.29–10.72) and 8.85 (95% CI, 6.26–12.51) in the 0–6, 6–12, 12–18, and 18–24 month periods. In the31 AMPLE trial at one year, 1.3% of patients treated with SC abatacept+MTX and 3.0% of patients treated with SC adalimumab+MTX discontinued medication for serious adverse events.
In the ACQUIRE trial,6 SC abatacept and IV abatacept percentages of suspension for serious adverse events was 1.1% and 1.9%, respectively. In the ACCOMPANY9 trial at 4 months, these rates were 5.9% for SC abatacept+MTX and 2.0% for SC abatacept monotherapy. In the ATTUNE8 trial, suspension rates for serious adverse events were 0.8% at 3 months and 3.3% in the accumulated period. These percentages were higher in patients who had previously failed TNF antagonists.
Serious InfectionsIn the pooled safety analysis,30 incidence rates per 100 patient-years of serious infections for SC abatacept in periods of 0–6, 6–12, 12–18, and 18–24 months were 2.42 (95% CI, 1.60–3.68), 2.12 (95% CI, 1.33–3.36), 1.28 (95% CI, 0.66–2.46) and 1.51 (95% CI, 0.68–3.36). Pneumonia and urinary tract infections were the most frequent serious infections. Perhaps these figures are somewhat lower than those reported in clinical trials with other biologics. These incidents show that the risk of serious infection does not increase over time. In the31 AMPLE trial at one year, 2.2% of patients treated with SC abatacept+MTX and 2.7% of patients treated with SC adalimumab+MTX had a serious infection.
Injection Site Skin ReactionsIn the pooled safety analysis,30 3% of patients treated with SC abatacept developed some skin reaction at the injection site. The rate of adverse events per 100 patient-years was 2.2. These percentages are somewhat lower than for etanercept (6.5% at 3 months and 10% per year) and adalimumab (19.5%–26.1% at 1 year).32–34 For the 0–6, 6–12, 12–18, and 18–24 months periods of exposure, the incidence rates per 100 patient-years for skin reaction at the injection site were 5.59 (95% CI, 4.24–7.38), 0.72 (95% CI, 0.32–1.59), 1.40 (95% CI, 0.77–1.59) and 0.30 In the AMPLE one year trial, 3.2% of patients treated with SC abatacept+MTX developed some kind of skin reaction at the injection site compared with 9.1% of patients treated with SC adalimumab+MTX.
In the ACQUIRE6 trial at 6 months, 2.6% of patients in the group of patients treated with SC abatacept had some kind of skin reaction at the injection site compared with 2.5% in the IV group treated with abatacept. The most common reactions in the SC abatacept group were pruritus and erythema. In the ACCOMPANY9 trial at 4 months, 5.9% of patients treated with SC abatacept+MTX developed cutaneous reactions at the site of injection compared to 8.2% of patients treated with SC abatacept. Systemic reactions 24h after the injection appeared in 7.8% of patients treated with combination therapy and 8.2% of patients treated with abatacept monotherapy.
Autoimmune EventsIn the pooled safety analysis,30 the rate of autoimmune events per 100 patient-years was 1.28 (95% CI, 0.9–1.75) in patients treated with SC abatacept and 1.99 (95% CI, 1.74–2.26) in patients treated with IV abatacept. Incidence rates for SC abatacept in the 0–6, 6–12, 12–18, and 18–24 month periods were 1.54 (95% CI, 0.91–2.60), 0.94 (95% CI, 0.47–1.88), 0 1.5 (95% CI, 0.85–2.63) and 2.00 (95% CI, 1.20–3.31). The AMPLE one year trial showed that 3.1% of patients treated SC abatacept+MTX developed some type of autoimmune event compared with 1.2% of patients treated with SC adalimumab+MTX.
In the ACQUIRE6 trial, 1% of patients treated with SC abatacept+MTX and 0.8% of patients treated with IV abatacept+MTX, respectively, developed autoimmune events. In ACCOMPANY9 trial at 4 months, none of the patients developed autoimmune events. In the ATTUNE8 trial, in the cumulative period, 1.6% of patients developed autoimmune events.
TumorsIn the pooled safety analysis,30 incidence rates per 100 patient-years for malignant tumors with SC abatacept in periods 0–6, 6–12, 12–18, and 18–24 months were 0.33 (95% CI, 0.11–1.02), 1.05 (95% CI, 0.55–2.01), 0.37 (95% CI, 0.12–1.14) and 0.65 (95% CI, 0.27–1.57). In the31 AMPLE trial at one year, 1.6% of patients treated with SC abatacept+MTX developed some kind of tumor compared with 1.2% of patients treated with SC adalimumab+MTX.
In the ACQUIRE6 trial at 6 months, 0.4% of patients in the group of patients treated with SC abatacept had a malignant or benign tumor compared with 0.7% in the IV abatacept group. The most common reactions in the SC abatacept group were pruritus and erythema. In the accumulated period of the ATTUNE8 trial, 1.6% of patients developed a tumor.
In conclusion, the safety profile in clinical trials with SC abatacept is similar to IV abatacept. Keep in mind that exposure in these trials is still short-term and it is necessary that this profile is complete with information that will come from observational studies generated by registries.
DiscussionTreatment with biologic therapies allow for 2 routes of administration, IV and SC. Each has its advantages and disadvantages, although there is no doubt that the SC route gives patients a much higher degree of autonomy compared to IV and exempts them from visiting the day hospital.
In this article, the efficacy and safety of SC abatacept, a biological agent that was approved for use in Spain IV 5 years ago, are reviewed. Abatacept is, to date, the only biological agent that allows both IV and SC administration, which is an added advantage for the patient, since at any given time one way may be more suitable for administration than the other.
