To analyse a cohort of pregnant patients with systemic lupus erythematosus and compare the outcomes of both the disease and pregnancy with the results of previous studies conducted in the same geographical area.
Patients and methodsRetrospective cohort study of 37 women with systemic lupus erythematosus (64 pregnancies) followed in a multidisciplinary unit. Comparative study with similar Spanish studies identified after literature search.
ResultsOur cohort was characterized by an older age and by the presence of non-Caucasian patients. Although we found no clinical differences, from the serological point of view our cohort presented a higher frequency of antiphospholipid antibodies. Patients included in this study were treated more frequently with antimalarials and low-dose aspirin. Systemic lupus erythematosus flare frequency was very similar between the different studies, and we did not identify clear predictors for them. Although the rate of live births was similar among studies, the obstetric outcome of our series was better with a very low rate of preeclampsia, preterm birth and low birth weight newborn. The only predictor of adverse obstetric event was age.
ConclusionsAlthough changes in the therapeutic attitude and planning of pregnancy in recent years have not had a direct impact on the rate of systemic lupus erythematosus flares during pregnancy, they have meant an improvement in the obstetric results. The introduction of new variables independent of the disease such as age at conception, socio-cultural origin, or the availability of multidisciplinary units should be considered in the results of future studies.
Analizar una cohorte de pacientes embarazadas con lupus eritematoso sistémico y comparar los desenlaces tanto de la enfermedad como del embarazo con los resultados de estudios previos realizados en la misma área geográfica.
Pacientes y métodosEstudio de cohortes retrospectivo de 37 mujeres con lupus eritematoso sistémico (64 embarazos) seguidas en una consulta multidisciplinar. Estudio comparativo con los estudios españoles similares identificados tras revisión bibliográfica.
ResultadosNuestra cohorte se caracterizó por una edad más elevada y por la presencia de pacientes de origen no caucásico. Aunque no encontramos diferencias clínicas relevantes, serológicamente nuestra cohorte presentó una mayor frecuencia de anticuerpos antifosfolípido. Las pacientes incluidas en este estudio fueron tratadas más frecuentemente con antipalúdicos y aspirina. La frecuencia de brotes fue muy similar entre los distintos estudios, y no identificamos predictores claros para los mismos. Aunque la tasa de nacidos vivos fue similar, el desenlace obstétrico de nuestra serie fue mejor, con una baja tasa de preeclampsia, parto pretérmino y recién nacido de bajo peso. El único predictor de acontecimiento obstétrico adverso fue la edad.
ConclusionesSi bien los cambios en la actitud terapéutica y la planificación del embarazo no han tenido un impacto directo sobre la tasa de reactivación del lupus eritematoso sistémico durante el embarazo, sí que han supuesto una mejoría en los resultados obstétricos. La introducción de nuevas variables independientes de la enfermedad como la edad en la concepción, la procedencia sociocultural, o la disponibilidad de unidades multidisciplinares deberán ser consideradas en los resultados de próximos estudios.
Systemic Lupus Erythematosus (SLE) is an autoimmune disease which primarily affects women of child-bearing ages.1 Pregnancy in women with SLE is accompanied by a significant increase in risk for the mother and the baby.2,3 The development of preeclampsia is particularly common as an obstetric complication in patients with SLE.4 This complication which puts the mother’s life at risk, also increases the risk of the baby in the form of increased premature birth, low birth weight and foetal death.5 Furthermore, pregnancy in SLE patients may be accompanied by an increased risk of developing new flares of the disease both during pregnancy and during the postpartum period. These are general mild but in certain cases they may significantly compromise the obstetric prognosis.6 Among the main predictors of disease flare and poorer obstetric result is age, disease activity, serological profile and a background of lupus nephritis (LN).6,7
Little information is available on a national scale, with few studies and outdated publications.8,9 However, in recent years, care of SLE patients during pregnancy has changed considerably10–12 and this has led to simultaneous improvement in the prognosis of these patients. It may therefore be of interest to know whether the prognosis of pregnancy in SLE patients has changed in recent years in Spain.
The essential aims of this study were to: (a) study the impact of pregnancy on the course of SLE, particularly in the frequency of reactivation and its possible predictors; (b) study the impact of SLE in the development of pregnancy, paying particularly interest to the incidence of obstetric complications and (c) compare the data of our cohort with previously published cohorts in Spain.
Patients and methodsPatientsA retrospective cohort study with 37 women who were followed up in the Autoimmune Gravidarum Pathology Unit (multidisciplinary unit jointly managed by a specialist in obstetrics and specialist in rheumatology) of a tertiary hospital. Patients were attended for the period 1/2005–4/2019. All patients fulfilled at least 4 criteria of the American College of Rheumatology 199713 classification and/or criteria of the Systemic Lupus International Collaborating Clinics de 2012.14 Antiphospholipid syndrome (APS) was diagnosed in keeping with the modified Sidney criteria.15 This study was approved by the ethics and research committee of Cantabria.
Data collectionPatients were assessed at different times: on pre-conception consultation (in its defect, visit prior to pregnancy), during pregnancy (months 0, 3, 6 and 9), and postpartum period (6 months). For assessment of disease activity and structural damage, prior to each pregnancy and in the postpartum visit, the SLEDAI16 scale was used and the Systemic Lupus International Collaborating Clinics/ACR Damage Index.17 During pregnancy the SLEDAI-P18 was used. During the scheduled visits routine tests were carried out: haemogram, kidney function, urine elements and sediment, and anti-dsDNA, C3, C4 tests. It was considered that a flare of SLE had occurred when there was an increase in the level of activity (according to SLEDAI/SLEDAI-P) scale > 4 points or when an increase in medication for disease control was called for.
To evaluate the impact of SLE on pregnancies the following obstetric events were studied: miscarriage (loss of gestation prior to week 22 or when the foetus was under 500 g in weight), voluntary termination of pregnancy, foetal death, preeclampsia, intrauterine growth retardation (IGR), prematurity (birth < 37 weeks), caesarean and newborn (NB) parameters (gestational age, weight, low weight NB [<2500 g], APGAR and pH). An adverse pregnancy outcome (APO) was understood to be the existence of any of the following pathologies: miscarriage, foetal death, preeclampsia, IGR, premature birth and low weight NB. Preeclampsia and IGR were defined in keeping with the widely accepted criteria.19,20
Statistical analysisStatistical analysis was completed using the statistical package SPSS 25.0 SPSS 25.0 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). In the descriptive analysis frequencies, percentages, and means were used and as a dispersion measurement, standard deviation. With regard to inferential analysis, in the univariate analysis the Chi-square test was applied to compare 2 qualitative variables, the Students’ t test or Mann Whitney U test to compare quantitative variables. All P values < .05 were considered statistically significant. A multivariate logistic regression analysis was performed with the “enter” and “conditional backwards” methods following the variable selection criteria proposed by Hosmer and Lemeshow,21 which initially included those who achieved a significant level (p < .25) in the univariate analysis.
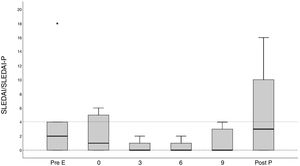
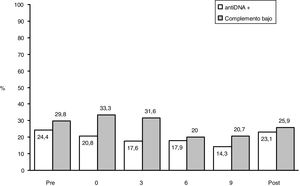
ResultsGeneral cohort descriptionThe cohort included 37 women with SLE who had had a total of 64 pregnancies. The general characteristics of the cohort and the treatments received are contained in Table 1. The mean age of the women when they conceived was 32.1 ± 5.04 years. Fifty five pregnancies were carried by Caucasian women and 9 by non Caucasian women (one of Asian original and the others Hispanic). 34 of the 64 pregnancies were unplanned. The patients with unplanned pregnancies were younger s (p = .002) and presented with a greater frequency of LN (p = .027). Patients presented with a high rate of cardiovascular risk factors, particularly a tobacco habit (37.5%). 17.2% of the women had suffered from a LN. in similar form to the rest of the country.22 In our cohort the rate of chronicity was low: 40 (74.1%) pregnancies with 0 points. In 31 (48.4%) pregnancies were APS positive and in 15 (23.4%) the anti-Ro/anti-La antibodies were positive. Eight women fulfilled the APS clinical criteria,15 with the rest being carriers of auto antibodies. The group with positive APS serology presented with more factors of cardiovascular risk, particularly obesity (16.7% vs. 0%, p = .008) and a tobacco habit (62.5% vs. 22.5%, p = .001). The pregnancies with positive APS serology received acetylsalicylic acid; more frequently (95.2% vs. 67.6%, p = .01) and HBPM (40.9% vs. 13.5%, p = .02.). Although we found no there to be no significant differences, pregnancies with positive APS serology presented with a lower rate of live NB (56.5% vs. 73%, p = .19). Globally, our cohort presented with a low level of activity (SLEDAI/SLEDAI-P ≤ 4) both at the time of the visit prior to pregnancy and during the same, with a slight increase in the postpartum period (Fig. 1.A). Similarly, this correlated with a low serological activity (normal levels of C3 and C4 and negative anti DNAn antibodies) (Fig. 1.B). Given that only 3 of the pregnancies presented with high levels of activity at the onset of pregnancy, the impact of the active disease could not be analysed.