However, the development of a drug by another route, SC in this case, than originally approved requires a virtually complete clinical development, as a different form of administration adopted initially means evaluating pharmacokinetics, determining the dose and scheduling for more appropriate management and finally demonstrating a degree of safety and efficacy that is at least similar to the administration route initially approved. Another added fact is immunogenicity. It has been shown that the development of anti-drug antibodies has a negative impact on drug efficacy.25,27 On the other hand, SC administration, by its nature, explained earlier in this article, is more likely to produce immunogenicity.
Apart from trials in phase II, in which the ideal dose and safety11 is determined, perhaps the most relevant study was ACQUIRE,6 which showed that 24 weeks of abatacept SC at the dose of 125mg weekly was not inferior than IV administration at the usual dose. All evaluated outcome measures were similar, with the main objective being ACR20, which obtained a response rate of 76% and 75.8% in the IV and SC groups, respectively. However, it should be noted that patients treated SC received an IV loading dose prior to the SC administration. Patients were openly followed receiving abatacept SC and compared with those of the AIM trial, IV22 abatacept study, which by design and features allowed comparing two populations with different routes of administration. While caution must be maintained when comparing populations of different trials, monitoring of both populations showed no differences between the main measures of rated activity.
Other topics discussed were whether or not to administer a loading IV dose prior to SC administration. In ACCOMPANY and ALLOW, the effectiveness of SC abatacept with and without a loading dose, respectively (Table 2), found no differences in the response. Again, and as discussed above, the results should be taken with caution, coming from different studies, however, no pharmacokinetic data supported using a loading dose.
Another fact that has attracted interest is whether the dose would depend on the patient's weight. The ACQUIRE study showed no difference in ACR20 response according to patient weight. Likewise SC use of abatacept in patients previously treated with IV formulations produced no loss of efficacy or increased adverse events.8 Another interesting development in the clinical aspect SC abatacept was to assess whether treatment discontinuation and subsequent restart would mean a loss of efficacy. The answer was that patients who had been treated for several weeks with placebo plus MTX, having previously received abatacept SC, lost efficiency, but recovered it with a response of equal intensity to that obtained when they were receiving the active drug.7 Another study indicated that SC administration of abatacept monotherapy may be a therapeutic option for patients who can not tolerate MTX.20 Finally, other studies8,23,28 have shown that abatacept SC has an identical capacity to its IV counterpart to produce an improvement in function as measured by HAQ.
One of the most interesting studies with abatacept SC has been conducted in a direct comparison with adalimumab.10 The study was designed to demonstrate non-inferiority in the ACR20 at one year of treatment with both drugs and also other, both clinical and radiological, secondary endpoints were studied. Abatacept was not shown to be inferior in efficacy or other secondary parameters measuring other activity or disability components. But, perhaps most importantly, was the fact that SC abatacept was found to be a drug with the same ability to prevent radiological progression than adalimumab. IV Abatacept has always been considered as a drug with less ability to slow structural damage compared with anti-TNF as a whole.35 If this new situation is due to the new formulation is or, conversely, the extrapolation of data done with IV abatacept led to conclusions which were not entirely accurate is a question that has no answer, but what is beyond doubt is that to speak emphatically of varying efficacy, or other outcome measures, the strongest proof is a direct comparison between drugs and not obtaining data through indirect means.
With regard to other aspects of interest, abatacept SC behaves like a scarcely immunogenic drug. Virtually in all studies of this new formulation (ACQUIRE, ALLOW, attune and ACCOMPANY), anti-drug antibody rates show no or merely symbolic rates and had no impact on the efficacy or safety of the patient.
Finally, in terms of safety concerns, and relating specifically to serious adverse events, different clinical studies have not demonstrated a significant increase in serious adverse events.6–9 Thus, in the combined safety study testing search phase (phase II) and III6–9 the rates of serious adverse were within the expected range. Moreover, in a combined safety analysis,30 incidence rates per 100 patient-years of serious infections were slightly lower than published and further demonstrate that there was no increase with time.
In the AMPLE study10 that compared abatacept with SC adalimumab, only 1.3% of patients treated with SC abatacept had serious adverse events compared with 3% in patients receiving adalimumab. With regard to skin reactions, only 3% developed clinical manifestations. These figures are somewhat lower than shown by etanercept and adalimumab, over 10%. The most common reactions are pruritus and erythema, and appear to have an upward tendency when abatacept is administered SC in monotherapy than when given in combination with MTX. With regard to autoimmune events, the occurrence rates were also very low, in any case not exceeding 2% from 6.8 to 10.30. The development of SC Abatacept did not arouse any alarm signals with respect to an increase in the tumor rate.
In summary, SC abatacept is presented as an effective and safe drug and, therefore, as an alternative to the many treatments currently available to the rheumatologist to treat RA. It also has the advantage of being the only biological agent that can be administered IV and SC, which may facilitate its use in certain patients.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that no experiments have been performed on humans or animals.
Data privacyThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
FinancingThis practical document has been financed through a contribution made to the Department of Rheumatology, La Paz University Hospital (AREPAZ), by Bristol Myers Squibb (Spain). The choice of experts was the sole responsibility of the project coordinator (EMM). No employee of the company participated in the scientific meetings or their development until the project was completed.
Conflict of InterestThe authors have no conflicts of interest
Please cite this article as: Mola EM, Balsa A, Martínez Taboada V, Marenco JL, Navarro Sarabia F, Gómez-Reino J, et al. Documento práctico para el uso de abatacept subcutáneo. Reumatol Clin. 2014;10:218–226.