General characteristics of study population.
| No. women/No. pregnancies | 37/64 |
|---|---|
| Age at diagnosis, mean (DE) | 25.2 (9.02) |
| Age at pregnancy, mean (DE) | 32.1 (5.04) |
| Caucasian, n (%) | 55 (85.9%) |
| Natural conception, n (%) | 60 (93.8%) |
| CV risk factors, n (%) | |
| Obesity | 4 (6.3%) |
| Tobacco habit | 24 (37.5%) |
| Diabetes mellitus | 1 (1.6%) |
| HBP | 3 (4.7%) |
| Dyslipidaemia | 1 (1.6%) |
| Treatment during pregnancy, n (%) | |
| Corticoids | 25 (39.1%) |
| Hydroxychloroquine | 41 (64.1%) |
| Azathioprine | 11 (17.2%) |
| Acetylsalicylic acid i | 45 (70.3%) |
| Low molecular weight heparin | 14 (21.9%) |
CV: cardiovascular; HBP: high blood pressure; No.: number; SD: standard deviation; %: percentage.
Clinical and serological evolution of the SLE in pregnant patients and during postpartum period.
(A) Evolution of the mean values of SLEDAI/SLEPDAI from the visit prior to pregnancy up until the postpartum period; (B) the percentage of patients with serology is shown (anti-DNA + and reduce complement) changed during the same period.
Post P: postpartum visit; Pre E: pre-conception visitor r visit prior to pregnancy.
In the overall cohort 12 flares were recorded during pregnancy (18.8% of pregnancies) and 12 during the postpartum period (30.8%). In Appendix B, supplementary tables 1 and 2 of the additional material the details of the disease flares are shown. In no case did they lead to an interruption of pregnancy. We were unable to identify any clear predictor of a flare in pregnancy, although being non Caucasian (8.2 [1–67], p = .049) and the use of corticoids during pregnancy (5.4 [0,99–29], p = .05)were the variables most associated with flares (Table 2).
Predictors (multivariate analysis) of adverse pregnancy outcomes (APO) and SLE flares during pregnancy and postpartum period.
| Predictors | ”enter” method | ”conditional” method | ||
|---|---|---|---|---|
| OR [IQE] | P | OR [IQE] | P | |
| Flare during pregnancy | ||||
| Non Caucasian | 5.53 [.5–56.8] | .15 | 8.2 [1.0–67] | .049 |
| CS during pregnancy | 4,4 [.7–26,1] | .10 | 5.4 [0.99–29] | .05 |
| Age at pregnancy | 1.18 [.95–1.5] | .13 | 1.18 [0.95–1.5] | .12 |
| Flare during postpartum period | ||||
| Non Caucasian | 10.53 [.7–158] | .09 | 7.9 [.6–101.7] | .11 |
| CS before pregnancy | 3.3 [.35–30.8] | .3 | 4.4 [.83–23] | .08 |
| APO | ||||
| HCQ at onset of pregnancy | 4.02 [.9–17.8] | .07 | 4.2 [.99–18] | .052 |
| CS during pregnancy | 3.2 [.4–23.4] | .25 | 2.99 [.81–11] | .09 |
| Age at pregnancy | 1.17 [.99–1.4] | .058 | 1.2 [1.02–1,4] | .025 |
APO: Adverse Pregnancy Outcome; CS during pregnancy: corticosteroids at any time during pregnancy; CS prior to pregnancy: corticosteroids before pregnancy; HCQ: hydroxychloroquine; IQR: interquartile range SLE: systemic lupus erythematosus; OR: odds ratio.
62.5% of pregnancies terminated with a live NB (Table 3). In general 26 APO (40.6%) were recorded. There was only one case of preeclampsia (1.6%). Fewer than 10% of patients had a preterm birth. The mean gestational age was slightly above 37 weeks and less than 10% of the NB was of low birth weight. In multivariate analysis, age was the only parameter associated with a higher risk of suffering an APO (1.2 [1,02–1,4], p = .025) (Table 2).
Main obstetric results in studies performed in Spain on systemic lupus erythematosus and pregnancy.
| De la Hera et al. | Carmona et al.8 | Cortés-Hernández et al.9 | |
|---|---|---|---|
| Miscarriage, n (%) | 18 (28.1%) | 3 (5%) | 15 (14.5%) |
| IIP. n (%) | 2 (3.1%) | 4 (6.7%) | 8 (8%) |
| Late foetal death, n (%) | 1 (1.6%) | 5 (8.3%) | 12 (11.6%) |
| Preeclampsia, n (%) | 1 (1.6%) | 5 (9.5%) | 2 (3.3%) |
| IGR, n (%) | 2 (3.1%) | 5 (9.4%) | 24 (35.3%) |
| Preterm birth, n (%) | 6 (9.4%) | 11 (20.8%) | 19 (28%) |
| Caesarean, n (%) | 14 (21.9%) | 14 (26.4%) | 13 (20%) |
| NB alive, n (%) | 40 (62.%) | 48 (75%) | 68 (66%) |
| Gestational age, mean (SD) or [range] | 37.8 (1.89) [31-41] | 37.0.4 (4.12) | 37 [24-41] |
| NB weight, mean (SD) or [range] | 2860 (538) [949-3900] | 2809 (918) | 2162 [800-4100] |
| LGAW, n (%) | 6 (9.3%) | 13 (24.5%) | ND |
| Neonatal lupus, n (%) | 0 (0%) | 2 (3.77%) | 1 (1.4%) |
IGR: Intrauterine Growth Retardation; IIP: Involuntary Interruption of pregnancy; LGAW: Low Gestational Age Weight; ND: No Data; NB: Newborn; SD: Standard Deviation.
A search was made on Pubmed introducing the following parameters [Systemic lupus erythematosus] and [pregnancy] and [Spain], with only 2 Spanish studies available which addressed the impact of SLE in pregnancy8,9 (Table 4): the Carmona et al.8 study published in 1999, and the Cortés-Hernández et al.9 study in 2002, but the cohort selected was studied until 1999.
General characteristics of studies performed in Spain on systemic lupus erythematosus and pregnancy.
| De la Hera et al. | Carmona et al.8 | Cortés-Hernández et al.9 | |
|---|---|---|---|
| N.o women | 37 | 46 | 60 |
| N.o pregnancies | 64 | 60 | 103 |
| Study period | 2005–2015 | 11/1985–12/1996 | 1984–1999 |
| Study type | Retrospective | Prospective | Prospective |
| SLE activity evaluation | SLEDAI-P scale | LACC scale | SLEDAI scale |
| Mean age at pregnancy (range) | 32.1 (19–42) | 28.6 (20–42) | 28 (18–42) |
| Active disease on conception, n (%) | 3 (4.7%) | 4 (6.7%) | 7 (11.6%) |
| SLE diagnosis during pregnancy (%) | 0% | 3.3% | 3.3% |
| Lupus nephritis, n (%) | 11 (17.2%) | 10 (16.7%) | 12 (20%) |
| Anti-Ro+, n (%) | 15 (23.4%) | 15 (25%) | 14 (23%) |
| APS + serology, n (%) | 31 (48.4%) | 16 (30.4%) | 17 (28%) |
| Corticoids at onset of pregnancy, n (%) | 23 (35.9%) | 9 (15%) | 38 (63%) |
| HCQ at onset of pregnancy, n (%) | 38 (59,4%) | 0 (0%) | 29 (48%)a |
| AZA at onset of pregnancy, n (%) | 8 (12.5%) | 2 (3.3%) | 3 (5%) |
| ASA at onset of pregnancy, n (%) | 45 (70.3%) | 16 (34.7%) | 16 (15.5%) |
APS: Antiphospholipid Syndrome; ASA: Acetylsalicylic Acid; AZA: azathioprine; HBP: High Blood Pressure; HCQ: Hydroxychloroquine; SLE: Systemic Lupus Erythematosus.
If we look at our cohort, mean age is 32.1 years, approximately 4 years more than the mean age of the other studies.8,9 From the serological viewpoint, the prevalence of anti-Ro/La antibodies is very similar among the 3 cohorts studied, although the frequency of APS serology is higher in ours. The same occurs with the frequency of LN, which is very similar in all 3 studies. From a therapeutic viewpoint, almost 60% of our overall cohort received antimalarial medication from onset, compared with 48% of the pregnancies in the Cortés-Hernandez et al.9 study and none in the Carmona et al.8study. The pregnancies of Cortés Hernández et al.9 received almost 30% more corticoids. From the viewpoint of obstetric outcomes (Table 3), in general, the results from our study are an improvement on those of the other studies in several aspects. Globally, the rate of live NB is very similar in the 3 series, although in ours early miscarriages are more frequent (possibly due to differences in study designs). The rate of foetal death and voluntary interruption of pregnancy is lower. Furthermore, our frequency of preeclampsia, IGR and pre-term birth is clearly lower, although the frequency of caesareans is very similar.
The Carmona et al.8 study makes a comparison between women with and without LN. As shown in Appendix B supplementary Table 3 of the additional material, the results are similar to ours. The LN group had more flare in pregnancy, more HBP, more premature births, a slightly lower gestational age and a lower NB weight. However, and despite the similarities in trends of both cohorts separately, when the study outcome is compared with ours, in general, our cohort presented with less HBP and fewer premature births, and the gestational age and NB weight of our patients with LN was higher.8
DiscussionThis study describes the outcome of pregnancy in patients with SLE in a Spanish cohort followed up in a multidisciplinary unit in the 21 st century. Although it is true that the global obstetric outcome is similar to that of previous cohorts, we did observe certain changes in the therapeutic attitude which accompanied an improvement in the obstetric outcome. Furthermore, and although the rate of flares during pregnancy and after birth continued to be within the average parameters of other series, most flares were controlled with treatment and did not significantly impact obstetric prognosis.
From an epidemiological viewpoint there are 2 essential aspects to highlight when comparing the different cohorts. One determining factor could be age at conception. If we look at our cohort, mean age is approximately 4 years older than the mean age of the other studies.8,9 The fact that our pregnancies are those of older women is somewhat intrinsic to social and employment motives of this century, and implies a higher risk of APO, regardless of other variable such as the level of activity or organic damage. Unlike that presented in other Spanish studies, in our cohort, 14% of pregnancies occurred in non Caucasian women. It is worth highlighting that non Caucasian women had mostly planned pregnancies. These women were characterised by having a higher frequency of LN and greater organic damage, a consequence of a higher prevalence of HBP. Furthermore, non Caucasian women were significantly younger (p = .004) than Caucasian ones, a factor which is also related to recent studies with a worse prognosis of SLE in the pregnancy.6,7 Due to increased seriousness, more medication was required, even the use of corticoids and immunosuppressants. Despite all of this, and probably due to the exhaustive control of these patients in a multidisciplinary unit, the obstetric outcomes did not differ significantly from those of the Caucasian women.
The percentage of women with a flare up during pregnancy ranged between 25%–65% from some studies to others.6,23 These discrepancies were essentially due to 3 aspects: differences in the definition of flare, the use of different parameters to measure SLE activity,24,25 and the different therapeutic approach of patients during pregnancy.26 What the studies do appear to conclude is that the majority of flares are mild to moderate in severity and mostly affect cutaneous, musculoskeletal, haematological and renal levels. This study shows a very similar flare rate to the 3 Spanish studies and in general a lower one to that described in the literature. This low rate may be related to the low proportion of patients with an active disease at the time of conception,6 a low number of patients diagnosed with SLE in pregnancy, and the significant way in which the 3 studies were conducted in specific units dedicated to the care of these patients.
From a serological viewpoint, the prevalence of specific autoantibodies with potential impact on pregnancy is highly similar to that of other studies except there is a greater prevalence of antiphospholipid antibodies. In pregnancies with patients who are the carriers of anti-Ro/La positive antibodies we did not detect any cases of neonatal lupos. This can be explained by 2 events. Firstly, the frequency of neonatal lupus described in carriers of these antibodies is of 1%–2%27. Purely statistical probability reasons it is not surprising that in our cohort no cases with this complication arose. Secondly, the use of HCQ was linked to a drop in the probability of developing a neonatal lupus, and in this sense, a significant proportion of our patients were found to be in treatment with anti-malarial medication before pregnancy, which may also have contributed to the absence of cases. It should also be noted that although only a statistically significant rise in IGR in the carriers of these antibodies was detected, we noted a trend28 in the presentation of a higher number of obstetric complications which cannot be ignored. More preterm births appear to present, along with a greater need for induction of births, more caesareans births and more complicated births. In view of these results it seems reasonable that, apart from the NB control because of the probability of developing neonatal lupus the specialists in charge of these patients were aware of the potential risk of obstetric complications at the end of pregnancy and the need for a more exhaustive monitoring during the last three months of pregnancy.
The higher percentage of antiphospholipid antibodies in our series may be explained by a higher sensitivity of the ELISA technique, which in the previous century did not depend on beta-2 glycoprotein I, nor did it measure the antibodies against it. Globally, although we observed a lower NB rate in patients with APS positive serology, the obstetric outcome obtained in our patients was very similar to that of the patient who did not present with these antibodies. The main sign was the presence of early miscarriages, with preeclampsia and late foetal death being very rare. These outcomes are obviously the consequence of a high percentage of patients who had received antiplatelet and/or anticoagulant therapy during pregnancy. One of the notable aspects and which was confirmed by previous studies to ours in groups of patients who were carriers of antiphospholipid antibodies29 is the high frequency of cardiovascular risk factors in the carriers of the APS serology and more specifically a high rate of obesity and tobacco habit. Pre-pregnancy control of these factors in patients with SLE would undoubtedly contribute to a better outcome.
Regarding obstetric outcomes, ours are an improvement in some aspects to those of other studies. The rate of live NB is very similar in the 3 series and although in ours early miscarriages were more common, the rate of foetal death and voluntary interruption of pregnancy was clearly lower. Also, although the rate of caesarean births was very similar our frequency of preeclampsia, IGR and preterm birth was lower than that of the other 2 cohorts. In this sense, it is possible that the lower frequency of preeclampsia could be related to higher use of acetylsalicylic acid.30 In our study we were not able to assess the possible protective effect of the hydroxychoroquine in the development of preeclampsia5 because only one patient developed it.
With the inherent limitations of a small sample size, this study shows that although changes to the therapeutic approach and planning of pregnancy events in recent years have not had a direct impact on the rate of reactivation of SLE during pregnancy, they have led to an improvement in the obstetric results of our patients. The introduction of new, independent variables of the disease, such as age at conception, sociocultural origin and availability of multidisciplinary units for treating patients with autoimmune diseases during pregnancy should be considered in the result of the next studies.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: de la Hera Madrazo M, Muñoz Cacho P, Riancho Zarrabeitia L, Álvarez Rodríguez L, Haya A, López-Hoyos M, et al. Embarazo y lupus eritematoso sistémico en España: ¿ha cambiado algo en el siglo xxi? Reumatol Clin. 2022;18:42–48.